Abstract
Public opinion surrounding the recreational use and therapeutic potential of cannabis is shifting. This review describes new work examining the behavioural and neural effects of cannabis and the endocannabinoid system, highlighting key regions within corticolimbic brain circuits. First, we consider the role of human genetic factors and cannabis strain chemotypic differences in contributing to interindividual variation in the response to cannabinoids, such as THC, and review studies demonstrating that THC-induced impairments in decision-making processes are mediated by actions at prefrontal CB1 receptors. We further describe evidence that signalling through prefrontal or ventral hippocampal CB1 receptors modulates mesolimbic dopamine activity, aberrations of which may contribute to emotional processing deficits in schizophrenia. Lastly, we review studies suggesting that endocannabinoid tone in the amygdala is a critical regulator of anxiety, and report new data showing that FAAH activity is integral to this response. Together, these findings underscore the importance of cannabinoid signalling in the regulation of cognitive and affective behaviours, and encourage further research given their social, political, and therapeutic implications.
Keywords: cannabis, phytocannabinoids, endocannabinoid system, THC, cannabidiol, CB1 receptor, AEA, FAAH, corticolimbic circuits, prefrontal cortex, amygdala, hippocampus, individual differences, decision-making, social interaction, emotional salience, associative learning, anxiety, elevated plus maze, striatum, nucleus accumbens, ventral tegmental area, dopamine, reward processing, COMT, NRG1, schizophrenia, executive function, Iowa Gambling Task, delay-discounting, risk-discounting
1. Introduction
In recent years, the social and political landscape surrounding cannabis use has been the focus of heightened scrutiny. Cannabis is the most widely used illicit drug with an estimated 180 million adults using the drug annually (SAMSHA, 2014; UNODC, 2015). However, a growing number of countries and jurisdictions have reformed cannabis laws so that personal consumption of the drug is no longer severely punishable (UNODC, 2015), while others have legalized its use for medicinal purposes. Recreationally, the drug is used for the “high” it produces, which includes feelings of relaxation and euphoria. However, these effects are biphasic, and in some individuals this high can manifest as anxiety, impaired cognition, and psychotic-like states such as in schizophrenia. Such adverse effects highlight the risks associated with cannabis exposure, with a number of studies showing that prolonged use may lead to adverse life outcomes and possible dependence in select users (American Psychiatric Association, 2013; Fergusson and Boden, 2008; Horwood et al., 2010). While for others, cannabis use may provide therapeutic benefits for the relief of pain, spasticity, nausea and vomiting.
The psychoactive effects of cannabis are primarily mediated by Δ9-tetrahydrocannabinol (THC), which is one of at least 70 phytocannabinoids found in the plant (Elsohly and Slade, 2005). THC binds to the presynaptic CB1 receptor that, together with CB2 receptors and the endogenous cannabinoids, 2-arachidonylglycerol (2-AG) and N-arachidonylethanolamine (anandamide; AEA), comprise the endocannabinoid system. CB1 receptors are located in key regions throughout corticolimbic brain networks, such as the prefrontal cortex (PFC) and amygdala, which functionally interact with subcortical dopamine pathways (Tan et al., 2014). As such, aberrations of the endocannabinoid system are increasingly recognized as etiological factors in several neuropsychiatric syndromes, including schizophrenia, anxiety, and mood disorders (Bossong and Niesink, 2010; D'Souza et al., 2005; Hillard and Liu, 2014; Lutz et al., 2015; Papini et al., 2015; Passie et al., 2012; Saito et al., 2013; Semple et al., 2005; Smit et al., 2004; Tan et al., 2014). These conditions may involve deficits in executive function, emotional processing and social behaviours, and/or co-morbidities with affective or addiction-related phenomena—in essence, broad deficits in behavioural processes mediated by corticolimbic circuits. Indeed, growing evidence from clinical and preclinical research demonstrates that CB1 receptor transmission within these networks strongly regulates the expression of cognitive and emotional behaviours (Arnold et al., 2012; Crane et al., 2013; Hajos and Freund, 2002; Hillard and Liu, 2014; Laviolette and Grace, 2006b; Lutz et al., 2015; Papini et al., 2015; Pattij et al., 2008; Tan et al., 2014)
In this review we will highlight key brain loci that are modulated by cannabinoid transmission, which may subserve the cognitive-impairing, pro-psychotic, and anxiety-regulating actions of cannabis. Given that not all individuals experience adverse effects, we will first describe factors that mediate interindividual variation in the behavioural response to cannabinoid drugs. This includes a number of identified gene variations, as well as different phytocannabinoids in the plant itself, which can modulate the neural and behavioural effects of THC. We will also review evidence suggesting that acute and/or regular THC exposure impairs frontal-cortical functioning, as evidenced by deficits in executive abilities following use. This section specifically focuses on the effects of cannabinoids in both clinical and preclinical models of decision-making, given that optimal cost/benefit decision-making is mediated by corticolimbic circuits. Focus will then shift to the endogenous cannabinoid system, and how CB1 signalling in the PFC and ventral hippocampus (vHIPP) modulates mesolimbic dopamine activity, dysregulation of which may underlie the emotional processing deficits observed in psychotic disorders like schizophrenia. Lastly, we will review data suggesting that variations in amygdalar endocannabinoid signalling could contribute to vulnerability to anxiety-related disorders and trait anxiety. Each section reflects a symposium presentation at the 2015 International Behavioural Neuroscience Society meeting in Victoria, BC, Canada, and all include background literature and new data; readers are guided to more comprehensive reviews on the subject throughout each section.
2. Individual differences in response to cannabis: contribution of genetic factors and strain differences
It is clear from human research that there is great interindividual variation in response to cannabis and the cannabinoids. For some, cannabis use is pleasurable and enhances creative thinking, while for others it may provoke anxiety, panic, memory loss and, in rare instances, psychotic-like states. In this section we will review research that seeks to explain variation in response to cannabinoid exposure. One explanation for this variation is genetic disposition, with some individuals being genetically prone to the adverse actions of cannabinoids, while others may be resilient. We will examine clinical and preclinical evidence that demonstrates specific genes modulate the neurobehavioural actions of THC. Another explanation for divergent cannabinoid response centres on the type of cannabis people choose to smoke. Cannabis’ psychoactive effects are primarily mediated by THC, but growing evidence highlights that other phytocannabinoids in the plant have unique properties that may modulate the actions of THC. Both human and clinical studies suggest cannabidiol (CBD) may protect against some of the adverse actions of THC on the brain. Therefore, cannabis with a balance of CBD and THC may be better tolerated than high THC varieties. We will also briefly review preclinical research that assesses whether non-psychoactive phytocannabinoids like CBD modulate the effects of THC, and the possible molecular mechanisms that explain these interactions.
2.1 Probing the genetic basis for interindividual response to cannabinoids
The isolation of genes that modulate cannabinoid action on the brain and behaviour may help explain why only a small proportion of cannabis users develop drug dependence or psychosis. Characterisation of vulnerability genes to cannabis-induced addiction or mental illness could pave the way for preventative approaches where those at genetically high risk could be forewarned of the potential danger of experimentation with cannabis. In addition, one of the major impediments to the therapeutic development of cannabinoid medicines is their tendency to increase the risk of addiction and psychosis. Therefore, a pharmacogenetic approach would allow those at risk of detrimental effects to be isolated, leaving low risk patients to benefit from cannabinoid-based therapy.
Genome-wide association studies (GWAS) and analysis of specific single nucleotide polymorphisms (SNPs) has yielded numerous candidate genes that may moderate the risk of developing cannabis-induced psychosis or addiction. Interestingly there is an emerging picture that genes implicated in the pathophysiology of schizophrenia also contribute to the risk of cannabis dependence and vice versa. For example, the largest GWAS to date examining risk genes for cannabis dependence isolated the novel antisense transcript RP11-206M11.7, the solute carrier family 35 member G1 gene (SLC35G1); and the CUB and Sushi multiple domains 1 gene (CSMD1) (see Table 1) (Sherva et al., 2016). Notably, CSMD1 is expressed in developing neurons, may regulate the complement system, and has been associated with increased schizophrenia risk (Schizophrenia Working Group of the Psychiatric Genomics, 2014). Other genes that have been linked to cannabis dependence are endocannabinoid system genes such as the cannabinoid receptor 1 gene (CNR1) and the fatty acid amide hydrolase gene (FAAH) (Buhler et al., 2015). Increased risk of cannabis-induced psychosis has been linked to the catechol-O-methyltransferase gene (COMT), the dopamine D2 receptor gene (DRD2), the brain derived neurotrophic factor (BDNF) gene and the protein kinase B (AKT1) gene (Caspi et al., 2005; Colizzi et al., 2015; Decoster et al., 2011; Di Forti et al., 2012; van Winkel, 2011).
Table 1.
Genes associated with increased risk of cannabis dependence and psychosis.
| Cannabis dependence genes | ||
| Gene | Function | SNPs |
| RP11-206M11.7 | Unknown | rs143244591 |
| SLC35G1 | Nucleotide sugar transport | rs146091982 |
| CSMD1 | Complement system regulation | rs77378271 |
| CNR1 | Endocannabinoid signaling | rs806380 |
| FAAH | Endocannabinoid metabolism | rs324420 |
| NRG1 | Neurotrophic factor | rs17664708 |
| MDR1 | Drug efflux pump | rs1045642 |
| Cannabis psychosis genes | ||
| Gene | Function | SNPs |
| DRD2 | Dopamine signaling | rs1076560 |
| BDNF | Neurotrophic factor | rs6265 |
| AKT1 | Kinase signaling | rs2494732 |
| COMT | Dopamine metabolism | rs4680 |
GWAS are limited because they cannot infer causation and they seldom probe the molecular, cellular and brain anatomical basis for gene-environment (G×E) interactions in cannabis use disorders. Animal studies have assisted in this regard, enhancing the biological plausibility of G×E interactions by allowing causative links to be drawn and increasing our understanding of the biological mechanisms involved. Here we will review examples of forward and back translation between human and animal research findings that have greatly improved our understanding of the biological bases of gene-cannabinoid interactions.
The identification of susceptibility genes for schizophrenia, such as neuregulin 1 (NRG1) and COMT, led to the development of animal models with targeted deletions of these genes. In a seminal study, mice with heterozygous deletion of Nrg1 (Nrg1 HET mice) displayed increased sensitivity to the behavioural actions of THC (Boucher et al., 2007a). Notably, THC selectively improved sensorimotor gating function in Nrg1 HET mice but not wild type mice, as measured in the prepulse inhibition of startle paradigm (PPI). The results with THC were also confirmed with a synthetic analogue of THC, CP 55,940, which acutely promoted PPI deficits in wild-type mice, but facilitated PPI in Nrg1 HET mice (Boucher et al., 2011). The atypical effect of THC on Nrg1 HET mice appeared to be dependent on stress and correlated with increased activation of stress circuitry in the brain, with pronounced effects in the lateral septum (Boucher et al., 2007b). Interestingly, more recent studies reported that stress decreased expression of Nrg1 in the brain (Brydges et al., 2014; Makinodan et al., 2012), and partial genetic deletion of Nrg1 altered behavioural and neurobiological responses to stress (Chohan et al., 2014a; Chohan et al., 2014b; Desbonnet et al., 2012; Taylor et al., 2011).
Nrg1 also moderates the neuroadaptive responses to repeated cannabinoid exposure. Nrg1 HET mice more rapidly developed tolerance to cannabinoid-induced hypothermia and locomotor suppression than wild-type mice (Boucher et al., 2011). Conversely, Nrg1 HET mice were resistant to development of tolerance to cannabinoid-induced anxiety-related behaviour. These effects again correlated with a selective induction of Fos transcription factors in the lateral septum of Nrg1 HET mice. This brain region interfaces with numerous corticolimbic components such as the prefrontal cortex, hippocampus, amygdala and hypothalamus. Repeated THC exposure during adolescence exacerbated the hyperlocomotor phenotype of Nrg1 HET mice in adulthood and altered the expression of various neurotransmitter receptors (Long et al., 2013). For example, THC exposure selectively increased NMDA receptor expression in the hippocampus of Nrg1 HET mice, which was associated with increased expression of proteins that transport and stabilise NMDA receptors at the synaptic membrane (Spencer et al., 2013). Studies also suggest Nrg1 influences the endocannabinoid system. Nrg1 modulated the effects of adolescent THC exposure on the expression of CB1 receptors (Long et al., 2013) and NAPE-PLD, an enzyme that synthesizes the endocannabinoid anandamide (Spencer et al., 2013). In addition, chronic Nrg1 exposure increased the expression of MAGL, which decreased levels of the endocannabinoid 2-AG and impaired long-term depression in hippocampal slices in vitro (Du et al., 2013).
The findings that Nrg1 modulates the effects of cannabinoids in mice have been translated to humans. For example, NRG1 SNPs were shown to worsen THC-induced information processing dysfunction (Stadelmann et al., 2010) and increase the risk of developing cannabis dependence (Han et al., 2012). Another example of a gene that influences cannabinoid action is COMT which encodes an enzyme that catabolises dopamine. Variation in COMT was first discovered to increase the risk of developing positive symptoms of schizophrenia in humans (Caspi et al., 2005; Pelayo-Teran et al., 2012; Radhakrishnan et al., 2014). In addition, SNP of COMT moderated THC-induced impairments in executive function (Verdejo-Garcia et al., 2007) and working memory (Tunbridge et al., 2015). COMT did not influence reversal learning deficits induced by inhalation of cannabis vapour (Spronk et al., 2016), highlighting that genetic modulation of THC’s effects may vary depending on the presence of other phytocannabinoids. However, this discrepancy may also be simply explained by the lower bioavailability of smoked cannabis compared to intravenous THC, or the greater variation conferred by intersubject variability in smoking dynamics such as inhalation volume and depth. The work of Waddington and colleagues subsequently back translated the human findings showing COMT knockout mice display altered behavioural sensitivity to cannabinoids (for a detailed review see O'Tuathaigh et al., 2014). Moreover their research enhanced our neurobiological understanding of the COMT-cannabinoid interaction by showing COMT mice were more vulnerable to various schizophrenia-relevant neurobiological changes promoted by cannabinoids, such as reductions in parvalbumin-positive GABAergic interneurons, and decreased dopaminergic cell size in the ventral tegmental area of the mesolimbic system.
It is also possible that variation in genes that influence the pharmacokinetics of cannabinoids might play a role in moderating the risk of developing cannabis dependence or psychosis. ABC transporters are drug efflux pumps expressed by blood brain barrier endothelial cells and function to move substrate drugs from brain tissue into the peripheral blood supply. Polymorphism of MDR1, the gene that encodes the ABC transporter P-glycoprotein (P-gp), increased the risk of developing cannabis dependence (Benyamina et al., 2009). Mouse studies then showed that THC is a P-gp substrate, as P-gp knockout mice displayed greatly enhanced brain concentrations and hypothermic effects of THC compared to wild-type mice (Spiro et al., 2012). Thus it is likely that SNP of MDR1 alters the brain uptake of THC in cannabis users, thereby influencing the risk of developing cannabis dependence. This provides yet another example of the importance of cross-translational research in improving our understanding of how genes contribute to the moderation of risk for cannabis use disorders.
2.2 Examining non-psychoactive phytocannabinoid modulation of the pharmacological effects of THC
Aside from genetic factors, individual differences in response to cannabis use are dictated by different cannabis plant strains with their varying compositions of phytocannabinoids. Concentrations of THC in cannabis have dramatically increased over the last 30 years and CBD levels have significantly decreased (Arnold et al., 2012; UNODC, 2015). Consistent with potency data collected in the US and UK, Australian street cannabis contains on average 15% THC with only 0.1 % CBD content (Swift et al., 2013). This may be of significant public health concern, as a growing body of evidence suggests that CBD may protect against the adverse actions of THC.
CBD content does not appear to modulate the subjective high caused by cannabis ingestion (Hindocha et al., 2015; Morgan et al., 2010a). Although, administration of high CBD:THC strains of cannabis reduced attentional bias and liking of drug related stimuli (Morgan et al., 2010a). Further, naturalistic and controlled human studies show that CBD attenuated THC-induced memory impairment, appetite stimulation, anxiety, and psychotic-like states (Englund et al., 2013; Hindocha et al., 2015; Morgan and Curran, 2008; Niesink and van Laar, 2013; Zuardi et al., 1982). Such protective effects of CBD have prompted the viewpoint that breeding CBD back into the plant may make cannabis “healthy” (Mechoulam and Parker, 2013). This issue also pertains to medicinal cannabis that is now legal in various jurisdictions around the world, as utilizing strains with balanced CBD to THC concentrations may maximize therapeutic endpoints while minimizing side effects.
The strength of preclinical research is that it allows controlled administration of various THC and CBD dose ratios, as well as providing mechanisms for their interactions (for a detailed overview of studies see Arnold et al., 2012). Animal studies have shown that CBD and THC interactions are highly complex and various factors need to be considered: the dose ratio, specific doses used, timing of administration (whether administered as a cocktail or temporally separated) and measured endpoint (Arnold et al., 2012; Zuardi et al., 2012). A number of studies indicate that a cocktail of CBD and THC may have greater therapeutic benefits than each of the compounds alone. For example, CBD has been shown to enhance the analgesic effects of THC (Varvel et al., 2006). In addition, combined subthreshold doses of CBD and THC additively disrupted reconsolidation of fear memory in rats (Stern et al., 2015), which may hold promise for cannabis-based pharmacotherapies in individuals with post-traumatic stress disorder.
Preclinical studies have also investigated whether CBD inhibits the adverse effects of THC. For example, CBD was shown to hinder the aversive effects of THC in rodents as measured in the conditioned place aversion paradigm and in animal models of anxiety (Todd and Arnold, 2016; Vann et al., 2008). However, there is conflicting evidence for whether CBD protects against THC-induced impairments in memory. Administration of CBD potentiated the impairing effects of THC on spatial memory in mice in the radial arm maze (Hayakawa et al., 2008), yet reversed the impairing effects of THC on spatial working memory in rats in a delayed-matching-to-place version of the Morris water maze (Fadda et al., 2004). The latter study used cannabis extracts, with a CBD-rich extract promoting less memory impairment than a THC-rich extract, raising the possibility that other phytocannabinoids need to be considered in examining interactions between THC and CBD.
Interactions between CBD and THC on behaviour may involve a multitude of both pharmacodynamic and pharmacokinetic mechanisms. With respect to CBD-induced augmentation of THC effects, combined THC and CBD exposure has been shown to increase CB1 receptor expression in the hippocampus and the hypothalamus, in a manner that correlated with greater memory-impairing and hypothermic effects of THC respectively (Hayakawa et al., 2008). A more recent study could not replicate this effect in rats, but showed that CBD treatment increases brain and blood levels of THC (Klein et al., 2011). ABC transporters, mentioned in section 2.1, might play a role in this phenomenon. CBD inhibits P-gp as well as the breast cancer resistance protein (BCRP) transporter, and THC is an ABC transporter substrate (Holland et al., 2008; Holland et al., 2007; Spiro et al., 2012). Therefore, CBD might enhance THC brain levels by inhibiting ABC-mediated transport of THC from the brain to the peripheral blood supply. Given that P-gp polymorphisms moderate risk for cannabis dependence (Benyamina et al., 2009), it is clear from the data reviewed thus far that both variation in the genetics of individual users and between strains of cannabis, particularly with respect to the THC:CBD ratio, will dictate the psychobehavioural effects of cannabis use.
CBD’s ability to hinder the effects of THC might be explained via cannabinoid receptor modulation. This could be achieved through indirect competition, as CBD inhibits fatty acid amide hydrolase (FAAH) which increases anandamide concentrations that would then compete with THC for the CB1 receptor (McPartland et al., 2015; Pertwee, 2008), although this may not be a very potent effect. Alternatively, an important new finding suggests CBD inhibits the effects of THC through negative allosteric modulation of the CB1 receptor (Laprairie et al., 2015), whereas earlier studies indicate a potential involvement of the serotonin system, particularly the 5-HT1A receptor (Russo et al., 2005; Scopinho et al., 2011). At a network level, clues on the brain circuitry involved in CBD’s ability to inhibit the neuropharmacological actions of THC are provided by a recent study. CBD administration robustly inhibited THC-induced c-Fos expression, a marker of neuronal activation, in the medial preoptic nucleus of the hypothalamus, the dentate gyrus of the hippocampus and periaqueductal gray (Todd and Arnold, 2016). These brains regions are implicated in the hypothermic, memory-impairing and anxiogenic actions of THC, respectively. Future work may further explore the precise corticolimbic network mechanisms involved in the interactions of THC with CBD. The subsequent sections of this review will focus on cannabinoid and endocannabinoid actions in different regions and functional domains of corticolimbic circuits.
Future studies could also expand beyond CBD and THC interactions to investigate the interactive effects of other phytocannabinoids such as cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC) and Δ9-tetrahydrocannabivarin (THCV). Few studies have been conducted in this area. An early study demonstrated that CBN and THC promoted synergistic depressant effects on animals (Takahashi and Karniol, 1975). More recently, THCV was shown to antagonise some of the effects of THC in mice, such as analgesia and hypothermia, due to THCV behaving as a cannabinoid receptor antagonist (Pertwee et al., 2007). The co-administration of CBC with THC promoted additive effects on catalepsy, analgesia and inflammation in mice, which might be attributable to CBC enhancing the brain concentrations of THC (DeLong et al., 2010). Clearly more studies are needed in this area, to carefully evaluate the pros and cons of cannabinoid combination psychopharmacology, and assist in the development of medicinal cannabis and cannabinoid therapies.
3. Cannabinoid modulation of prefrontal cortical function: focus on decision-making
As described above, genetic vulnerabilities and strain composition can dictate inter-individual response to cannabinoids. This is especially true for the cognitive-impairing effects of the drug, where some individuals are more susceptible to deficits in executive functioning following acute or regular cannabis use. Indeed, the influence of cannabis on the neurocognitive domains of attention, learning, and inhibitory control have been thoroughly investigated and reviewed elsewhere (see Crane et al., 2013). In contrast, relatively little research has investigated the effect of cannabis on decision-making processes. In its most basic form, decision-making involves evaluating and forming preferences amongst available options, making a choice, evaluating its outcomes, and subsequently using this information to guide future behavior (Ernst and Paulus, 2005). Outside the routine decisions of day-to-day life, optimal cost/benefit decision-making becomes crucial when choices carry with them the possibility of substantial gain or loss; not unsurprisingly, perturbations in decision-making are observed in almost every mental illness (Goschke, 2014). The effects of cannabis are particularly important to consider given that heavy use is associated with impaired decision-making, as evidenced by persistent use despite negative physical, psychological, social, and legal outcomes (American Psychiatric Association, 2013). Neuroimaging studies have shown that prolonged cannabis use is associated with altered activity in the corticolimbic circuitry (i.e. PFC (Wesley et al., 2011), striatum (van Hell et al., 2010), amygdala (Gruber et al., 2009)) subserving cost/benefit decision-making (Floresco et al., 2008). In this section, we will review data from human and preclinical literature on cannabinoid modulation of decision-making, and will identify important questions the field must address in light of changing attitudes towards cannabis use.
3.1 Human studies on the effects of cannabis on decision-making
Naturalistic studies in regular cannabis users have assessed decision-making in a variety of tasks following periods of short (e.g. 12–18 h) or extended abstinence (e.g. >25 days). One such task is the Iowa Gambling Task (IGT), designed to assess real-life decisions involving uncertainty, reward, and punishment. In this paradigm, participants earn as much fictitious money as possible by choosing cards from four decks, each of which leads to varying amounts of monetary gain or loss as determined by set probabilistic schedules (Bechara et al., 1994; Bechara et al., 1999). Cannabis users displayed impaired decision-making in the IGT, being less able to bias behaviour towards the advantageous decks throughout a session (Moreno et al., 2012; Wesley et al., 2011; Whitlow et al., 2004). Functional imaging revealed that while users did not differ behaviourally from non-users during the initial strategy stage, they displayed reduced activity in the anterior cingulate and medial prefrontal cortices in response to losses (Wesley et al., 2011). Strikingly, neural responses to early IGT losses predicted future improvements in task performance in controls, but not cannabis users, suggesting that impaired decision-making may arise from an insensitivity to losses, consistent with reports that regular and heavy cannabis users show abnormalities in affective and reward processing (Gruber et al., 2009; Martin-Soelch et al., 2009; Nestor et al., 2010). Indeed, computational modelling of IGT performance in cannabis users reveals that these individuals tend to be under-influenced by loss magnitude, treating each loss as a constant and minor negative outcome regardless of the size of the loss (Fridberg et al., 2010; but see Bishara et al., 2009).
By contrast, a number of studies have shown that both adolescent and adult cannabis users show intact decision-making performance in the IGT, and often perform better than other drug-using groups, including cocaine and MDMA users (Dougherty et al., 2013; Gonzalez et al., 2012; Hermann et al., 2009; Quednow et al., 2007; Vaidya et al., 2012; Verdejo-Garcia et al., 2007). Notably, while Hermann et al. (2009) found no difference in IGT performance between cannabis users and controls, high levels of THC metabolites in hair were associated with poor performance in the final block of the task. In another study where cannabis users did not differ from controls in IGT performance, worse performance was associated with increased symptoms of cannabis dependence (Gonzalez et al., 2012). These studies suggest that while cannabis users as a group may not differ from control subjects in IGT performance, a “threshold effect” may exist, whereby decision-making impairments are prominent in heavy, but not casual, users (Bolla et al., 2005). Support for this notion comes from data showing that IGT deficits in heavy cannabis users persist following a 25-day abstinence period, and correspond to altered patterns of neural activity in the cerebellum, lateral orbitofrontal cortex, and dorsolateral PFC, relative to controls (Bolla et al., 2005).
Naturalistic studies have also assessed decision-making in tasks other than the IGT, in which subjects must decide between two options where the more preferred reward is discounted by temporal delay, risk, or physical effort. For example, adolescent and young adult, but not older adult, cannabis users demonstrate impaired decision-making in such a delay-discounting task, opting for smaller-sooner versus larger-later rewards (Dougherty et al., 2013; Johnson et al., 2010; Moreno et al., 2012). Furthermore, cannabis users make riskier selections in a two-choice gambling task, and are less willing to exert physical effort for large monetary rewards (Griffith-Lendering et al., 2012; Lane et al., 2005a; but see Gilman et al., 2015). Collectively, these findings together with those from IGT studies indicate that cannabis use is associated with impaired decision-making, the severity of which is likely related to age and frequency of use.
Although the acute effects of cannabis on decision-making have received less empirical attention, impairments in various tasks are evident under experimental conditions. Generally no impairments in IGT performance have been observed immediately following, or up to 2.5 h post-cannabis (up to 13% THC) ingestion (Ramaekers et al., 2006; Vadhan et al., 2007); however, the sensitivity of the IGT to detect acute drug effects has been called into question, and decision-making impairments following cannabis intake are observed in other tasks. For example, Lane et al., (2005 a,b) employed a task in which subjects chose between a non-risky option associated with guaranteed small monetary reward, and a risky option where payouts were larger, but uncertain. Administration of joints containing 3.58% THC increased selection of the risky option, and made subjects persist on this option following a win or loss relative to placebo (Lane et al., 2005b). This profile suggests that THC-induced risky decision-making may be due to an altered sensitivity to reward and loss outcomes, similar to the deficits thought to underlie the impaired IGT performance in chronic cannabis users described above (Fridberg et al., 2010; Wesley et al., 2011 but see McDonald et al., 2003). In contrast, THC did not affect delay-discounting of monetary rewards, but decreased the selection of larger rewards discounted by physical effort (Cherek et al., 2002; McDonald et al., 2003). Such acute challenge studies allow us to understand the basic cognitive processes that may be impinged upon by regular exposure to cannabis, including affective and reward processing; these emotionally-guided behaviours will be examined in more detail in section 4 with respect to the downstream effects of disrupted PFC cannabinoid signalling on subcortical dopamine activity.
An overview of the human literature reveals that regular or acute cannabis exposure is associated with impaired decision-making in some conditions but not others. These discrepancies likely relate to differing methodologies, such as the inclusion criteria used to select chronic users, the different dosing regimens used to administer THC, or by insufficient sample sizes required to detect moderate effects. In addition, issues of causation are difficult to tease apart in naturalistic human studies, as it is unknown whether repeated cannabis use per se produces decision-making impairments, or if impaired decision-making precedes, and possibly influences, the development of regular use (Bechara, 2005; Wang et al., 2013). Such issues can be better addressed in cross-translational research using preclinical models.
3.2 Animal studies on cannabinoid modulation of decision-making
The use of animal models of decision-making allows unparalleled experimental control relative to their human counterparts. In these cost/benefit decision models, rodents are typically given the choice of two options, where one is associated with small food reward and a nominal response cost, while the other option offers more reward but at a larger expense. As in human decision-making paradigms, the costs associated with a given decision can vary, and a substantial body of work has established that valuations involving delay, risk, and effort costs are subserved by overlapping, yet distinct, circuitries (Ghods-Sharifi et al., 2009; Hosking et al., 2014; Rudebeck et al., 2006; Winstanley et al., 2003).
Research using such models has shown that administration of THC modifies decision-making involving delay costs, eliciting rats to increase choice of the larger reward option as the delay to its receipt is lengthened (Wiskerke et al., 2011). This effect was subsequently antagonized by pre-treatment with rimonabant, a CB1 receptor antagonist/inverse agonist, indicating the THC-induced shift in choice was CB1 receptor-dependent. Notably, administration of WIN 55, 212-2, a synthetic CB1-prefering agonist, did not affect delay-based choice in the same task (Pattij et al., 2007), demonstrating that phyto- and synthetic cannabinoids with similar actions at the CB1 receptor can produce divergent behavioural effects. In contrast, direct infusions of arachidonyl-2-chloroethylamide (ACEA), a potent synthetic CB1 receptor agonist, into the orbitofrontal cortex (OFC) produced a robust shift towards the low reward option on a T-maze equivalent of the operant delay-discounting task (Khani et al., 2015). This effect is similar to that observed following post-training lesions of the OFC on the same task, and might suggest that presynaptic intra-OFC CB1 receptor activation induces decision-making deficits by dampening glutamate release in this region (Rudebeck et al., 2006).
Cannabinoid modulation of effort-based decision-making has been investigated using a T-maze task where rats must scale a 30 cm barrier to obtain the larger reward. Infusions of ACEA, but not the synthetic CB1 receptor antagonist AM251, into the anterior cingulate cortex (ACC) caused a robust shift in choice towards the small, unobstructed reward option, suggesting that rats were less willing to exert physical effort for the preferred reward (Khani et al., 2015). Lesions of the ACC, but not OFC, produce similar deficits in effortful decision-making, and when considered with the delay-discounting data above, it appears that CB1 receptors in distinct prefrontal regions are necessary when choosing between options varying in delay or effort (Rudebeck et al., 2006). Notably physical costs, such as scaling a barrier or pressing a lever, are typically employed to assess effort-based decision-making in rodents, but these tasks do not address costs that are cognitive in nature. This distinction is important, not only because the two forms of decision-making are subserved by somewhat dissociable neurobiological mechanisms, but because cognitive costs are more representative of the effort costs faced by humans in an industrialized society (Hosking et al., 2015). Indeed, links between cannabis use and impaired education, economic, and employment outcomes may reflect a fundamental deficit in effortful decision-making, whereby cannabis use decreases the willingness to expend the greater cognitive load associated with gaining lucrative prospects (Fergusson and Boden, 2008; Horwood et al., 2010; Hyggen, 2012).
A recently developed rodent Cognitive Effort Task (rCET) (Cocker et al., 2012), in which effort costs vary by the amount of visuospatial attention required to complete low- or high-reward trials, provided a behavioural model in which these issues could be addressed. THC administration dose-dependently decreased choice of the difficult, high-reward option requiring accurate detection of a brief (0.2 s) light stimulus amid a five-hole array; correspondingly, rats shifted choice to the easier, low-reward option where the light stimulus was presented for a longer duration (1 s) (Silveira et al., 2016). Importantly, the lack of effect of THC on attentional accuracy suggests that the choice shift was not due to an inability to complete high-reward trials. Moreover, THC-induced choice impairments were correlated with CB1 receptor density in the medial PFC, indicating that prefrontal CB1 receptors may contribute to THC-induced alterations in effortful decision-making. These findings complement those of Khani et al., (2015), where CB1 receptor agonism in the anterior cingulate cortex decreased physical effort-based decision-making. Interestingly, unlike the effects of THC, CB1 agonism by administration of WIN 55, 212-2 did not shift choice on the rCET, similar to findings noted earlier in a delay-based choice task (Pattij et al., 2007; Wiskerke et al., 2011). These discrepancies are likely related to the distinct pharmacodynamic profiles of these drugs (Felder et al., 1995), and/or differences in the intracellular signalling pathways they recruit, with THC a potent recruiter of the arrestin2 pathway, and WIN 55, 212-2 a recruiter of the classical G-protein Gαi/o and Gβγ pathways (Laprairie et al., 2014). These data emphasise that not all forms of cannabinoid receptor activation produce converging effects, and animal studies aiming to model the psychoactive effects of cannabis would do well to administer THC in lieu of its synthetic counterparts. In contrast, modulating endogenous cannabinoid tone, via CB1 receptor inverse agonism, or by inhibition of endogenous anandamide hydrolysis by FAAH, did not affect choice on the rCET (Silveira et al., 2016). Taken together with the null effects of CB1 antagonism reported with the delay and physical effort tasks, it appears that endocannabinoid signalling does not tonically regulate decision-making, at least under non-pathological conditions (Khani et al., 2015; Pattij et al., 2007; Wiskerke et al., 2011).
Overall, the preclinical literature suggests that THC induces decision-making deficits when choices involve delay and effort (both physical and cognitive) costs, and that this effect is mediated by CB1 receptors in distinct frontal cortical regions. However, to date no animal studies have investigated the role of the cannabinoid system in decisions involving risk costs (Ghods-Sharifi et al., 2009; Orsini et al., 2015b), despite the existence of validated models. Additionally, a rodent analogue of the IGT has been developed, but to our knowledge cannabinoid drugs have yet to be investigated on this task (Zeeb et al., 2009). Given its potential to parse out the specific neural processes mediating poor IGT performance in regular cannabis users, this would be a worthwhile avenue to pursue. Preclinical models could also benefit research assessing how CBD may moderate THC-induced decision-making impairments. As discussed in section 2.2, ratios of THC/CBD in cannabis can vary widely (Swift et al., 2013; Syed et al., 2014), CBD can attenuate THC-induced memory and attentional impairments (Morgan et al., 2010a; Morgan et al., 2010b), and both agents differentially influence brain activity when performing cognitive or emotional processing tasks (Bhattacharyya et al., 2015; Bhattacharyya et al., 2010; Borgwardt et al., 2008; Fusar-Poli et al., 2009). However, only one human study has attempted to relate decision-making impairments to CBD concentrations in hair (Hermann et al., 2009), and no studies have assessed how it might modulate THC’s effects. We addressed this gap in the literature by testing rats on the rCET following CBD administration (Silveira et al., 2016). While CBD had no effect in isolation, it modestly attenuated THC-induced decision-making impairments in “slacker” rats (i.e. those that preferred the cognitively easy, low-reward option), when administered at a 1:1, but not 1:10, ratio matching that in cannabinoid therapeutics. This finding both highlights the dose by ratio interactions that mediate the psychoactive effects of co-administered phytocannabinoids, and underscores the importance of considering interindividual differences in the diverse response to cannabis observed in the population, as discussed in section 2. Finally, it is important to note the various cognitive, emotional, and motivational subprocesses that interact in decision-making, many of which are also mediated by cannabinoid transmission (Orsini et al., 2015a). For example, the ability to form action-outcome (A–O) contingencies, which is crucial as subjects associate different choices with their anticipated outcomes, is dependent on dorsomedial striatal CB1 receptors (Goodman and Packard, 2015; Hart et al., 2014). Indeed, as will be discussed below, cannabinoid transmission in downstream limbic and striatal regions can have a profound impact on executive processes primarily attributed to the prefrontal cortex.
4. Cannabinoid modulation of mesocorticolimbic dopamine transmission: focus on emotional salience and memory formation
Cannabis-induced deficits in frontally-mediated executive processes, such as decision-making described in section 3, arise not only from local modulation of PFC activity but also its effects on downstream components of corticolimbic networks. Indeed, emerging evidence is pointing to a critical role for dysregulated cannabinoid signalling in both the PFC and vHIPP as potential underlying mechanisms associated with aberrant dopaminergic signalling, a cardinal feature of neuropsychiatric syndromes including schizophrenia. Several post-mortem studies demonstrate that individuals with schizophrenia have highly abnormal CB1 receptor expression patterns in the PFC (Dalton et al., 2011; Dean et al., 2001; Eggan et al., 2010; Volk et al., 2014). Furthermore, neuroimaging evidence suggests there are widespread alterations in CB1 receptor levels in patients with schizophrenia (Ceccarini et al., 2013; Ranganathan et al., 2016; Wong et al., 2010), including significantly reduced levels in the hippocampus (Ranganathan et al., 2016). While PFC dysfunction is primarily thought to underlie cognitive deficits, hippocampal dysfunction is associated with both positive and negative (e.g. blunted affect, social withdrawal) symptomology (Hajos and Freund, 2002; Nguyen et al., 2014). Literature concerning the role of endocannabinoid dysfunction in schizophrenia and the potential for cannabis to induce psychosis has been extensively reviewed elsewhere (Arnold et al., 2012; Bossong and Niesink, 2010; Chadwick et al., 2013; Gage et al., 2016; Radhakrishnan et al., 2014; Saito et al., 2013; Semple et al., 2005; Smit et al., 2004; Volk and Lewis, 2016). In this section, we review recent preclinical work showing that CB1 receptor transmission in the PFC and vHIPP is capable of functionally controlling mesolimbic dopaminergic activity states in the context of regulating emotional processing and memory formation. Given their breadth and complexity, we first provide an overview of the relevant circuits before moving on to the electrophysiological and behavioural data; avenues for further research are also discussed.
4.1 Prefrontal-cortical cannabinoid regulation of subcortical dopamine transmission
The mammalian PFC is a complex neural region that shares reciprocal functional connections with the mesolimbic dopamine pathway, including the ventral tegmental area (VTA) and nucleus accumbens (NAc). For example, VTA dopamine neurons send functional inputs to the PFC and, conversely, pyramidal output neurons from the PFC send glutamatergic projections to both dopaminergic and non-dopaminergic neurons in the VTA (Carr and Sesack, 2000a, b). Functionally, the CB1 receptor regulates the presynaptic release of both GABA and glutamate within the PFC and can thus regulate the activity states of pyramidal and interneuron populations (Domenici et al., 2006; Szabo and Schlicker, 2005). As such, CB1 receptor-mediated changes in PFC neuronal activity can profoundly influence the activity states of the mesolimbic dopamine system (Draycott et al., 2014). In addition, GABAergic interneurons in the PFC normally provide inhibitory, feedforward inputs to pyramidal output neurons. Naturally occurring endocannabinoids, such as 2-AG, are synthesized by pyramidal neurons and travel to CB1 receptor-expressing GABAergic terminals; CB1 receptor activation then leads to disinhibition of pyramidal neuron activity. In other words, cannabinoid transmission in the PFC serves to regulate an inhibitory, GABAergic ‘braking’ mechanism on excitatory outputs to VTA dopaminergic neurons. Given the importance of dopaminergic disturbances in schizophrenia and other psychiatric disorders, an important implication is that localised dysfunction in PFC cannabinoid signalling may alter the activity states of GABAergic interneurons versus pyramidal neurons and, in turn, lead to downstream dysregulation of mesolimbic dopaminergic activity.
Preclinical research has characterized a bi-directional effect of CB1 receptor signalling in the PFC during the processing of emotional salience and memory formation. For example, using an olfactory fear conditioning procedure wherein rats receive pairings of either sub-threshold (normally non-salient) or supra-threshold (highly salient) footshock stimuli, with olfactory cues paired with footshock delivery or its absence, activation of PFC CB1 receptors robustly potentiated the emotional salience of normally non-salient fear-related associative memories when measured behaviourally (e.g. fear-related freezing) and in terms of PFC neuronal activity (Laviolette and Grace, 2006a; Tan et al., 2010; Tan et al., 2011). More specifically, acute overstimulation of the local cannabinoid system with CB1 receptor agonists, including WIN 55, 212-2, strongly increased PFC neuronal associative responses to fear-related conditioned cues and shifted neuronal activity from tonic to bursting modes during affective processing (Laviolette and Grace, 2006a; Tan et al., 2010). In contrast, pharmacological blockade of PFC CB1 receptor activity attenuated the formation of supra-threshold fear memories at both the behavioural and neuronal levels (Laviolette and Grace, 2006a; Tan et al., 2010; Tan et al., 2011). Together, this evidence shows that aberrant cannabinoid activation can potently modulate PFC neuronal network activity and simultaneously distort affective processing, similar to disturbances observed in schizophrenia and mood disorders.
A critical question remains as to how PFC cannabinoid signalling modulates VTA dopaminergic transmission in the context of emotional salience processing. In terms of cannabinoid-dependent regulation of the PFC-VTA pathway, acute THC administration has been reported to both decrease GABA release and increase dopamine release in the PFC (Pistis et al., 2002). In addition, administration of either synthetic CB1 receptor agonists or THC increased PFC pyramidal neuron activity while simultaneously blocking the inhibitory effects of VTA dopaminergic inputs (Pistis et al., 2001), suggesting that CB1 receptor activation can ramp up the excitability of PFC neurons through both local and distal circuit interactions. A recent study characterized a biphasic role for PFC CB1 receptor transmission in the regulation of emotional salience and memory formation via effects on VTA dopaminergic activity (Draycott et al., 2014). Using single unit, in vivo extracellular recordings of VTA dopaminergic neurons in concert with intra-PFC infusions of WIN 55, 212-2, high levels of CB1 receptor stimulation decreased spontaneous and burst firing activity of VTA dopamine neurons, whereas low levels of stimulation potentiated these parameters. Interestingly, these biphasic effects reflected divergent effects on emotional memory formation, with intra-PFC WIN 55, 212-2 infusions at high doses blocking the formation of normally salient (supra-threshold) fear memory, and lower doses potentiating normally non-salient (sub-threshold) fear memory (Draycott et al., 2014). Furthermore, the behavioural effects of high levels of PFC CB1 receptor stimulation were dependent on a GABAB receptor substrate directly in the VTA, whereas those of low levels of CB1 activation were dopamine-dependent (Draycott et al., 2014). Thus, relative CB1 receptor tone in the PFC appears to induce either emotional blunting, or potentiation of normally non-salient stimuli via direct actions on subcortical dopamine neurons.
These preclinical findings complement clinical evidence demonstrating that acute cannabis exposure can induce psychotic episodes consistent with hyper-dopaminergic activity (D'Souza et al., 2004). By contrast, repeated cannabis exposure is associated with blunted levels of mesolimbic dopaminergic activity, as indexed by a reduced capacity for dopamine synthesis in the ventral striatum of heavy users (Bloomfield et al., 2014). Indeed, the influence of local cannabinoid signalling over subcortical emotional processing regions corresponds to the top-down executive control function of the PFC, emphasising the importance of downstream factors in cannabinoid modulation of complex cognitive processes, like decision-making discussed in section 3. Beyond the PFC, however, recent studies also reveal an important functional role for hippocampal CB1 receptor transmission in regulating subcortical dopamine activity and emotionally-guided behaviours.
4.2 Identification of ventral hippocampal cannabinoid transmission as a functional regulator of mesolimbic dopamine activity
The vHIPP is a critical neural region implicated not only in the modulation of dopaminergic transmission (Floresco, 2007; Floresco and Grace, 2003) but in neuropsychiatric-related pathology as demonstrated in both preclinical and clinical studies (Grace et al., 2007; Nguyen et al., 2014; Sams-Dodd et al., 1997; Strange et al., 2014). Anatomically, the vHIPP serves as a nexus point with complex functional connections with the NAc, VTA and PFC (Calhoon and O'Donnell, 2013; Grace et al., 2007; Sotres-Bayon et al., 2012). Located mainly on inhibitory, GABAergic presynaptic terminals within the vHIPP (Takacs et al., 2015), CB1 receptor activation reduces GABAergic tone and thereby increases the output activity of principal neurons to their targets, including the NAc (Hajos and Freund, 2002). Specifically, through excitatory projections to the NAc shell, vHIPP output neurons have been shown to modulate dopamine neuronal activity via both direct and indirect pathways to VTA cell bodies. For example, vHIPP stimulation increases the spontaneous activity of VTA dopamine neurons via excitatory glutamatergic projections (Floresco et al., 2001). The notion that abnormal mesolimbic dopamine transmission might be secondary to hippocampal dysfunction in schizophrenia is relatively well-established (Schmajuk, 2001); however, a potentially critical involvement of vHIPP CB1 receptor transmission in modulating schizophrenia-like affective and cognitive disturbances associated with dopamine dysregulation has only recently come to light (Loureiro et al., 2015).
Similar to the work in the PFC reviewed above, a combination of in vivo electrophysiology and behavioural approaches were used to assess the effects of vHIPP CB1 receptor stimulation. Intra-vHIPP infusions of WIN 55, 212-2 increased both spontaneous and burst firing activity of isolated VTA dopamine neurons, yet attenuated the spontaneous activity rates of non-dopaminergic (presumably GABAergic) neurons (Loureiro et al., 2015). Thus, cannabinoid activity in the vHIPP appears capable of modulating VTA neuronal network activity through both dopaminergic and non-dopaminergic circuits, similar to the effects of CB1 receptor signalling in the PFC. In contrast, the effects of vHIPP CB1 receptor stimulation were not biphasic and only led to activation of the VTA dopamine system (Loureiro et al., 2015). At the behavioural level, stimulation of CB1 receptors in the vHIPP caused a range of affective and cognitive abnormalities. For example, vHIPP CB1 receptor activation potentiated the reward salience of normally non-salient conditioning doses of morphine in a conditioned place preference paradigm, and disrupted social interaction behaviours by diminishing both the motivation for sociability as well as recognition memory (Loureiro et al., 2015). With respect to reward processing, the effects of intra-vHIPP CB1 receptor activation differed sharply with those of intra-PFC CB1 receptor activation. Contrasting reports that PFC CB1 receptor stimulation switches a normally behaviourally-rewarding dose of morphine into an aversive stimulus (Ahmad et al., 2013), CB1 receptor stimulation in the vHIPP was found to potentiate opiate-reward salience (Loureiro et al., 2015). Notably, the behavioural effects of intra-vHIPP CB1 receptor stimulation were blocked by dopamine receptor antagonism within the NAc, indicating that the ability of intra-vHIPP CB1 receptor stimulation to disrupt rewarding and social interaction behaviours is mediated through a dopaminergic signal directly in the mesolimbic pathway (Loureiro et al., 2015). Therefore, CB1 receptor signalling within both the PFC and vHIPP can strongly, albeit through distinct neural mechanisms, modulate the activity states of mesolimbic dopamine neurons and induce corresponding changes in affective/reward-related processing and social interaction/cognition behaviours.
Nevertheless, many critical questions remain for future study. For example, given that the vHIPP and PFC share reciprocal connections, it will be interesting to examine how the PFC may regulate subcortical transmission between the vHIPP and mesolimbic dopamine system. Recent evidence demonstrates that the PFC can strongly regulate the ability of the vHIPP to control neuronal activity states in the NAc by gating vHIPP→NAc excitatory projections (Belujon and Grace, 2008). Thus, how might top-down PFC influences on the mesolimbic pathway control the ability of vHIPP CB1 receptor signalling to regulate neuronal and behavioural effects within the VTA or NAc? In addition, given that the NAc receives and integrates functional inputs from both the PFC and VTA, how might the biphasic effects of PFC CB1 receptor activity control neuronal activity states directly within the NAc, and how might these differential effects modulate phasic mesolimbic dopamine release? Characterizing and comparing the effects of cannabinoid transmission within the PFC and vHIPP on dopaminergic activity states will yield important new insights into how dysregulation of the endocannabinoid system, induced by either exposure to cannabis or due to intrinsic, genetically-determined endocannabinoid disturbances, may underlie neuropsychiatric symptoms in disorders such as schizophrenia.
Finally, it is important to note a critical role for the amygdala in regulating emotional processing and associative learning behaviours, with its inextricable anatomical and functional connections to the PFC, vHIPP and mesocorticolimbic dopamine networks (for a detailed overview of studies see Laviolette and Grace, 2006b; Tan et al., 2014). These data were not reviewed herein as complementary work regarding amygdalar endocannabinoid signalling in regulating anxiety is reviewed in the next section.
5. Cannabinoid modulation of amygdalar function: focus on anxiety and fatty acid amide hydrolase activity
Accumulating evidence suggests that the endocannabinoid system is involved in the regulation of anxiety (Korem et al., 2016), and it has long been recognized that cannabis use produces significant effects on anxiety in humans (Patel and Hillard, 2009). Anxiety is a negative emotional state that serves an important protective function, but can become pathological when the intensity of cognitive and behavioral responses does not correlate with actual danger. Hyperactivity in the circuits that induce anxiety can result from repetitive stress, and/or traumatic life events, which can lead to psychopathologies including generalized anxiety, phobias, hypervigilance and other somatic signs of fear (Calhoon and Tye, 2015). Several corticolimbic brain regions are involved in the detection and assessment of environmental stimuli as being potentially harmful, including the amygdala and bed nucleus of the stria terminalis (BNST), as well as the vHIPP and medial PFC discussed in section 4. In the following section, we first review evidence demonstrating a key role for amygdalar cannabinoid signalling in the regulation of anxiety in both humans and animals. We will then describe new data from studies looking specifically at the modulation of endocannabinoid activity in the amygdala in anxiety-like behaviours in mice.
5.1 Modulation of anxiety by endocannabinoid signalling in the basolateral amygdala
The basolateral amygdala (BLA) receives sensory information in the form of excitatory inputs from the thalamus and sensory cortices (McDonald, 1998). Processing of this sensory information in the BLA results in formation of associations between previously neutral stimuli that predict an outcome that has positive or negative valence through Hebbian learning mechanisms (Maren and Quirk, 2004). The emotional valence that is attached to the stimuli determines whether fear or reward pathways are activated by BLA outputs (Namburi et al., 2015). When the situation is predictive of threat, the BLA activates the central amygdala (CeA) and BNST which, through projections to the hypothalamus and brainstem, provide information to autonomic, neuroendocrine and motor systems to coordinate physiological and behavioral responses to cope with the threat (Calhoon and Tye, 2015).
CB1 receptors are present in the presynaptic terminals of both GABAergic and glutamatergic neurons in the amygdala (Katona et al., 2001; Marsicano and Lutz, 1999). Thus, as in the PFC, endocannabinoid signalling inhibits the release of both glutamate and GABA in the amygdala, which is consistent with biphasic effects of CB1 receptor agonists on anxiety-like behaviors in preclinical models (Patel and Hillard, 2006). For example, selective deletion of CB1 receptors from cortical glutamatergic axons results in loss of the anxiolytic effects of low doses of CB1 receptor agonists while the converse occurs when CB1 receptors are deleted from GABAergic terminals (Rey et al., 2012). Furthermore, CB1 receptor agonist treatment synergizes with restraint stress to increase c-Fos expression in the CeA, which is consistent with a pro-anxiety role for CB1 receptor on GABA terminals (Patel et al., 2005). In contrast to the biphasic effects of direct CB1 receptor agonists, inhibition of FAAH activity, which results in increased concentrations of AEA, exerts a monophasic effect on anxiety-like behaviors in mice (Patel and Hillard, 2006) and does not synergize with stress to promote CeA activation (Patel et al., 2005).
Considerable evidence collected from biochemical, behavioral and genetic studies supports the hypothesis that FAAH activity contributes to amygdalar activation by an acute stress exposure (Gunduz-Cinar et al., 2013a). For example, the potent inhibitor of FAAH, URB597, induces anxiolytic effects in mice in a CB1 receptor-dependent manner (Busquets-Garcia et al., 2011; Kathuria et al., 2003). Similarly FAAH knockout mice exhibit reduced expression of anxiety-related behaviors in stressful environments (Naidu et al., 2007). Acute exposure to stress results in a reduction of AEA concentrations in the amygdala (Rademacher et al., 2008) which results in the activation of the hypothalamic-pituitary-adrenal axis (Hill et al., 2009). Together, these data lead to the hypothesis that AEA concentrations in the amygdala serve as a gate that inhibits stress responses; and that the catabolism of AEA by FAAH results in a permissive state in which stress can elicit an anxiety response (Hill et al., 2009). Recent studies demonstrate that the stress effect on AEA concentrations is the result of corticotrophin releasing factor-mediated increases in FAAH activity (Gray et al., 2015). In relation to the impact of chronic stress, repeated exposure to restraint stress increases amygdalar FAAH Vmax and is associated with a significant suppression of AEA concentrations (Rademacher et al., 2008). Additionally, repeated immobilization results in a significant increase in anxiety-like behavioral responding and dendritic arborisation in the amygdala—effects that do not occur in FAAH knockout mice (Hill et al., 2013). These and other studies support a critical role for FAAH in the regulation of acute anxiety and in the anxiogenic consequences of chronic stress exposure (Gunduz-Cinar et al., 2013a).
Human studies are in accord with a critical role for FAAH in the regulation of amygdalar function. Indeed, complementing data reviewed in section 2, genetic variation in FAAH may contribute to individual differences in reactivity to negative stimuli, including stress. Specifically, a polymorphism in the gene for FAAH (rs324420) has been described that results in FAAH protein that is more labile to degradation (Chiang et al., 2004; Sipe et al., 2002). An imaging study examined the contribution of this polymorphism to amygdalar reactivity evoked by fearful faces in humans and found that individuals with the rare allele demonstrate reduced reactivity (Gunduz-Cinar et al., 2013b). Mice genetically engineered to mimic this human polymorphism exhibit reduced FAAH activity, increased amygdalar AEA and reduced anxiety-like behaviors (Dincheva et al., 2015). These studies lead to the hypothesis that individual differences in FAAH activity could contribute to susceptibility to the development of anxiety disorders.
5.2 Elucidating the influence of age and sex on amygdalar FAAH modulation of anxiety
Evidence suggests that FAAH activity exhibits a development trajectory, demonstrating interesting differences during adolescence that contribute to anxiety-like behaviors (Lee et al., 2016). Although less is known, there is a tendency for FAAH protein to increase in aged mice, particularly in mice null for the CB1 receptor (Maccarrone et al., 2001). Circumstantial evidence also supports the hypothesis that FAAH becomes a more significant regulator of cardiovascular function and peripheral inflammation (Batkai et al., 2007), together with hippocampal signalling and inflammation (Murphy et al., 2012), as animals age. These data are consistent with an age-related increase in FAAH activity. In support of this notion, AEA concentrations decrease with aging in the spinal cord, thalamus and cortex (Bishay et al., 2013). Moreover, post-mortem studies in human tissues demonstrate that FAAH expression steadily increases with age in the dorsolateral region of the PFC (Long et al., 2012).
We carried out a set of studies to determine the extent to which FAAH contributes to changes in anxiety-like behaviors in old female and male mice. Male and female mice on the ICR (CD-1) background from three different age ranges, 4–6 (adolescent/young adult), 20–29 (mid-adult) and 60–75 (late-adult) weeks, were exposed to the elevated plus maze (EPM) to evaluate anxiety-like behavior as previously described (Patel and Hillard, 2006). Twenty-four hours after the EPM test, brains were harvested and FAAH activity was determined by measuring the conversion of AEA to ethanolamine as described (Patel et al., 2003). Female mice were gonadally-intact and had never been pregnant. Although not determined, it is likely that the female mice in the oldest group were no longer cycling through estrous stages.
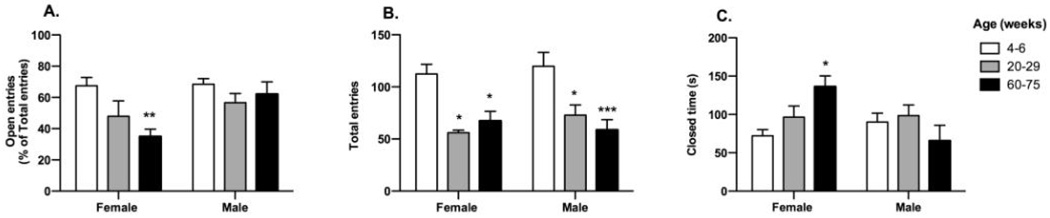
Behavior of male and female mice in each of the three age ranges was examined in the EPM. The percent of total entries that occurred into the open arms were calculated for each age and sex group and were examined using two-way ANOVA with age and sex as between-subjects factors (Fig 1A). Percent entries into the open arm were affected by both age (F2,33 = 5.50; p < 0.01) and sex (F1,33 = 7.20; p < 0.05) with a significant interaction (F2,33 = 3.40; p < 0.05). Post hoc tests reveal that female adolescent/young adult mice exhibit a greater percentage of open entries compared with female late adult mice. Two-way ANOVA also revealed an effect of age on total arm entries (Fig 1B; F2,33 = 15.00; p < 0.001), with the youngest group showing more entries than either of the other groups in both male and female mice. Two-way ANOVA also demonstrated a significant interaction between sex and age in the time spent in the closed arms of the EPM (Fig 1C; F2,33 = 5.40, p < 0.01). The female oldest mice spent a markedly longer time in closed arm exploration than the female youngest and the male oldest mice. In the time spent in the open arms, two-way ANOVA demonstrated a significant interaction between sex and age (F2,33 = 3.60, p < 0.05; data not shown). Post hoc tests revealed that the female adolescent/young adult mice spent more time in open arm exploration compared with female mid-adult and late-adult group.
Figure 1.
Elevated plus maze behaviour in male and female mice at different ages. ICR mice were purchased from Harlan, Madison, WI, and were divided in three age groups of each sex (5–7 mice/group) as shown. Prior to use, mice were group housed on a 12 h light/dark cycle with lights on at 6:00 AM. All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. The EPM consists of four arms 30 cm long and 5 cm wide, two open without walls and two closed by 30 cm high walls; the apparatus is elevated 40 cm above the floor (San Diego Instruments). Experiments began by placing a single mouse on the central platform facing an open arm: behaviors were recorded for 5 min as they explored the maze. Data were analyzed using video-based EthoVision System data analysis software (EthoVision 3.1; Noldus Information Technology). A: Percent entries into the open arms were calculated as the number of open arm entries/(open + closed arm entries) ×100. B: Total entries into open and closed arms. C: Total time spent in the closed arms. * p<0.05; ** p<0.01 and *** p<0.005 compared to the 4–6 week old group.
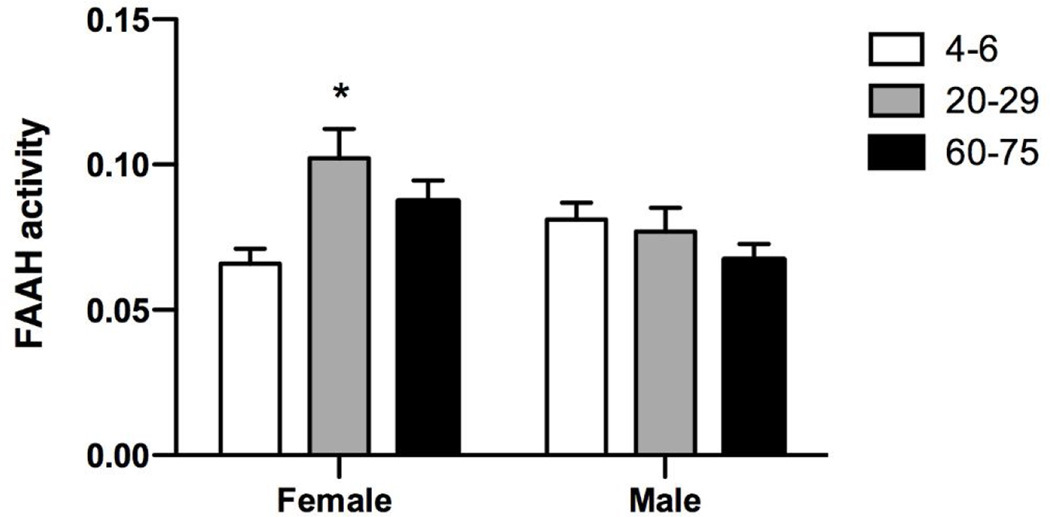
FAAH activity was measured in membranes harvested from the amygdala 24 h after the EPM. Two-way ANOVA also demonstrated a significant interaction between sex and age (Fig 2; F2,33 = 5.40, p < 0.01). FAAH activity was greater in the female mid-adult mice compared to the female adolescent/young adult and the male late-adult mice. In male mice, FAAH activity was not different in any of the age ranges examined.
Figure 2.
Amygdalar FAAH activity in male and female mice at different ages. Twenty-four hours after EPM, mice were decapitated; brains were removed and frozen on dry-ice. The amygdala was dissected on dry-ice, weighed and membranes were harvested as described previously (Hillard et al., 1995). Membranes were incubated with 0.2 nM [3H]AEA for 10 min. FAAH activity was calculated as the ratio of dpm (disintegration per minute) in the aqueous phase and the total dpm (aqueous + organic). *p<0.05 compared to 4–6 week old group.
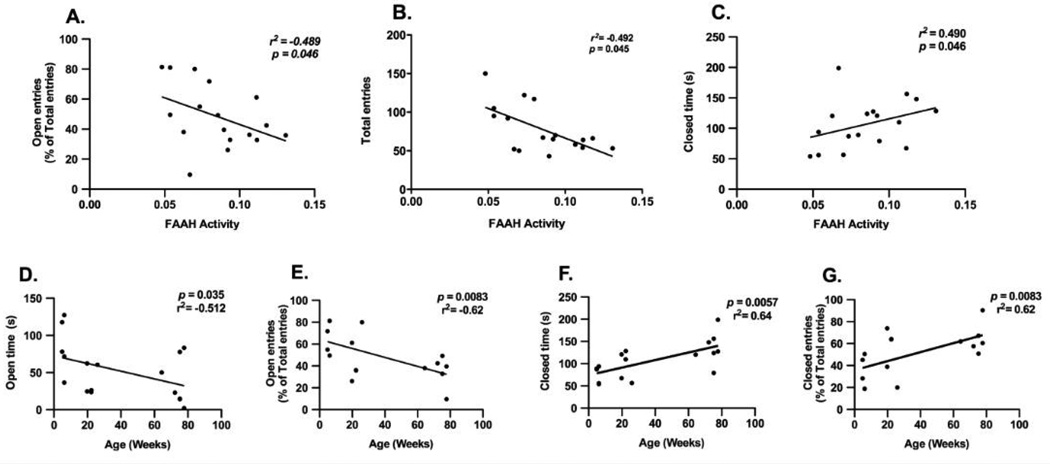
Correlation analyses were carried out between the parameters determined in the EPM and amygdala FAAH activity. In female mice, significant, negative correlations occurred between amygdalar FAAH activity and the percentage of open entries (Fig 3A; r2 = −0.489, p < 0.05) and the total number of entries (Fig 3B; r2 = −0.492, p < 0.05) while a positive correlation between amygdalar FAAH activity and closed time (Fig 3C; r2 = 0.490, p < 0.05) was demonstrated. No significant correlations were seen between EPM parameters and FAAH activity in male mice (data not shown).
Figure 3.
Correlational analyses were made using Spearmańs bivariate test between FAAH activity in the amygdala and the following EPM parameters in female mice of all three ages: (A) percent entries into the open arms, (B) total arm entries, and (C) time spent in the closed arms. The correlation between EPM parameters and age in female mice were: (D) time spent in the open arms, (E) percent entries into the open arms, (F) time spent in the closed arms and (G) percent entries into the closed arms.
Our findings, that anxiety-like behaviors are increased in late-adult female mice compared to adolescent/young adult female but not male mice, are consistent with earlier studies (Frick et al., 2000; Lamberty and Gower, 1992) although the opposite has also been reported (Chen et al., 2007). A wide range of EPM behaviors, together with FAAH activity in the same mice, allowed us to examine the contribution of FAAH activity in the EPM behaviors. In the female mice, increased anxiety-like behaviors correlated with increased amygdalar FAAH activity, which supports the hypothesized role of FAAH as a critical regulator of anxiety. In fact, approximately 50% of the variation in the number of entries, percent entries into the open arms and time in the closed arms can be explained by FAAH activity. However, these correlations were confined to female mice. Notably, the menopause and post-menopausal periods in mice are marked with depressed mood and anxiety-like behaviour, which are related to fluctuations in endogenous estrogen and progesterone levels (Guimaraes et al., 2015). We have reported that old, menopausal mice are more anxious than young mice. Indeed, Lamberty and Gower (1992) showed anxiogenic-like behavior in aged female NMRI mice (17–22 months old). Taken together, the present findings suggest that aging increases anxiety, possibly due to a decrease in the levels of ovarian steroid hormones estrogen and progesterone, in addition to increasing FAAH activity. Indeed, there is evidence for an interaction between gonadal hormones and FAAH activity.
There is an estrogen response element in the FAAH gene that inhibits FAAH transcription upon translocation of the estrogen receptor to the nucleus (Hill et al., 2007; Waleh et al., 2002). It has been suggested that the anxiolytic effects of estrogen are due, at least in part, to an elevation of AEA and, thus, CB1 receptor signalling. Data to support this hypothesis include findings that a CB1 antagonist reversed the anxiolytic effect of estradiol in rats (Hill et al., 2007). With regard to the present findings, estrogen levels would be expected to be lower in older females, which would result in disinhibition of FAAH expression and, thus is consistent with the hypothesis that the anxiolytic effects of estrogen are mediated by AEA. However, although there was a trend, FAAH activity was not significantly greater in the aged compared to youngest group of female mice. Given that we did not measure estrogen levels and have made somewhat arbitrary divisions in the age groups, we cannot make any conclusions from the present studies regarding this potential mechanism.
Together, these results are consistent with the hypothesis that endocannabinoid signalling is part of an endogenous anxiolytic neuromodulator system and suggest that variations in the endocannabinoid system, particularly resulting from changes in FAAH, could contribute to different vulnerability to anxiety-related disorders and trait anxiety in females compared to males.
6. Summary and conclusions
In this review we have shown that cannabinoid signalling across corticolimbic networks can have profound effects on cognitive and affective behaviours. Effects arise following acute administration of cannabis and related cannabinoids, via interactions with CB1 receptors in cortical and subcortical regions. However, endogenous cannabinoid tone in the amygdala, hippocampus, and PFC also appears to regulate fundamental emotional and reward-related processes. Understanding cannabis’ psychoactive effects and the factors which might minimize adverse reactions is crucial given the widespread use of cannabinoid drugs worldwide. By the same token, understanding how endocannabinoid signalling contributes to basic neural functioning, and how it might be disrupted in pathological states such as schizophrenia and anxiety, suggest a viable therapeutic target for these disorders.
As described in section 2, both human genetic influences and strain differences in the blend of the phytocannabinoids THC and CBD in the plant interact at the level of individual users, leading to a range of psychobehavioural responses. From a psychiatric standpoint, understanding genetic vulnerability for psychotic episodes or cannabis dependence may pave the way for preventative approaches in which “high risk” individuals could be warned of the risks associated with ongoing cannabis use. Alternatively, modifying the composition of cannabis itself may offer utility in curbing the potential for adverse side effects, with phytocannabinoids like CBD evidenced to attenuate some of THC’s anxiogenic and cognitive-impairing actions. While concentrations of THC in street cannabis continue to rise, accompanied by a concomitant decline in CBD levels (Burgdorf et al., 2011; Swift et al., 2013), cannabinoid therapeutics, such as nabiximols (Sativex), purposefully contain equal quantities of THC and CBD (Syed et al., 2014). Thus the composition of cannabis and cannabinoid preparations varies widely as a function of intended use, contributing to vastly different effects. Most clinical and preclinical studies to date have focused on THC exclusively, yet in the interest of ecological validity, ongoing research will benefit by manipulating the ratio of THC relative to other phytocannabinoids present in cannabis.
From a public health and policy perspective, regulating the dose and phytocannabinoid composition of cannabis requires special consideration, given that low doses of THC can induce relaxation and euphoria, but high doses may result in anxiety, psychotic episodes, and impaired cognition (Crane et al., 2013). In section 3, we reviewed evidence for impaired decision-making in regular cannabis users; this is most likely mediated by repeated THC exposure which has been shown to acutely impair this cognitive domain in clinical and preclinical models. As suggested, the poor education and economic life outcomes reported amongst regular cannabis users might reflect decision-making deficits, and such diminished outcomes would place undue burden on the welfare state (Fergusson and Boden, 2008; Horwood et al., 2010; Hyggen, 2012). Given the growing popularity of cannabis concentrates and synthetic cannabinoids, together with evidence that cannabis users report greater risk-taking in social, health, and ethical domains, it is clear that THC-induced deficits in cognitive ability may have severe societal consequences (Gilman et al., 2015; Raber et al., 2015). These could be mitigated by either raising public awareness of the negative outcomes associated with regular use, or regulating the composition of cannabis so that side effects are minimized, as described above.
Importantly, cannabis-induced psychoactive effects reflect the “hijacking” of an endogenous cannabinoid system involved in regulating a variety of reward, emotional, and prosocial processes. Current findings, reviewed in section 4, demonstrate important functional distinctions between modulation of mesolimbic dopaminergic activity states by CB1 receptor stimulation in the PFC versus the vHIPP (Ahmad et al., 2013; Draycott et al., 2014; Loureiro et al., 2015). Similarly, the evidence reviewed in section 5 indicates that endocannabinoid signalling in the amygdala, particularly via FAAH activity, is a critical regulator of anxiety in a manner that appears both sex- and age-dependent, adding another layer of interindividual variation in terms of endogenous cannabinoid tone. Interestingly, modulation of both inhibitory and excitatory neurons in the PFC and amygdala seems to underlie biphasic behavioural effects, whereas current data suggests primarily monophasic effects of vHIPP CB1 receptor signalling. Moreover, administration of synthetic cannabinoid ligands, phytocannabinoids and modulation of endocannabinoid tone, such as through FAAH inhibition, are seen to have divergent outcomes across different behavioural modalities. Taken together, a complicated picture of cannabinoid modulation of corticolimbic function emerges, offering the prospect of site-specific cannabinoid therapeutics via exploitation of regional differences and designer drugs. Indeed, cannabinoid therapeutics, such as CBD and FAAH inhibitors, have been investigated for the treatment of schizophrenia and anxiety, respectively (Batista et al., 2014; Iseger and Bossong, 2015).
Although brief, this review has highlighted the importance of continued research on cannabinoid transmission in a variety of domains, including recreational cannabis and related cannabinoids, given changing social and political attitudes towards their use, as well as medicinal cannabis, in light of its potential to ameliorate neuropsychiatric disorders amongst other medical conditions. For these directives to be achieved, it is imperative that clinical and preclinical research into the cannabinoids be accessible, having long been impeded by strict legislation, with the overarching goals of minimizing harm and maximizing therapeutic potential.
Highlights.
Corticolimbic circuits mediate cognitive and emotional behavioural processes
Endocannabinoid activity, and thus exogenous cannabinoids, modulate their function
The prefrontal cortex, amygdala and ventral hippocampus are key neural loci
Individual variation in cannabis response is an important consideration
Both human and animal studies are vital components of such research
Acknowledgments
MMS receives a scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) and WKA is supported by the philanthropic contributions of the Gillespie family; MMS and WKA gratefully acknowledge the guidance of Dr. Catharine Winstanley and Prof. Iain McGregor. The Friends of the University of Navarra Foundation (FADA) supported M. Celorrio during a visit to the Medical College of Wisconsin. The experiments were funded by NIH grant R01 DA026996 and by the Research and Education Component of the Advancing a Healthier Wisconsin Endowment of the Medical College of Wisconsin and PI14/02070 from the Spanish Government (Plan estatal I+D+I 2013–2016 and ISCIII-FEDER).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad T, Lauzon NM, de Jaeger X, Laviolette SR. Cannabinoid transmission in the prelimbic cortex bidirectionally controls opiate reward and aversion signaling through dissociable kappa versus mu-opiate receptor dependent mechanisms. J Neurosci. 2013;33:15642–15651. doi: 10.1523/JNEUROSCI.1686-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington D.C.: American Psychiatric Association; 2013. [Google Scholar]
- Arnold JC, Boucher AA, Karl T. The Yin and Yang of Cannabis-induced Psychosis: the Actions of Delta 9-Tetrahydrocannabinol and Cannabidiol in Rodent Models of Schizophrenia. Curr Pharm Des. 2012;18:5113–5130. doi: 10.2174/138161212802884726. [DOI] [PubMed] [Google Scholar]
- Batista LA, Gobira PH, Viana TG, Aguiar DC, Moreira FA. Inhibition of endocannabinoid neuronal uptake and hydrolysis as strategies for developing anxiolytic drugs. Behav Pharmacol. 2014;25:425–433. doi: 10.1097/FBP.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Batkai S, Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Cravatt BF, Csiszar A, Ungvari Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2007;293:H909–H918. doi: 10.1152/ajpheart.00373.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamina A, Bonhomme-Faivre L, Picard V, Sabbagh A, Richard D, Blecha L, Rahioui H, Karila L, Lukasiewicz M, Farinotti R, Picard V, Marill C, Reynaud M. Association between ABCB1 C3435T polymorphism and increased risk of cannabis dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1270–1274. doi: 10.1016/j.pnpbp.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Falkenberg I, Martin-Santos R, Atakan Z, Crippa JA, Giampietro V, Brammer M, McGuire P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015;40:1343–1352. doi: 10.1038/npp.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, CM OC, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishara AJ, Pleskac TJ, Fridberg DJ, Yechiam E, Lucas J, Busemeyer JR, Finn PR, Stout JC. Similar Processes Despite Divergent Behavior in Two Commonly Used Measures of Risky Decision Making. J Behav Decis Mak. 2009;22:435–454. doi: 10.1002/bdm.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishay P, Haussler A, Lim HY, Oertel B, Galve-Roperh I, Ferreiros N, Tegeder I. Anandamide deficiency and heightened neuropathic pain in aged mice. Neuropharmacology. 2013;71C:204–215. doi: 10.1016/j.neuropharm.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology. 2014;231:2251–2259. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, Fraccaro V, Atakan Z, Martin-Santos R, O'Carroll C, Rubia K, McGuire PK. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Delta9-tetrahydrocannabinol. Psychopharmacology. 2007a;192:325–336. doi: 10.1007/s00213-007-0721-3. [DOI] [PubMed] [Google Scholar]
- Boucher AA, Hunt GE, Karl T, Micheau J, McGregor IS, Arnold JC. Heterozygous neuregulin 1 mice display greater baseline and Delta(9)-tetrahydrocannabinolinduced c-Fos expression. Neuroscience. 2007b;149:861–870. doi: 10.1016/j.neuroscience.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Boucher AA, Hunt GE, Micheau J, Huang X, McGregor IS, Karl T, Arnold JC. The schizophrenia susceptibility gene neuregulin 1 modulates tolerance to the effects of cannabinoids. Int J Neuropsychopharmacol. 2011;14:631–643. doi: 10.1017/S146114571000091X. [DOI] [PubMed] [Google Scholar]
- Brydges NM, Seckl J, Torrance HS, Holmes MC, Evans KL, Hall J. Juvenile stress produces long-lasting changes in hippocampal DISC1, GSK3ss and NRG1 expression. Mol Psychiatry. 2014;19:854–855. doi: 10.1038/mp.2013.193. [DOI] [PubMed] [Google Scholar]
- Buhler KM, Gine E, Echeverry-Alzate V, Calleja-Conde J, de Fonseca FR, Lopez-Moreno JA. Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict Biol. 2015;20:845–871. doi: 10.1111/adb.12204. [DOI] [PubMed] [Google Scholar]
- Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117:59–61. doi: 10.1016/j.drugalcdep.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, O'Donnell P. Closing the gate in the limbic striatum: prefrontal suppression of hippocampal and thalamic inputs. Neuron. 2013;78:181–190. doi: 10.1016/j.neuron.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000b;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, De Hert M, Van Winkel R, Peuskens J, Bormans G, Kranaster L, Enning F, Koethe D, Leweke FM, Van Laere K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. NeuroImage. 2013;79:304–312. doi: 10.1016/j.neuroimage.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Chadwick B, Miller ML, Hurd YL. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Front Psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GH, Wang C, Yangcheng HY, Liu RY, Zhou JN. Age-related changes in anxiety are task-specific in the senescence-accelerated prone mouse 8. Physiol Behav. 2007;91:644–651. doi: 10.1016/j.physbeh.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD, Dougherty DM. Possible amotivational effects following marijuana smoking under laboratory conditions. Exp Clin Psychopharmacol. 2002;10:26–38. doi: 10.1037//1064-1297.10.1.26. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Chohan TW, Boucher AA, Spencer JR, Kassem MS, Hamdi AA, Karl T, Fok SY, Bennett MR, Arnold JC. Partial genetic deletion of neuregulin 1 modulates the effects of stress on sensorimotor gating, dendritic morphology, and HPA axis activity in adolescent mice. Schizophr Bull. 2014a;40:1272–1284. doi: 10.1093/schbul/sbt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan TW, Nguyen A, Todd SM, Bennett MR, Callaghan P, Arnold JC. Partial genetic deletion of neuregulin 1 and adolescent stress interact to alter NMDA receptor binding in the medial prefrontal cortex. Front Behav Neurosci. 2014b;8:298. doi: 10.3389/fnbeh.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–1837. doi: 10.1038/npp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizzi M, Iyegbe C, Powell J, Ursini G, Porcelli A, Bonvino A, Taurisano P, Romano R, Masellis R, Blasi G, Morgan C, Aitchison K, Mondelli V, Luzi S, Kolliakou A, David A, Murray RM, Bertolino A, Di Forti M. Interaction Between Functional Genetic Variation of DRD2 and Cannabis Use on Risk of Psychosis. Schizophr Bull. 2015;41:1171–1182. doi: 10.1093/schbul/sbv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 2013;23:117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9- tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2011;36:1620–1630. doi: 10.1038/npp.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- Decoster J, van Os J, Kenis G, Henquet C, Peuskens J, De Hert M, van Winkel R. Age at onset of psychotic disorder: cannabis, BDNF Val66Met, and sex-specific models of gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:363–369. doi: 10.1002/ajmg.b.31174. [DOI] [PubMed] [Google Scholar]
- DeLong GT, Wolf CE, Poklis A, Lichtman AH. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010;112:126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, O'Tuathaigh C, Clarke G, O'Leary C, Petit E, Clarke N, Tighe O, Lai D, Harvey R, Cryan JF, Dinan TG, Waddington JL. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene×environment interaction. Brain Behav Immun. 2012;26:660–671. doi: 10.1016/j.bbi.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, Sirianni M, La Cascia C, Stilo SA, Marques TR, Handley R, Mondelli V, Dazzan P, Pariante C, David AS, Morgan C, Powell J, Murray RM. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–816. doi: 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature communications. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, Zieglgansberger W, Lutz B, Rammes G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, Shannon EE, Acheson A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. 2013;226:307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draycott B, Loureiro M, Ahmad T, Tan H, Zunder J, Laviolette SR. Cannabinoid transmission in the prefrontal cortex bi-phasically controls emotional memory formation via functional interactions with the ventral tegmental area. J Neurosci. 2014;34:13096–13109. doi: 10.1523/JNEUROSCI.1297-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Kwon IK, Kim J. Neuregulin-1 impairs the long-term depression of hippocampal inhibitory synapses by facilitating the degradation of endocannabinoid 2-AG. J Neurosci. 2013;33:15022–15031. doi: 10.1523/JNEUROSCI.5833-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg A, Brenneisen R, Holt D, Feilding A, Walker L, Murray RM, Kapur S. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19–27. doi: 10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropharmacology. 2004;47:1170–1179. doi: 10.1016/j.neuropharm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–976. doi: 10.1111/j.1360-0443.2008.02221.x. discussion 977-968. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Dopaminergic regulation of limbic-striatal interplay. J Psychiatry Neurosci. 2007;32:400–411. [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, Porrino L, Stout JC. Cognitive Mechanisms Underlying Risky Decision-Making in Chronic Cannabis Users. J Math Psychol. 2010;54:28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O'Carrol C, Atakan Z, Zuardi AW, McGuire PK. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: Epidemiologic evidence. Biol. Psychiatry. 2016;79:549–556. doi: 10.1016/j.biopsych.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Calderon V, Curran MT, Evins AE. Young adult cannabis users report greater propensity for risk-taking only in non-monetary domains. Drug Alcohol Depend. 2015;147:26–31. doi: 10.1016/j.drugalcdep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34:962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J, Packard MG. The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiol Learn Mem. 2015;125:1–14. doi: 10.1016/j.nlm.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Goschke T. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. Int J Methods Psychiatr Res. 2014;23(Suppl 1):41–57. doi: 10.1002/mpr.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, Hill MN. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith-Lendering MF, Huijbregts SC, Vollebergh WA, Swaab H. Motivational and cognitive inhibitory control in recreational cannabis users. J Clin Exp Neuropsychol. 2012;34:688–697. doi: 10.1080/13803395.2012.668874. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes RA, Asth L, Engelberth RC, Cavalcante Jde S, Soares-Rachetti Vde P, Gavioli EC. Spontaneous failure of the estrous cycle induces anxiogenic-related behaviors in middle-aged female mice. Physiol Behav. 2015;147:319–323. doi: 10.1016/j.physbeh.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013a;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Macpherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie TS, Gorka AX, Alapafuja SO, Nikas SP, Makriyannis A, Poulton R, Patel S, Hariri AR, Caspi A, Moffitt TE, Kunos G, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013b;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Han S, Yang BZ, Kranzler HR, Oslin D, Anton R, Farrer LA, Gelernter J. Linkage analysis followed by association show NRG1 associated with cannabis dependence in African Americans. Biol Psychiatry. 2012;72:637–644. doi: 10.1016/j.biopsych.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol Learn Mem. 2014;108:104–118. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, Egawa T, Kitamura Y, Uchida N, Nishimura R, Egashira N, Iwasaki K, Fujiwara M. Cannabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- Hermann D, Lemenager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision making of heavy cannabis users on the Iowa Gambling Task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. Eur Addict Res. 2009;15:94–98. doi: 10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, McEwen BS. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psych. 2013;18:1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Campbell WB. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem. 1995;64:677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Liu QS. Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des. 2014;20:3795–3811. doi: 10.2174/13816128113196660735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. 2015;25:325–334. doi: 10.1016/j.euroneuro.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland ML, Allen JD, Arnold JC. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1) Eur J Pharmacol. 2008;591:128–131. doi: 10.1016/j.ejphar.2008.06.079. [DOI] [PubMed] [Google Scholar]
- Holland ML, Lau DT, Allen JD, Arnold JC. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br J Pharmacol. 2007;152:815–824. doi: 10.1038/sj.bjp.0707467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood LJ, Fergusson DM, Hayatbakhsh MR, Najman JM, Coffey C, Patton GC, Silins E, Hutchinson DM. Cannabis use and educational achievement: findings from three Australasian cohort studies. Drug Alcohol Depend. 2010;110:247–253. doi: 10.1016/j.drugalcdep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology. 2014;39:1558–1567. doi: 10.1038/npp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Floresco SB, Winstanley CA. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 2015;40:1005–1015. doi: 10.1038/npp.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyggen C. Does smoking cannabis affect work commitment? Addiction. 2012;107:1309–1315. doi: 10.1111/j.1360-0443.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015;162:153–161. doi: 10.1016/j.schres.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani A, Kermani M, Hesam S, Haghparast A, Argandona EG, Rainer G. Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost-benefit decision making. Psychopharmacology. 2015;232:2097–2112. doi: 10.1007/s00213-014-3841-6. [DOI] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS. Cannabidiol potentiates Delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218:443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Korem N, Zer-Aviv TM, Ganon-Elazar E, Abush H, Akirav I. Targeting the endocannabinoid system to treat anxiety-related disorders. Basic Clin. Physiol. Pharmacol. 2016;27:193–202. doi: 10.1515/jbcpp-2015-0058. [DOI] [PubMed] [Google Scholar]
- Lamberty Y, Gower AJ. Age-related changes in spontaneous behavior and learning in NMRI mice from middle to old age. Physiol Behav. 1992;51:81–88. doi: 10.1016/0031-9384(92)90206-h. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Steinberg JL. Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addict Behav. 2005a;30:815–828. doi: 10.1016/j.addbeh.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005b;30:800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the type 1 cannabinoid receptor. Br J Pharmacol. 2015 doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Dupre DJ, Denovan-Wright EM. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem. 2014;289:24845–24862. doi: 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci. 2006a;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006b;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, Hill MN, Lee FS. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav. 2016;15:108–124. doi: 10.1111/gbb.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. Transmembrane domain Nrg1 mutant mice show altered susceptibility to the neurobehavioural actions of repeated THC exposure in adolescence. Int J Neuropsychopharmacol. 2013;16:163–175. doi: 10.1017/S1461145711001854. [DOI] [PubMed] [Google Scholar]
- Long LE, Lind J, Webster M, Weickert CS. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Renard J, Zunder J, Laviolette SR. Hippocampal cannabinoid transmission modulates dopamine neuron activity: impact on rewarding memory formation and social interaction. Neuropsychopharmacology. 2015;40:1436–1447. doi: 10.1038/npp.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Attina M, Bari M, Cartoni A, Ledent C, Finazzi-Agro A. Anandamide degradation and N-acylethanolamines level in wild-type and CB(1) cannabinoid receptor knockout mice of different ages. J Neurochem. 2001;78:339–348. doi: 10.1046/j.1471-4159.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Kobel M, Stoecklin M, Michael T, Weber S, Krebs B, Opwis K. Reduced response to reward in smokers and cannabis users. Neuropsychobiology. 2009;60:94–103. doi: 10.1159/000239685. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker L. Towards a better cannabis drug. Br J Pharmacol. 2013;170:1363–1364. doi: 10.1111/bph.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Estevez AF, Zaldivar F, Montes JM, Gutierrez-Ferre VE, Esteban L, Sanchez-Santed F, Flores P. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol Depend. 2012;124:355–362. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192:306–307. doi: 10.1192/bjp.bp.107.046649. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010a;35:1879–1885. doi: 10.1038/npp.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected] Br J Psychiatry. 2010b;197:285–290. doi: 10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Murphy N, Cowley TR, Blau CW, Dempsey CN, Noonan J, Gowran A, Tanveer R, Olango WM, Finn DP, Campbell VA, Lynch MA. The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J Neuroinflammation. 2012;9:79. doi: 10.1186/1742-2094-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R, Morrissey MD, Mahadevan V, Cajanding JD, Woodin MA, Yeomans JS, Takehara-Nishiuchi K, Kim JC. Parvalbumin and GAD65 interneuron inhibition in the ventral hippocampus induces distinct behavioral deficits relevant to schizophrenia. J Neurosci. 2014;34:14948–14960. doi: 10.1523/JNEUROSCI.2204-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesink RJ, van Laar MW. Does Cannabidiol Protect Against Adverse Psychological Effects of THC? Front Psychiatry. 2013;4:130. doi: 10.3389/fpsyt.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Gantois I, Waddington JL. Genetic dissection of the psychotomimetic effects of cannabinoid exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:33–40. doi: 10.1016/j.pnpbp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015a;58:147–167. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. J Neurosci. 2015b;35:1368–1379. doi: 10.1523/JNEUROSCI.3586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini S, Sullivan GM, Hien DA, Shvil E, Neria Y. Toward a translational approach to targeting the endocannabinoid system in posttraumatic stress disorder: a critical review of preclinical research. Biol Psychol. 2015;104:8–18. doi: 10.1016/j.biopsycho.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passie T, Emrich HM, Karst M, Brandt SD, Halpern JH. Mitigation of posttraumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug Test Anal. 2012;4:649–659. doi: 10.1002/dta.1377. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Role of endocannabinoid signaling in anxiety and depression. In: Kendall D, Alexander S, editors. Behavioral Neurobiology of the Endocannabinoid System. Berlin: Springer Verlag; 2009. pp. 347–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, Falck JR, Nithipatikom K, Campbell WB, Hillard CJ. The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. 2003;139:1005–1013. doi: 10.1038/sj.bjp.0705334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Schepers I, Gonzalez-Cuevas G, de Vries TJ, Schoffelmeer AN. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology. 2007;193:85–96. doi: 10.1007/s00213-007-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, Schoffelmeer AN. Cannabinoid modulation of executive functions. Eur J Pharmacol. 2008;585:458–463. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- Pelayo-Teran JM, Suarez-Pinilla P, Chadi N, Crespo-Facorro B. Gene-environment interactions underlying the effect of cannabis in first episode psychosis. Curr Pharm Des. 2012;18:5024–5035. doi: 10.2174/138161212802884609. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, Martin BR, Razdan RK. The psychoactive plant cannabinoid, Delta9-tetrahydrocannabinol, is antagonized by Delta8- and Delta9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Pistis M, Porcu G, Melis M, Diana M, Gessa GL. Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur J Neurosci. 2001;14:96–102. doi: 10.1046/j.0953-816x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA ("Ecstasy") Psychopharmacology. 2007;189:517–530. doi: 10.1007/s00213-005-0256-4. [DOI] [PubMed] [Google Scholar]
- Raber JC, Elzinga S, Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J Toxicol Sci. 2015;40:797–803. doi: 10.2131/jts.40.797. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacol. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Wilkinson ST, D'Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Front Psychiatry. 2014;5:54. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Cortes-Briones J, Radhakrishnan R, Thurnauer H, Planeta B, Skosnik P, Gao H, Labaree D, Neumeister A, Pittman B, Surti T, Huang Y, Carson RE, D'Souza DC. Reduced brain cannabinoid receptor availability in schizophrenia. Biol Psychiatry. 2016;79:997–1005. doi: 10.1016/j.biopsych.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Saito A, Ballinger MD, Pletnikov MV, Wong DF, Kamiya A. Endocannabinoid system: potential novel targets for treatment of schizophrenia. Neurobiol Dis. 2013;53:10–17. doi: 10.1016/j.nbd.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- SAMSHA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmajuk NA. Hippocampal dysfunction in schizophrenia. Hippocampus. 2001;11:599–613. doi: 10.1002/hipo.1074. [DOI] [PubMed] [Google Scholar]
- Scopinho AA, Guimaraes FS, Correa FM, Resstel LB. Cannabidiol inhibits the hyperphagia induced by cannabinoid-1 or serotonin-1A receptor agonists. Pharmacol Biochem Behav. 2011;98:268–272. doi: 10.1016/j.pbb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Sherva R, Wang Q, Kranzler H, Zhao H, Koesterer R, Herman A, Farrer LA, Gelernter J. Genome-wide Association Study of Cannabis Dependence Severity, Novel Risk Variants, and Shared Genetic Risks. JAMA Psychiatry. 2016;73:472–480. doi: 10.1001/jamapsychiatry.2016.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira MM, Adams WK, Morena M, Hill MN, Winstanley CA. Δ9-Tetrahydrocannabinol decreases willingness to exert cognitive effort in male rats. J. Psychiatry Neurosci. 2016;41(6):150363. doi: 10.1503/jpn.150363. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction. 2004;99:425–430. doi: 10.1111/j.1360-0443.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JR, Darbyshire KME, Boucher AA, Kashem MA, Long LE, McGregor IS, Karl T, Arnold JC. Novel molecular changes induced by Nrg1 hypomorphism and Nrg1-cannabinoid interaction in adolescence: A hippocampal proteomic study in mice. Front Cell Neurosci. 2013;7:1–13. doi: 10.3389/fncel.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro AS, Wong A, Boucher AA, Arnold JC. Enhanced brain disposition and effects of Delta9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PLoS One. 2012;7:e35937. doi: 10.1371/journal.pone.0035937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, Van der Schaaf ME, Cools R, De Bruijn ER, Franke B, van Wel JH, Ramaekers JG, Verkes RJ. Acute effects of cocaine and cannabis on reversal learning as a function of COMT and DRD2 genotype. Psychopharmacology. 2016;233:199–211. doi: 10.1007/s00213-015-4141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann AM, Roser P, Arning L, Gallinat J, Epplen JT, Juckel G. Acute effects of delta9-tetrahydrocannabinol on the auditory evoked mismatch negativity are modulated by the NRG1 gene. Pharmacopsychiatry. 2010;43:194–195. doi: 10.1055/s-0030-1254088. [DOI] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, Takahashi RN, Bertoglio LJ. Delta9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25:958–965. doi: 10.1016/j.euroneuro.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Swift W, Wong A, Li KM, Arnold JC, McGregor IS. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PloS one. 2013;8:e70052. doi: 10.1371/journal.pone.0070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YY, McKeage K, Scott LJ. Delta-9-tetrahydrocannabinol/cannabidiol (Sativex®): a review of its use in patients with moderate to severe spasticity due to multiple sclerosis. Drugs. 2014;74:563–578. doi: 10.1007/s40265-014-0197-5. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol. 2005:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Takacs VT, Szonyi A, Freund TF, Nyiri G, Gulyas AI. Quantitative ultrastructural analysis of basket and axo-axonic cell terminals in the mouse hippocampus. Brain Struct Funct. 2015;220:919–940. doi: 10.1007/s00429-013-0692-6. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Karniol IG. Pharmacologic interaction between cannabinol and delta9-tetrahydrocannabinol. Psychopharmacologia. 1975;41:277–284. doi: 10.1007/BF00428937. [DOI] [PubMed] [Google Scholar]
- Tan H, Ahmad T, Loureiro M, Zunder J, Laviolette SR. The role of cannabinoid transmission in emotional memory formation: implications for addiction and schizophrenia. Front Psychiatry. 2014;5:73. doi: 10.3389/fpsyt.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex. 2010;20:1486–1496. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Chi N, Bechard M, Laviolette SR. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J Neurosci. 2011;31:5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Taylor AR, Markham JA, Geurts AM, Kanaskie BZ, Koenig JI. Disruption of the neuregulin 1 gene in the rat alters HPA axis activity and behavioral responses to environmental stimuli. Physiol Behav. 2011;104:205–214. doi: 10.1016/j.physbeh.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and delta9-tetrahydrocannabinol in mice: implications for medical cannabis. Br J. Pharmacol. 2016;173:53–65. doi: 10.1111/bph.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Dunn G, Murray RM, Evans N, Lister R, Stumpenhorst K, Harrison PJ, Morrison PD, Freeman D. Genetic moderation of the effects of cannabis: Catechol-O-methyltransferase (COMT) affects the impact of Delta9-tetrahydrocannabinol (THC) on working memory performance but not on the occurrence of psychotic experiences. J Psychopharmacol. 2015;29:1146–1151. doi: 10.1177/0269881115609073. [DOI] [PubMed] [Google Scholar]
- UNODC. World Drug Report, United Nations publication, Sales No. E.15.XI.6. 2015 [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. J Clin Exp Neuropsychol. 2007;29:357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Block RI, O'Leary DS, Ponto LB, Ghoneim MM, Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012;37:618–629. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: an fMRI study. Eur Neuropsychopharmacol. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- van Winkel R. Family-based analysis of genetic variation underlying psychosisinducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157. doi: 10.1001/archgenpsychiatry.2010.152. [DOI] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology. 2006;186:226–234. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Eggan SM, Horti AG, Wong DF, Lewis DA. Reciprocal alterations in cortical cannabinoid receptor 1 binding relative to protein immunoreactivity and transcript levels in schizophrenia. Schizophr Res. 2014;159:124–129. doi: 10.1016/j.schres.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. The role of endocannabinoid signaling in cortical inhibitory neuron dysfunction in schizophrenia. Biol. Psychiatry. 2016;79:595–603. doi: 10.1016/j.biopsych.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291:203–210. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PloS one. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Stoop N, Schetters D, Schoffelmeer AN, Pattij T. Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PloS one. 2011;6:e25856. doi: 10.1371/journal.pone.0025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, Ye W, Dannals RF, Ravert HT, Nandi A, Rahmim A, Ming JE, Grachev I, Roy C, Cascella N. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Hallak JE, Crippa JA. Interaction between cannabidiol (CBD) and (9)-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology. 2012;219:247–249. doi: 10.1007/s00213-011-2495-x. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]