Abstract
RATIONALE
A method was needed to accomplish solid phase extraction of a large urine volume in a convenient way where resources are limited, towards a goal of metabolome and xenobiotic exposome analysis at another, distant location.
METHODS
A porous extraction paddle (PEP) was set up, comprising a porous nylon bag containing extraction particles that is flattened and immobilized between two stainless steel meshes. Stirring the PEP after attachment to a shaft of a motor mounted on the lid of the jar containing the urine accomplishes extraction. The bag contained a mixture of nonpolar and partly nonpolar particles to extract a diversity of corresponding compounds.
RESULTS
Elution of a urine-exposed, water-washed PEP with aqueous methanol containing triethylammonium acetate (conditions intended to give a complete elution), followed by MALDI-TOF/TOF-MS, demonstrated that a diversity of compounds had been extracted ranging from uric acid to peptides.
CONCLUSION
The PEP allows the user to extract a large liquid sample in a jar simply by turning on a motor. The technique will be helpful in conducting metabolomics and xenobiotic exposome studies of urine, encouraging the extraction of large volumes to set up a convenient repository sample (e.g. 2 g of exposed adsorbent in a cryovial) for shipment and re-analysis in various ways in the future, including scaled-up isolation of unknown chemicals for identification.
Keywords: Solid phase extraction, bag, paddle, urine, mass spectrometry
We were confronted with the challenge of discovering xenobiotics in urine samples furnished by pregnant women at a location (Puerto Rico) which is remote from our laboratory (in Boston) where the mass spectrometer for such analysis is located. The long term goal or our project is to better understand the impact of exposure to environmental chemicals on preterm birth. Some other considerations were as follows. (1) It was important to collect large urine samples both to measure an average exposure and to establish, for each urine, a corresponding abundant repository sample (especially for the purpose of scaling up selected unknown compounds in the future for identification). Each urine is to be collected at a designated point (first trimester) in the pregnancy, making each corresponding repository sample unique and precious, so we wanted to have plenty of this sample from each pregnant women. (2) Neither storage nor shipment of large urine samples was realistic, so extracts much lower in volume needed to be formed. (3) The resources available to conduct the extraction steps where the urine was collected were limited.
To meet these challenges, we developed a “porous extraction paddle (PEP)” for simple solid phase extraction of a large (1.8 L) urine sample. In this technique, a porous bag filled with solid phase extraction particles (analogous to a tea bag) is flattened and immobilized between two pieces of stainless steel mesh so that extraction can be accomplished by stirring the PEP in the sample with a motor. The PEP bag then can be mailed to our laboratory for chemical analysis.
Here we present a prototype PEP and characterize its performance for the extraction first of a dye from aqueous acetic acid, and then for extraction of some representative endogenous and spiked compounds in a large volume (1.8 L) of acidified urine. We demonstrate, as was a main goal, that a PEP containing a mixed adsorbent can extract a diversity of compounds which are at least partly nonpolar from urine, a property shared by many xenobiotics. This minimal degree of purification, as intended here, means that one or more subsequent sample cleanup steps will usually be required to adequately minimize matrix effects in a subsequent detection step. For example, in a companion article,[3] we describe a subsequent weak anion exchange step to enable the detection of nonpolar sulfates in urine. Thus, as practiced here, the PEP mainly is providing a broad-scope extraction. A scaled-down version of the technique also is briefly described in which a small, particle-filled bag is rigidified in a vial containing a liquid sample, and extraction is accomplished by shaking.
Other bag techniques for extraction have been as follows: XAD-2 or OASIS HLB particles in a porous polyester bag for tumbling in a sample,[2] Tenax TA particles in a mulberry paper bag for headspace extraction, [3] activated carbon particles in a fiberglass bag for passive extraction of environmental water in dye tracing,[4] and a polypropylene membrane bag containing cyclohexane for headspace extraction.[5] Further, Lee and coworkers demonstrated the usefulness and versatility of housing adsorbent particles in a small polypropylene bag with very small pores (0.2-μm) to minimize undissolved matrix interferences from mixtures such as a slurry of soil,[6] or from tissues.[7]
EXPERIMENTAL
The “Mixed Particulate Adsorbent (MP-adsorbent)” contained equal amounts of the following six adsorbents, all from Supelco (Bellefante, PA, USA): “Silica-octadecyl”, 50 μm mean particle size, DSC-18/SP19381; “Silica-phenyl”, 50 μm, DIS-Ph/SP13625; Hydrophilic-Modified Styrene Polymer, 55–60 μm, HLB SPE/media; “Silica-ethylphenyl sulfonate”, 50 μm, DSC-SCX/SP18321; Polyamide Resin, 50–160 μm, DSC-DPA-6S/SP 10627; and “Silica-propyltrimethylamine”, 50 μm, DSC-SAX/SP18957. The MP-adsorbent was made homogeneous by dry-tumbling a mixture of equal amounts by weight of the six adsorbents for 5 minutes using a rotary evaporator at atmospheric pressure. Also the carbon adsorbent, Carboxen-1003, 350–860 μm, I124475, was from Supelco. The Tefzel Tiewraps (T-TEFZEL-04) were from Tiewraps.com (San Diego, CA, USA). Acetic acid, methanol and isooctane were from Fisher Scientific (Suwanee, GA, USA). Malachite Green (a carcinogen) as an HCl salt, α-cyano-4-hydroxycinnamic acid (CCA), triethylammonium acetate (TEAA) and reserpine were from Sigma Aldrich (St. Louis, MO, USA). Thiamine-d3 HCl (TD3) was from Toronto Research Chemicals (Toronto, Canada, M3J2J8). L-carnitine: HCl, O-octanyl (N-methyl-d3), that we will designate as OCD3, was from Cambridge Isotope Laboratories (Tewksbury, MA, USA). The Empore SDB-XC Disk, a polystyrenedivinylbenzene membrane, was from 3M Purification Inc. (St. Paul, MN, USA). Microcentrifuge tubes, pipette tips, and HPLC grade acetonitrile (ACN) were from Fisher Scientific (Pittsburgh, PA, USA). Pooled human urine was collected under IRB approval. The FEP bags (7 × 6 × 0.005 inches) and ClipNSeal LFB 16–19 assemblies were from Welch Fluorocarbon (Dover, NH, USA).
Malachite green solutions
To a 100 mL volumetric flask was added 10 mg of Malachite Green followed by addition up to the mark with 4% acetic acid. Ten microliters of this stock solution was added to 1.8 mL of 4% acetic acid for the “Mini-PEP” extractions, and 10 mL of the stock solution was added to 1.8 liters of the same solvent for the PEP extractions.
Porous extraction paddle (PEP)
The nylon mesh (N25, 25 μm pore) used to make the bags was from Industrial Products Corp. (Minneapolis, MN, USA). A Model 530–166S S/S CRYOBAND, from Accu-Seal (San Marcos, CA, USA), was used to heat-seal two pieces of nylon mesh together to make a bag (seal three sides, add adsorbent, and then seal the last side; outside dimensions 6.5 × 7.0 cm, inside 6.0 × 6.5 cm, using these automated conditions: 80 psi clamping for 3 sec at 243°C, then release the clamp when the temperature drops to 82°C). The 0.5 gallon glass jar (2000 MJ-PC) was from Industrial Glassware (Millville, NY, USA). The other components and construction of the stirring motor-mounted PEP, where the motor is attached to the lid of a jar (PEP assembly), are described in Supplemental Materials (Appendix A and B).
Cleaning a PEP
The PEP was washed by soaking in the following series of solutions (each total volume was 1.4 L): (1) acetone (solvent A, 6 h), (2) isooctane: methanol: acetone, 0.25:0.25:0.50, v/v/v. overnight; (B, 3), methanol: water:ammonia, 0.20:0.80:0.025, v/v/v (C, 3 h); (4) methanol:water:acetic acid, 0.20:0.80:0.012, v/v/v (D, 3h); and (5) methanol:water, 0.20:0.80, v/v (E, ≥ 3h). Between each transfer, solvent was removed from the PEP by shaking. A FEP bag (7 × 6 × 0.005 inches), was washed with 10 mL of the following series of solvents for 1 minute each: A, B, methanol, E, and water. A washed PEP (containing 1 mL of residual water) was charged with a PEP (still dripping wet with solvent E), and sealed with a ClipNSeal until use.
Extraction of Malachite Green from acidified water with a PEP
A 0.5 gallon jar was charged with 1.8 L of 0.5 μg/mL Malachite Green in 4% acetic acid (urine will be preserved in this way). A PEP assembly, containing 2.0 g of MP-adsorbent in a nylon bag, was screwed onto the jar, and the motor was plugged into an electrical outlet, giving 190 RPM. Aliquots were taken with time for measurement of absorbance, to define the half-life for dye adsorption. Similarly, a PEP containing an Empore SDB-XC membrane, and a PEP containing Carboxen-1003, were stirred separately in equivalent samples, and absorbance measurements were made.
Extraction of Malachite Green from acidified water with a Mini-PEP
A nylon bag was built with outside dimensions of 0.6 × 3 cm, and inside dimensions of 0.3 × 2.5 cm, that contained 10 mg of MP-adsorbent. The bag was placed in a 2-mL vial, and 1.8 mL of a solution of 0.5 μg/mL Malachite Green (A = 0.09 at 617 nm) in 4% acetic acid was added. Capping the vial immobilized the bag. The vial (n = 5) was shaken at 230 oscillations per minute for a period of time ranging up to 20 minutes. The absorbance of the solution in each vial was measured to define the half-life for dye adsorption.
PEP extraction of compounds from acidified urine followed by qualitative mass spectrometry analysis
Pooled human urine (1.8 L) comprising 4% acetic acid as a preservative was PEP-extracted with 2 g of MP-adsorbent for 30 h at 190 RPM. After washing the PEP by similar stirring in water for 1 h, patting dry with Kimwipes, and further drying the recovered bag in a desiccator containing anhydrous calcium sulphate, 5 mg of rehomogenized adsorbent was taken and vortexed in 0.2 mL of 40% aqueous acetonitrile containing 20 mM triethylammonium acetate for 20 min followed by centrifugation (4000 x g for 10 sec). Two microliters of supernatant was mixed with CCA matrix (5 mg/mL, ACN: water, 1:1) in 1: 10 volume ratio, and 0.7 μL/spot of the mixture was applied to the MALDI plate. Analysis was done on a Model 5800 MALDI-TOF/TOF-MS (AB SCIEX, Foster City, CA) in both negative and positive ion modes with a delay time of 150 ns, and 400 laser pulses were averaged to generate a spectrum. MS/MS was performed with air, a mass resolution window of 400, and the metastable-ion suppressor on.
Recovery of spiked compounds from acidified urine
Two equivalent 1.8 L urine samples were set up from a pooled urine. The first one (blank urine) was extracted as above with a PEP assembly followed by a similar sequence of steps (see below) leading to MALDI-TOF-MS spectra. The second urine sample (spiked urine) was spiked with 400 nmol each of TD3, OCD3 and reserpine prior to corresponding extraction and post-extraction steps. In more detail, an aliquot (5.4 mg) of MP adsorbent from the blank urine was processed as follows: shaken in 0.1 mL of 20 mM TEAA in 30% ACN for 20 min at 250 RPM followed by centrifugation and recovery of the supernate. This step was repeated with 0.1 mL of 20 mM TEA in 70% ACN. One μL of the combined supernant was added to 9 μL CCA Matrix followed by depositing 0.7 μL on the MALDI plate three times and acquiring MALDI-TOF-MS spectra (see below). The spiked urine sample was processed in the same way, except a second 1:9 sample/CCA matrix solution was spiked further with 2 pmol each of thiamine, OCD3 and reserpine prior to similar analysis by MALDI-TOF-MS. Since 0.7 μL of 10 μL was spotted, the amount of the second spike was 140 fmol per MALDI spot. Thiamine rather than TD3 was used for the second spike since we ran out of TD3; nevertheless the second spike with thiamine met our needs. Each such spectrum was the average of 1200 laser shots in the positive reflector mode with a delay time of 120 ns. The observed peak heights for the spiked and doubly-spiked standards were normalized against the peak height of endogenous 9-decenoylcarnitine at m/z 314.232 on the same MALDI spot.
RESULTS
Description of the PEP
A picture of a motor-mounted (stirring motor) PEP assembly is shown in Figure 1. In use, the PEP assembly is screwed onto a jar of a sample and plugged into an electrical outlet. The black lid with a PTFE liner fits a 0.5 gallon jar. On the top of the lid the stirring motor is mounted. The lid contains a hole to accommodate the PTFE shaft (vertical) that connects the PEP to the motor. The PEP has four parts: (1) a white PTFE bar (horizontal), functioning as a cage (mesh/bag) holder, with a hole centered and part-way through on the top (into which the above shaft is press-fitted); this bar also has a rectangular slot from one end to the other (into which the cage fits that comprises the screen and bag) located on the side of the bar, opposite this hole; (2) a pair of stainless steel meshes forming the relatively flat sides of the cage; (3) a porous, heat-sealed, nylon bag containing 2.0 g of a Mixed Particulate Adsorbent (MP-adsorbent, to extract a broad range of compounds); and (4) five Tefzel Tiewraps that both secure the bag in the cage and lock the edge of the cage into the slot of the PTFE bar. In this way the entire PEP cage, including the MP-adsorbent inside, is rigidified, and no plastic (except the nylon bag) contacts the sample to be extracted. We selected a motor that stirs the PEP at about 190 RPM in a 0.5 gallon jar nearly full of water (2 cm below the top) corresponding to 1.8 L. Under these conditions, the cage holder (horizontal PTFE bar) maintains the orientation of the cage.
Figure 1.
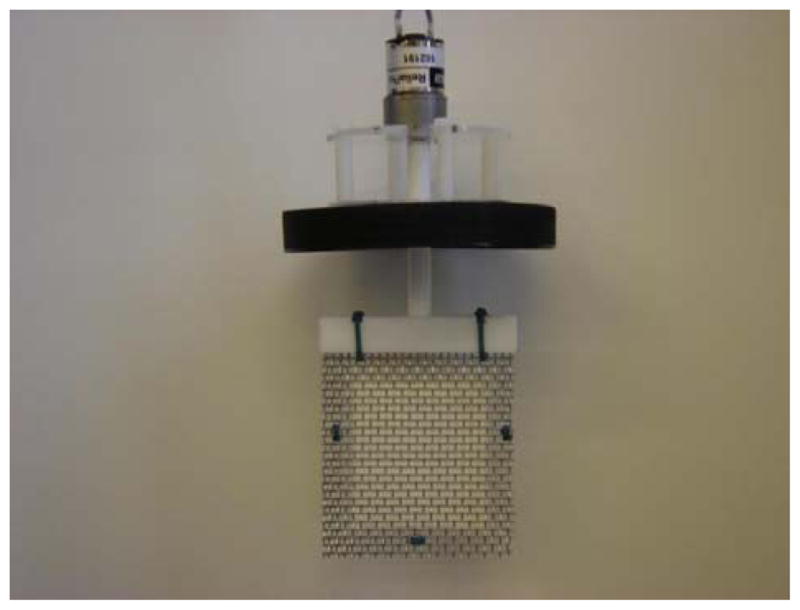
Photograph of a porous extraction paddle (PEP) having the following components from top to bottom: stirring motor; four off-sets (vertical white rods), black lid (for a 0.5 gallon jar, not shown); shaft; horizontal PTFE bar into which the shaft is press-fitted at the top of the bar, with a slot on the lower side that accommodates the cage, and with two vertical slots for two Tiewraps; porous nylon bag (6.0 × 6.5 cm) containing 2 g of MP-adsorbent that is sandwiched between two sheets of stainless steel mesh (each 8.0 × 8.7 cm), where the meshes are held together further with three additional Tiewraps
A stainless teel woven mesh with an intermediate wire thickness (0.025 inch) was selected for the cage of the PEP for two reasons. First, it was easy to cut with hand held shears. Second, it had enough flexibility so that varying amounts of bagged adsorbent could be immobilized inside while still allowing the top edges of theses meshes to be fully and easily squeezed together to fit into a given slot of the PTFE mounting bar that engages the shaft of the motor.
As a model system to conveniently study the properties of this prototype PEP, a visibly-green solution of Malachite Green in 4% acetic acid (initial absorbance at 617 nm was 0.09) was selected. We prepared three bags with little effort (intentionally) to spread the MP-adsorbent uniformly. This gave the average adsorption curve B in Figure 2, revealing a poor precision in the speed of removal of the dye during the early part of the experiment. Nevertheless, each bag achieved essentially complete removal of the dye after 30 hours. Thus, it is not important to spread the adsorbent uniformly in the bag. The half-life for extracting the dye, considering curve B, is about 0.6 h. Each of these three, irregularly-packed bags exhibited a unique, irregular pattern of color at the end of the experiment, and the pattern was different between the opposite sides of the same bag.
Figure 2.
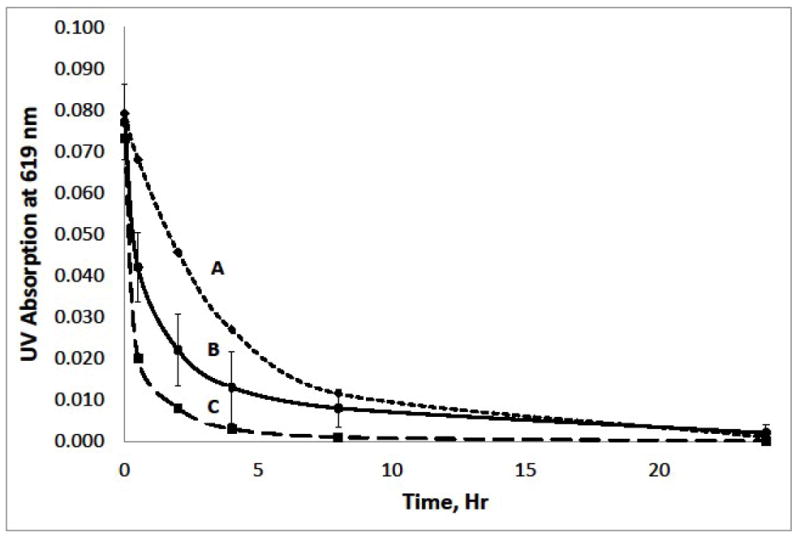
Extraction of Malachite Green dye from 4% acetic acid with a PEP. A, adsorbent in the cage is an Empore SDB-XC membrane; B, MP-adsorbent (2.0 g) packed nonuniformly in a cage-immobilized porous nylon bag, n=3; and C, Carboxen-1003 adsorbent (2.0 g) packed in a cage-immobilized porous nylon bag.
When a bag was prepared in a cage with care to spread the packing as evenly as possible (by using the edge of a ruler) the color distribution on each side of the bag was much more homogeneous. The color was darker throughout one side of the bag relative to the other, even though each side mostly was relatively homogenous in color. Probably this is largely because the paddle was not completely symmetric in its construction, nor could it be stirred in a completely symmetric way in the jar. Even a perfect paddle gives a complex flow regime in a simple container.[8] We speculate that there is some tangential flow of solvent along each side of the bag from the higher pressure zone (region of the bag surface on each side that pushes solvent) to the lower pressure zone (adjacent region on the same side where each bag surface retreats from the solvent).
In these (and other experiments about to be described), the level of the dye solution was set to 2 cm below the upper rim of the jar (right at its neck) before the PEP was inserted. No vortex-induced funnel of air formed upon stirring under these conditions (at 190 RPM). If the initial level of dye solution instead was 3 cm below the rim at the outset, a funnel of air formed that reached the top of the bar of the PEP, but not the cage. We did not study extraction of the dye under this latter condition.
We also tested 2.0 g of a carbon packing (Carboxen-1003) having particles mostly in the range of 350 to 860 μm in diameter. This decreased the extraction half-life for the dye to about 0.23 h (curve C in Figure 2). The black color of the carbon packing prevented visible inspection of the dye on this packing at the end of the experiment.
Membrane PEP
We similarly tested a PEP containing an Empore SDB-XC (polystyrenedivinylbenzene) membrane. This gave curve A in Figure 2, where the half-life for the extraction is seen to be about 2.4 h. At the end of the extraction, the color was basically homogeneous on each side of the bag and one side of the bag was only slightly darker than the other. Only one of the two forward vertical edges was visibly darker.
Mini-PEP
A mini-PEP was set up by placing a small porous nylon bag containing 10 mg of the MP-adsorbent in a 2 ml vial. Malachite Green solution (1.8 mL, same concentration as above) was added and the cap was screwed on to immobilize the bag. The appearance of the vial at this point is shown in Figure 3A. This and four equivalent vials were shaken for times up to 20 min, and the absorbance of the solutions were measured, resulting after the longer times in a dyed Mini-PEP and a colorless solution, as shown at the 8 min point in Figure 3B. The half-life for adsorption of the dye was about 20 seconds. In principle, a small bag of adsorbent can also be immobilized at the bottom of an open vial in various ways that would permit a pipet tip or tube to reach directly to the bottom of the vial. A Mini-PEP set up in this way would permit a process of solid phase extraction to be accomplished by pipetting and shaking steps that could be automated, and would thereby enable the PEP to be applied to small sample volumes. However, for the analysis of small liquid samples by mass spectrometry, many techniques such as on-line extraction or automated handling of solid phase extraction cartridges are widely employed and convenient for many applications.
Figure 3.
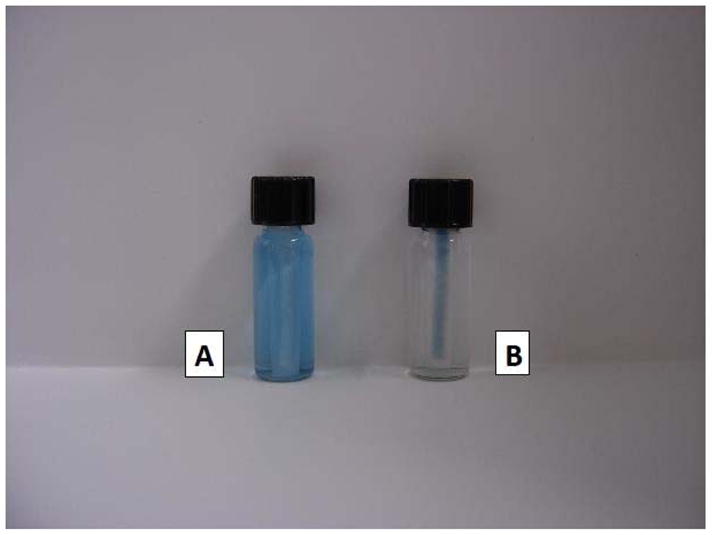
Photograph of vials for extraction of Malachite Green dye with a mini-PEP. A, vial containing a porous nylon bag which, in turn, contains 10 mg of MP-adsorbent, and also a solution (1.8 mL) of Malachite Green in 4% acetic acid that has just been added. B, equivalent vial after shaking at 230 oscillations per minute for 8 minutes.
Qualitative analysis of endogenous compounds in urine
Pooled human urine (1.8 L) was extracted with a PEP containing 2 g of MP-adsorbent. As pointed out earlier, a large volume of urine was selected to provide an average exposure to xenobiotics and to enable formation of a significant repository sample. A mixed particle (MP) adsorbent, with each component either partly or fully nonpolar, was selected since many xenobiotics are nonpolar. Further, the amount (2 g) of the MP-adsorbent was small relative to the volume of urine, in order to set up a competitive extraction that favored recovery of highly nonpolar xenobiotics.
Acetic acid was selected as the preservative mainly because we intend the woman to form the large volume of urine in her home, and also because of its low cost in a highly purified form. While some analytes will degrade at low pH, the same products will tend to form in different samples. Some analytes are more stable at a low pH.
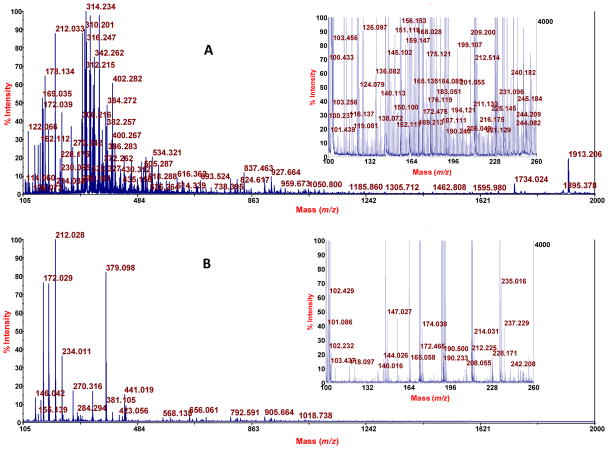
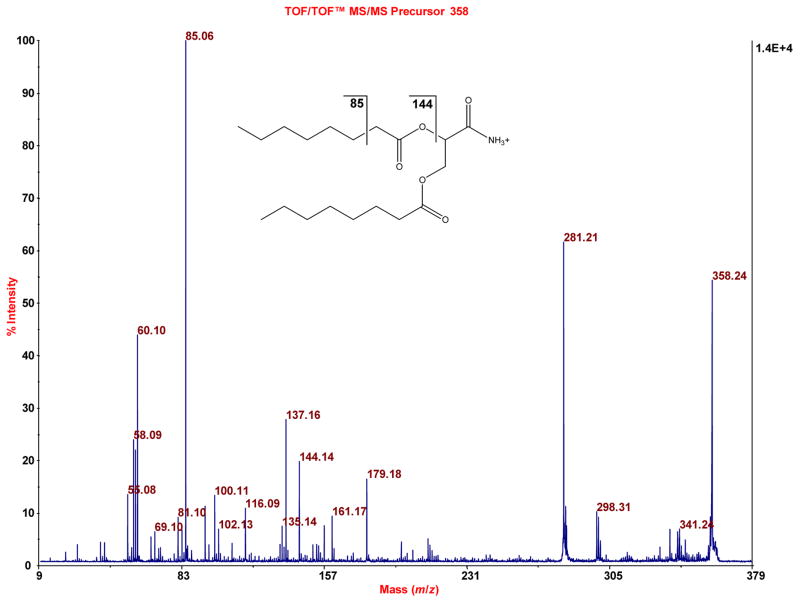
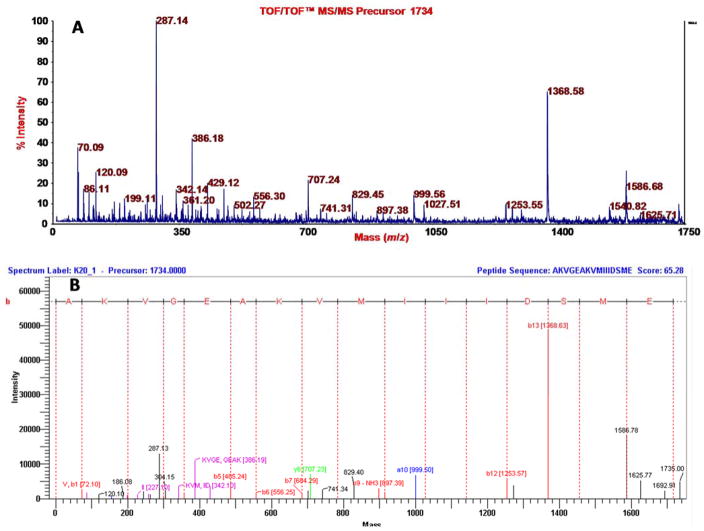
Elution of an aliquot (5.4 mg) of the recovered, washed (only with water, in order to retain even analytes that are only slightly nonpolar), dried PEP adsorbent with aqueous acetonitrile containing triethylammonium acetate in two steps was done towards a goal of fully or at least highly eluting the PEP. Analysis of an aliquot (2 μL of 200 μL) by MALDI-TOF-MS showed many mass spectral features as seen in Figure 4A relative to a blank spectrum (CCA matrix only) in Figure 4B. The insets show 25x amplification to bring up lower intensity peaks. Some of the peaks were subjected to TOF/TOF analysis. Representative spectra (chosen to represent the structural variety of known compounds detected) are as follows, where the assignments are based on the accurate mass measurements provided by MALDI-TOF-MS analysis; the fragmentation data obtained by MALDI-TOF/TOF-MS analysis; and use of METLIN: sulfate, glucuronide conjugate of apigenin or genistein, which are highly polar isomers (Figure 5); uric acid, a slightly nonpolar compound (Figure 6); vitamin B1, a moderately nonpolar compound (Figure 7); and 2,3-dioctanylglyceramide, a highly nonpolar compound (Figure 8). Some peptides were also detected (fragmentation gave iminium ions), for example a peptide derived from the light chain of kinesin (Figure 9). Thus, a PEP can extract a diversity of compounds from urine. At this stage the analysis was qualitative. For quantitative analysis in the future work of analytes that appear to correlate with preterm birth, it will be important to employ stable isotope internal standards. As described in the next paragraph, the method of standard additions can be used for quantitation of selected analytes in explorative studies, although this technique is tedious.
Figure 4.
MALDI-TOF-MS Spectra of a PEP extract of urine (A) and of a matrix (CCA) blank (B). For A, 1.8 L of urine was extracted with a PEP containing 2.0 g of MP-adsorbent; then 5 mg of the washed, dried, and re-homogenized adsorbent was eluted with 40% aqueous acetonitrile containing triethylammonium acetate followed by combination of an aliquot of the supernatant after centrifugation with MALDI matrix. Insets: higher sensitivity setting.
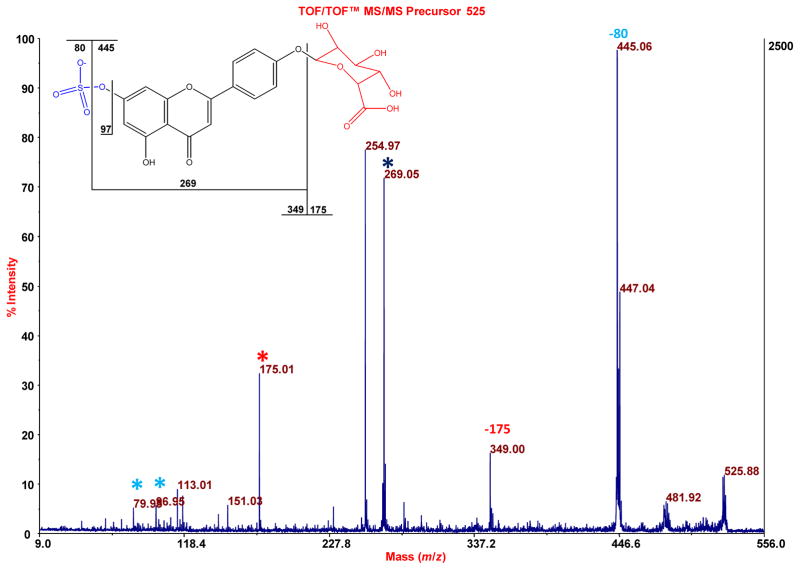
Figure 5.
MALDI-TOF/TOF-MS of m/z 525.030 that is observed in the MALDI-TOF-MS spectrum of Figure 4A (where the sample tested was an eluate of a urine-exposed PEP). This accurate mass fits both a sulfate, glucuronide conjugate of apigenin and genistein (exact mass 525.034), as does the fragmentation pattern seen here. For illustration purposes, the structure of apigenin-7-sulfate-4′-glucuronide is shown.
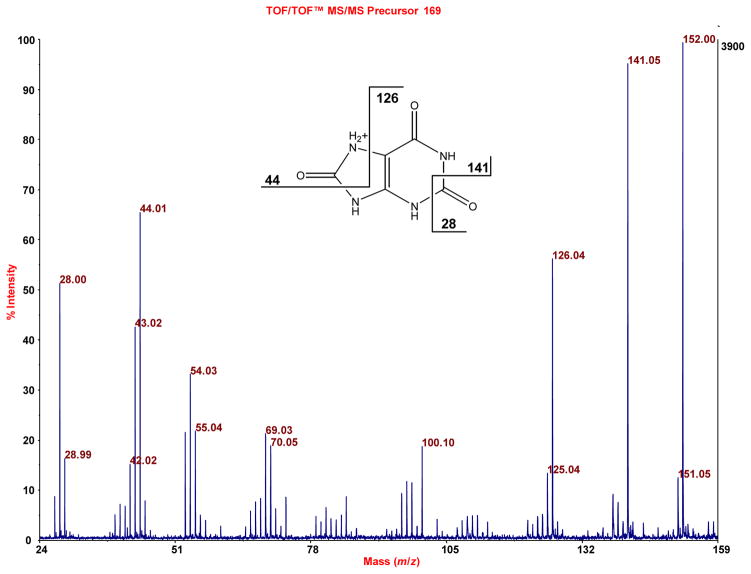
Figure 6.
Similar to Figure 5, MALDI-TOF/TOF-MS indicates the presence of uric acid in the sample of Figure 4A, when the accurate mass 169.035 (exact mass of uric acid is 169.036) is subjected to TOF/TOF analysis.
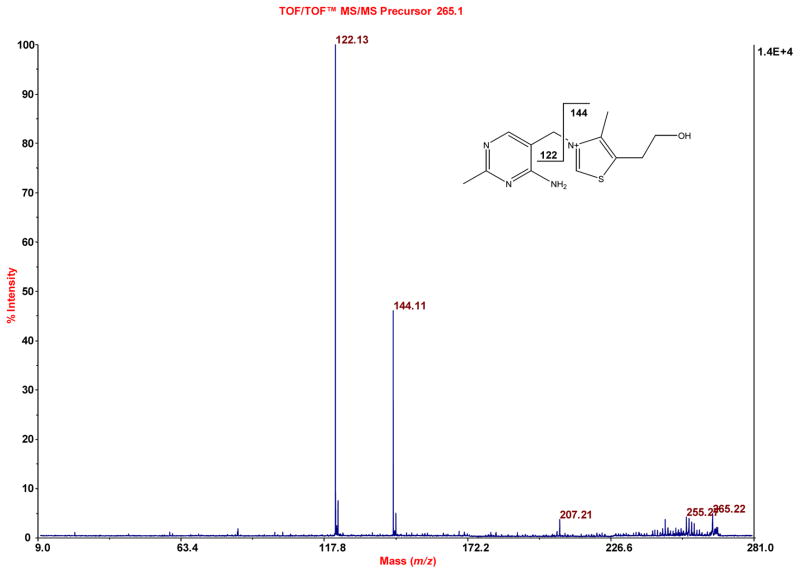
Figure 7.
Similar to Figure 5, evidence for vitamin B1 is obtained when the accurate mass 265.110 (exact mass of vitamin B1 is 265.112) is subjected to TOF/TOF analysis.
Figure 8.
Similar to Figure 5, evidence for 2,3-dioctanoylglyceramide is obtained, when the accurate mass 358.264 (exact mass is 358.259) is subjected to TOF/TOF analysis.
Figure 9.
Similar to Figure 5, TOF/TOF (A) and annotation (B) data are shown for a peptide having a mass of 1734, which is derived from kinesin light chain and has the sequence AKVGEAKVMIIIDSME based on employing DeNova Explorer for the annotation.
Quantitative analysis of spiked compounds in urine
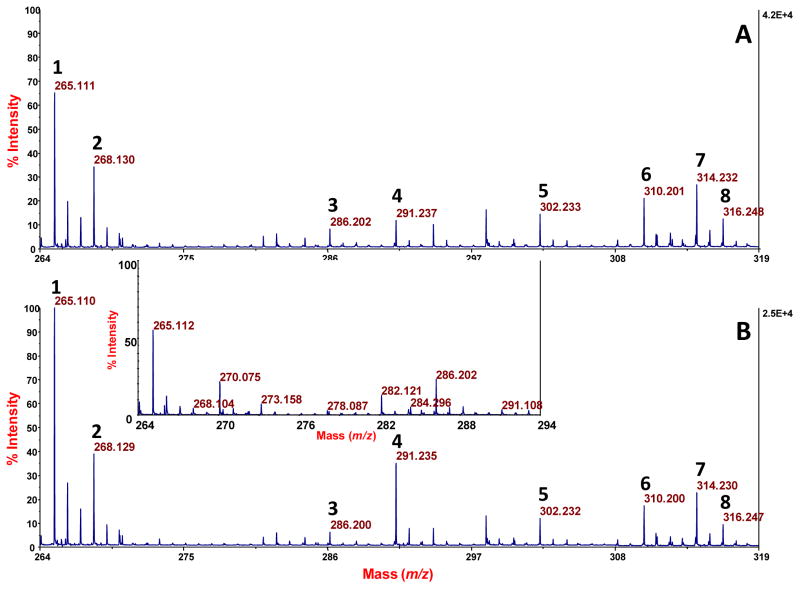
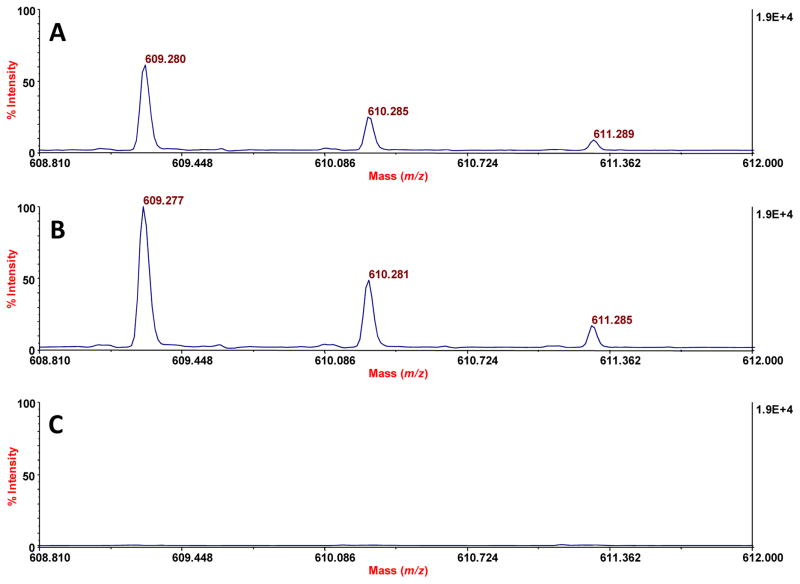
To evaluate the performance of the competitive PEP technique quantitatively when applied to 1.8 L of urine, we tested corresponding unspiked (blank) and spiked urines. The latter sample was spiked with 400 nmol each of thiamine-d3 HCl (TD3), L-carnitine: HCl, O-octanyl[N-methyl-d3] (OCD3), and reserpine. The peaks that we observed for these compounds in the spiked urine by MALDI-TOF-MS (Figures 10A and 11A) were normalized by ratioing them to the peak for endogenous 9-decenyl carnitine, and then calibrated by the method of standard additions (Figures 10B and 11B). No peaks for these compounds were observed in the blank urine (Inset in Figure 10; Figure 11). The absolute recoveries of these three spiked standards, based on the method of standard additions, were as follows: 26 ± 3.8% for TD3, 15 ± 1.9% for OCD3, and 52 ± 3.3% for reserpine (n = 3 in each case). The lower yield for OCD3 may be caused, in part, by competitive adsorption with other acylcarnitines, and the higher yield for reserpine may reflect its stronger adsorption competitively (to the PEP) due to its greater nonpolar bulk. Consistent with a 52% recovery of reserpine, direct analysis of the urine after the PEP extraction showed that about 60% of the reserpine had been removed. The noise level for such direct analysis did not allow a meaningful, similar assessment of the other two spiked compounds. This overall data is consistent with our plans to preferentially enrich nonpolar compounds. We made no effort to optimize the yields of these compounds, as by selecting the best adsorbent for a given analyte of interest, or by applying the PEP to a smaller volume of the urine.
Figure 10.
MALDI-TOF-MS spectra of spiked and nonspiked urine samples after PEP extraction, elution, and combination with CCA matrix. Assignment of peaks: 1, thiamine; 2, TD3; 3, O-octanolycarnitine; 4, OCD3; 5, nonanoylcarnitine; 6, decatrienoylcarnitine; 7, 9-decenolycarnitine; 8, decanolycarnitine. A. Spectrum from a urine that was spiked with 400 nmol each of TD3, OCD3, and reserpine. B. Similar spectrum where a second spike of 140 fmol per spot of thiamine, OCD3 and reserpine was done just prior to MALDI-TOF-MS analysis. Inset: spectrum from a blank urine, lacking peaks for TD3 and OCD3. (Note: data for reserpine is shown in Figure 11.)
Figure 11.
MALDI-TOF-MS spectra similar to that of Figure 10, except here the data for recovery of reserpine is shown. Assignment of peaks: 609.280, protonated reserpine; 610.285 and 611.289, isotopic peaks for protonated reserpine. A. Spectrum from a urine that was spiked with 400 nmol of reserpine. B. Similar spectrum where a second spike (140 fmol) was done just prior to MALDI-TOF-MS analysis. C. Spectrum from a blank urine, lacking peaks for protonated reserpine.
Potential to resist clogging
Solid phase extraction becomes more complicated when particulates are present. Commonly a sample is centrifuged or filtered to remove particulates prior to solid phase extraction, usually because the particulates are not of interest, or because they tend to clog the extraction device. In other cases it is important to recover the particulates. While the ability of the PEP to resist clogging was not studied here, clogging will depend on the nature of the particulates. No doubt the PEP can be designed or operated to resist clogging. This is because the PEP adsorbent is spread out; the pressure across the bed can be relatively low; tangential flow in principle can be enhanced; and the direction of rotation of the PEP can be switched back and forth.
CONCLUSION
The solid phase extraction technique presented here, termed a “porous extraction paddle or PEP”, provides a simple way to perform solid phase extraction of a relatively large volume of urine (e.g. 1.8 L). The technique is flexible in the size of the bag and its content of adsorbent media; anticipated to be resistant to clogging by particulates; and can be conducted with low cost equipment (stirring motor). An application of the PEP is reported in an accompanying article.[1]
Supplementary Material
Acknowledgments
This work was supported by NIH Grant P42WS017198 from NIEHS. The authors thank Supelco for donating the particulate adsorbents, Accu-Seal for donating a bag sealer, and Industrial Fabrics for donating the nylon mesh.
Footnotes
Electronic supplementary material. The online version of this article contains supplementary material, which is available to authorized user.
Conflict of interest All authors declare they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Internal Review Board at Northeastern University.
References
- 1.Yao Y, Wang P, Shao G, Anzalota Del Toro LV, Codero J, Giese RW. Nontargeted analysis of the urine nonpolar sulfateome: a pathway to the nonpolar exposome. 2016 doi: 10.1002/rcm.7726. submitted to Rapid Commun. Mass Spectrom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magner JA, Alsberg TE, Broman D. Bag-SPE-a convenient extraction method for screening of pharmaceutical residues in influent and effluent water from sewage treatment plants. Anal Bioanal Chem. 2009;395:1481. doi: 10.1007/s00216-009-3099-8. [DOI] [PubMed] [Google Scholar]
- 3.Won M-M, Cha EJ, Yon O-K, Kim NS, Kim K, Lee D-S. Use of head space mulberry paper bag micro solid phase extraction for characterization of volatile aromas of essential oils from Bulgarian rose and Provence lavender. Anal Chim Acta. 2009;631:54. doi: 10.1016/j.aca.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Aley TJ. 2012 www.ozarkundergroundlab.com.
- 5.Hauser B, Shcellin P, Popp P. Membrane-assisted solvent extraction of triazines, organchlorine, and organophosphorus compounds in complex samples combined with large-volume injection-gas chromatography/mass spectrometric detection. Anal Chem. 2004;74:6029. doi: 10.1021/ac0492923. [DOI] [PubMed] [Google Scholar]
- 6.Basheer C, Alnedhary AA, Madhava Rao BS, Lee HK. Determination of carbamate pesticides using micro-solid-phase extraction combined with high-performance liquid chromatography. J Chromatogr A. 2009;1216:211. doi: 10.1016/j.chroma.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Sajid M, Basheer C, Narasimhan K, Choolani M, Lee H-L. Application of microwave-assisted micro-solid-phase extraction for determination of parabens in human ovarian cancer tissues. J Chromatogr B. 2005;1000:192. doi: 10.1016/j.jchromb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Siba S. Flow patterns of liquids in agitated vessels. AIChE Journal. 1958;4:48589. doi: 10.1002/aic.690040419. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.