Abstract
Purpose
To investigate white matter (WM) structural alterations using diffusion tensor imaging (DTI) in obstructive sleep apnea (OSA) patients, with or without residual sleepiness, following adherent continuous positive airway pressure (CPAP) treatment. Possible quantitative relationships were explored between the DTI metrics and two clinical assessments of somnolence.
Methods
Twenty nine male patients (30–55 years old) with a confirmed diagnosis of OSA were recruited. The patients were treated with CPAP therapy only. The Psychomotor Vigilance Task (PVT) and Epworth Sleepiness Scale (ESS) were performed after CPAP treatment and additionally administered at the time of MRI scan. Based on the PVT results, the patients were divided into a non-sleepy group (lapses≤5) and a sleepy group (lapses > 5). DTI was performed at 3T, followed by an analysis using tract-based spatial statistics (TBSS) to investigate the differences in fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (λ1), and radial diffusivity (λ23) between the two groups.
Results
A higher MD (p < 0.05) was observed in the sleepy group than the non-sleepy group in the whole-brain TBSS analysis in the WM. The increased MD (17.8% of the fiber tracts; p<0.05) was caused primarily by an elevated λ23. Axial diffusivity (λ1) exhibited no significant difference (p > 0.17). The alterations in FA or MD of individual fiber tracts occurred mainly in the internal/external capsule, corona radiata, corpus callosum, and sagittal stratum regions. The FA and MD values correlated with the PVT and ESS assessments from all patients (R ≥ 0.517, p < 0.05).
Conclusions
Global and regional WM alterations, as revealed by DTI, can be a possible mechanism to explain why OSA patients with high levels of CPAP use can have differing responses to treatment. Compromised myelin sheath, indicated by increased radial diffusivity, can be involved in the underlying WM changes.
INTRODUCTION
Obstructive sleep apnea (OSA), characterized by repeated obstructions of the upper airway with intermittent hypoxic exposure, occurs in at least 5% of the population.1 This ventilatory condition has multiple detrimental physiological and psychological consequences, including intermittent hypoxemia, fragmented sleep at night, and excessive daytime sleepiness.2 Besides cardiovascular and metabolic comorbidities, OSA patients also show brain alterations especially in areas responsible for cognitive, autonomic, or emotional functions,3 leading to compromised work efficiency and decreased life quality. Intermittent hypoxemia can result in oxidative stress and ischemia-reperfusion injury in the brain, which has been recognized as a major factor contributing to the pathogenesis of OSA and its comorbidities.4
Continuous positive airway pressure (CPAP) is a primary non-surgical approach for treating OSA.5 It has been demonstrated in several studies that CPAP treatment can alleviate daytime sleepiness,6–8 reduce nocturnal symptoms,9 and improve mental acuity after 12 months of treatment.10 However, CPAP therapy does not fully reverse wake impairment in all patients with daytime sleepiness.6,11–13 For example, among OSA patients receiving daily CPAP treatment for more than 6 hours, 34% reported daytime sleepiness and 65% had objectively measured hyper-somnolence.13 It is also reported that 25% of the newly-diagnosed patients who are treated with CPAP remain symptomatic and sleepy during daytime.13 Thus, millions of OSA patients continue suffering from daytime sleepiness despite CPAP therapy. The mechanism underlying the difference in response to treatment remains unclear. We hypothesize that patients with residual sleepiness following CPAP therapy may have structural alterations in specific brain regions when compared to those responding to CPAP treatment.
MRI is a powerful tool to investigate brain structural and functional changes in many pathologic and neuropsychologic processes, as well as provide feedback following therapeutic intervention. Recent MRI studies on OSA patients have suggested gray matter (GM) structural alterations, such as focal volume reductions in the hippocampus,14, 15 cortical GM,15 thalamus and basal ganglia.16 Improvement in cognitive function was also observed in conjunction with GM volume increase after CPAP treatment.14 Additionally, metabolic changes in the hippocampus of OSA patients have been detected, as well as the partial reversal after CPAP treatment.17
With the advent of diffusion tensor imaging (DTI), studies on white matter (WM) alterations associated with OSA have also emerged. DTI is particularly powerful as it provides a set of quantitative parameters that can be related to tissue microstructural changes, including myelin or axonal alterations. Using fractional anisotropy (FA), a prevalent DTI parameter,18 changes in the corpus callosum, cingulum,19 and parietal and prefrontal WM areas10 have been demonstrated in OSA patients. In addition, altered mean diffusivity (MD), another popular DTI parameter, has also been reported in multiple brain regions.10, 20 Recently, Castronovo et al. 10 reported that WM brain structures susceptible to hypoxemia can change in OSA patients before, during, and after a 12-month CPAP treatment. However, the question of why some patients respond to CPAP therapy whereas others do not remains intriguing.
The present study aims at extending the application of DTI from characterizing OSA-induced brain changes to understanding the mechanism of the differential response to CPAP treatment in OSA patients. Such a study is expected to provide new insights into the pathogenesis of this disorder.
METHODS
Participants
With approval of the Institutional Review Board and written informed consent, 29 male treatment-naïve patients (age 30–55) with OSA were recruited. Inclusion criteria were: a polysomnographically-confirmed diagnosis of severe OSA (Apnea-Hypopnea Index [AHI] ≥40 with duration > 5% of the time 21); prescribed CPAP as the only treatment (no medication was provided); and full adherence to nightly use of CPAP (≥6h/night) for at least 30 days. Exclusion criteria were: age > 60 years; sleep disorders other than OSA; night shift workers; psychiatric disorders; hypertension (> 150/90mmHg); diabetes; use of neuro adrenergic, monoamine, or sedative medications; history of stroke, epilepsy, head trauma, or brain surgical operation; brain structural abnormalities (such as infarction, hemorrhage, or tumors); or contraindications to MRI. The CPAP device has a microprocessor that measures daily mask-on time and AHI along with software to download and import the data into the study database.
Assessments
The PVT was performed at Visit 1 and at the time of the MRI scan. Based on the PVT results, participants were divided into a non-sleepy group (no daytime sleepiness, both PVT lapses ≤5; n = 17, and age = 46.1±8.8 years) and a sleepy group (experiencing daytime sleepiness, PVT lapses > 5; n = 12, and age = 45.6±7.3 years). Besides sleepiness, neurocognitive function, mood, and rest/activity measured by actigraphy were assessed. Participants also completed an ESS test to evaluate self-reported daytime somnolence.
Image Acquisition
Images were acquired on a 3-Tesla MRI scanner (Discovery MR750, General Electric Health Care, Waukesha, Wisconsin, USA) using a commercial 32-channel head coil (Nova Medical, Inc., Wilmington, MA). The subjects were padded with flexible foam to limit head motion during the imaging session. Using an axial T1-weighted three-dimensional brain volume imaging (3D-BRAVO) sequence (repetition time or TR = 12.0ms, echo time or TE = 5.2ms, flip angle = 13°, inversion time or TI = 450ms, matrix size = 384×256, voxel size = 1.2×0.57×0.69 mm, and scan time = 2min 54sec), high-resolution anatomic images were obtained to exclude possible lesions specified in the exclusion criteria. DTI data were then obtained in the axial plane using a spin-echo echo planar imaging sequence with the following parameters: TR= 4500ms, TE = 89.4ms, field of view = 20×20cm2, matrix size = 160×132, slice thickness = 3mm, slice spacing = 1mm, NEX = 1, b-values = 0, 1000 s/mm2, gradient direction = 27, and scan time = 8min 29sec.
Data Analysis
The diffusion tensor images were processed using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (http://www.fmrib.ox.au.uk/fsl), or FSL.22 Voxel-wise statistical analysis of the images was performed using tract-based spatial statistics (TBSS)23 with the following steps. First, the brain was extracted using the brain extraction tool. An FSL “eddy” tool was applied as a preventive measure to reduce inconsistent image distortion. After generating the FA maps using the FMRIB diffusion toolbox, the images from all subjects were aligned to an FA standard template through a nonlinear co-registration. The aligned FA maps were then averaged to produce a group mean image, which was used to generate an FA skeleton highlighting the tracts common to the entire group. For each subject, an FA threshold of 0.2 was used before projecting the aligned FA map onto this skeleton. The resulting skeletonized FA maps were then fed into a voxel-wise group-level analysis.24 In addition to FA, diffusivity maps based on MD, axial diffusivity (λ1; the principal eigenvalue), and radial diffusivity (λ23; the average of the two remaining eigenvalues) were also generated using the same steps outlined above. Using an FSL permutation test (FSL Randomize Tool with 500 permutations), FA, MD, λ1, and λ23 were tested for differences between the means of the sleepy and non-sleepy groups. A significance level of p < 0.05 was used to declare difference between the patient groups.
To investigate regional WM changes, the Johns Hopkins University (JHU) WM tractography atlas25 in FSL was used as a standard for WM parcellation. The entire WM was parceled into 48 regions of interest (ROIs) using the 1mm JHU-ICBM labels (Fig. 1). FA and MD were calculated by averaging the pixel values in each ROI and reported as mean ± standard deviation, followed by a statistical analysis described in the following sub-section. In order to investigate the possible relationships between the DTI metrics and the clinical findings, multiple linear regression analyses were performed between the ROI-based FA or MD values of specific fiber tracts (such as the corpus callosum, corona radiata, internal/external capsule, sagittal stratum, and cingulum) and the ESS or PVT results for all 29 patients. The statistical analysis is detailed below.
Fig. 1.
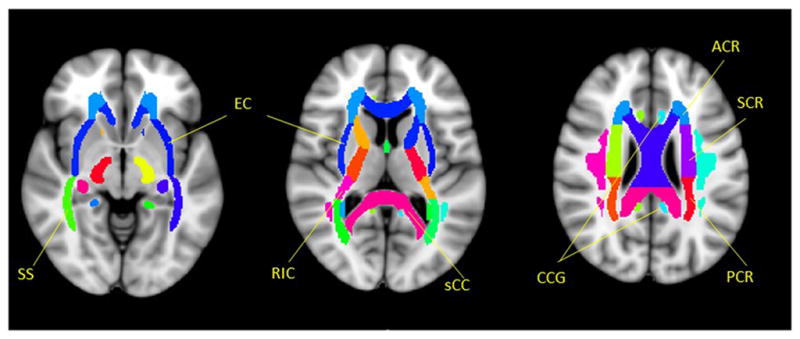
Selected ROIs in individual fiber tracts according to a JHU-ICBM-labels-1mm template. SS - Sagittal striatum (including inferior longitudinal fasciculus and inferior fronto-occipital fasciculus); EC - External capsule; RIC - Retrolenticular part of internal capsule; sCC - splenium of Corpus Callosum; CCG - Cingulum (cingulate gyrus); ACR - Anterior corona radiata; SCR - Superior corona radiata; PCR - Posterior corona radiata.
Statistical Analyses
Using SPSS 17.0 software (IBM Corporation, Armonk, New York), the demographics, biophysical, and the two sleepiness scores were compared between the sleepy and non-sleepy subjects using a Mann-Whitney U test (Table 1). A significant level was set at p < 0.05. To investigate the differences in the average FA and MD values between the two patient groups in the ROI analysis, a Mann-Whitney U-test was employed by setting p < 0.05 to claim significance. False discovery rate (FDR) correction26 was used to correct the multiple comparisons. In the correlation analysis between the DTI metrics and the clinical ESS or PVT results, body mass index (BMI) and age were included as covariates. The Pearson correlation coefficient R and p-values were evaluated with a statistical significance set at p < 0.05.
Table 1.
Sample Characteristics.
| Variables | Sleepy group (n = 12) | Non-sleepy group (n = 17) | p-value† |
|---|---|---|---|
| Age range (years) | 30–55 | 34–54 | NA |
| Age (years) | 45.6±7.3 | 46.1±8.8 | 0.867 |
| BMI (kg/m2) | 36.8±6.0 | 32.1±5.9 | 0.095 |
| Systolic pressure (mmHg) | 130.4±11.6 | 129.1±16.6 | 0.843 |
| Diastolic pressure (mmHg) | 78.4±5.0 | 74.3±12.8 | 0.399 |
| Handedness (right) | 12 | 17 | NA |
| AHI index | 58.8±43.0 | 43.1±31.7 | 0.305 |
| Nadir SpO2 (%) | 84.9±8.1 | 83.7±11.8 | 0.795 |
| SpO2 Baseline (%) | 91.5±5.8 | 91.2±4.3 | 0.881 |
| Days of CPAP use (>6h/day) | 30 | 33.5 | 0.411 |
| Mean CPAP pressure (cmH2O) | 10.6±1.6 | 9.8±3.0 | 0.504 |
| ESS | 8.9±5.0 | 5.7±4.5 | 0.127 |
| PVT lapses | 17.4±21.7 | 1.9±1.7 | 0.012 |
| Mean Reaction Time | 398.7±165.1 | 271.5±28.3 | 0.007 |
Data are expressed as mean ± standard deviation unless otherwise indicated.
Significant statistical difference is shown in bold (using p < 0.05 as a threshold).
p-values were obtained using a Mann-Whitney U-test between groups.
NA: Not Available.
RESULTS
Demographic And Physiologic Data
As summarized in Table 1, no significant differences in age (p = 0.867), BMI (p = 0.095), SpO2 baseline (p = 0.881), and AHI (p = 0.305) were observed between the sleepy and non-sleepy groups. However, the PVT lapses and the Reaction Time were significantly higher or longer in the sleepy compared to the non-sleepy subjects (p = 0.012 and 0.007, respectively)
Whole-brain DTI Comparisons Among Groups
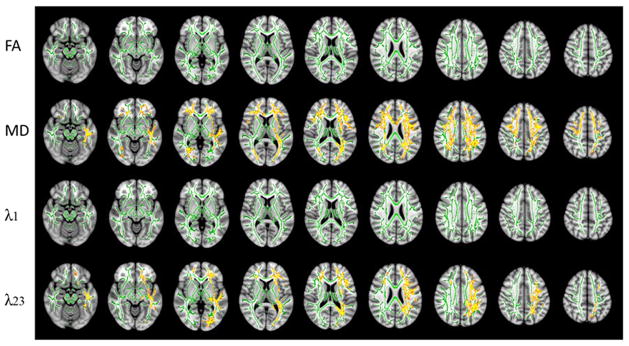
A higher MD (p < 0.05) was observed in the sleepy group than the non-sleepy group in the whole-brain TBSS analysis (Fig. 2). Evaluation of the individual eigenvalues illustrated that the increase in MD was caused primarily by an elevated radial diffusivity λ23, rather than changes in axial diffusivity λ1. It was revealed that 17.8% (24520/137832 voxels) and 10.2% (14100/137832 voxels) of the fiber tracts exhibited increased MD and radial diffusivity, respectively, in the sleepy group compared with the non-sleepy group (Fig. 2) (p < 0.05). In contrast, FA and axial diffusivity did not show significant difference between the two groups.
Fig. 2.
DTI results from an FSL-TBSS analysis showing possible alterations in DTI metrics including FA, MD, λ1 and λ23 between the sleepy and the non-sleepy groups. Green: mean FA skeleton (threshold = 0.2) without significant change. Red-yellow: fibers with increased DTI metrics in the sleepy group when compared to the non-sleepy group (p < 0.05).
Regional Differences In Specific Fiber Tracts Between The Groups
Figure 3 illustrates the FA and MD differences (p < 0.05 with false discovery rate correction) in specific fibers of the brain between the two groups. In the sleepy group, significantly decreased FA was observed (Fig. 3a) in the left retrolenticular part of the internal capsule (RIC.L), left posterior corona radiata (PCR.L), and left sagittal stratum (SS.L). In addition, increased MD was observed in the splenium of corpus callosum (sCC), RIC.L, left anterior corona radiata (ACR.L), left superior corona radiata (SCR.L), SS.L, and left external capsule (EC.L), as shown in Fig. 3b. Overall, the atlas-based analyses on individual fiber tracts suggested that the FA reduction and MD elevation occurred primarily in the corpus callosum, internal/external capsule, corona radiata, and sagittal stratum regions. It is interesting to note that most of the changes were seen in the left hemisphere. To further demonstrate the reasons behind the observed FA or MD alterations, radial and axial diffusivities of four selected fiber tracts are shown in Table 2. While the radial diffusivity showed statistically significant differences between the two groups, axial diffusivity did not, indicating FA and MD alterations in Fig. 3 were caused predominantly by radial diffusivity, which was consistent with the results in Fig. 2.
Fig. 3.
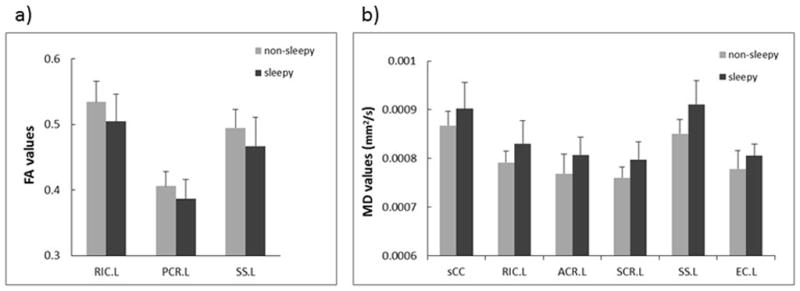
Difference (p < 0.05, FDR corrected) in mean FA (a) and MD (b) in selected WM fiber tracts between the sleepy and non-sleepy groups. MD is expressed in units of mm2/s. “L” denotes left hemisphere. The abbreviations for the individual fiber tracts are defined in the caption of Fig. 1.
Table 2.
Axial and radial diffusivity values in four selected fiber tracts.
| λ1 (axial diffusivity, ×10−3mm2/s) | λ23 (radial diffusivity, ×10−3mm2/s) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sleepy | Non-sleepy | p-value† | Sleepy | Non-sleepy | p-value† | |
| sCC | 1.60±.065 | 1.57±.037 | 0.258 | 0.553±.057 | 0.516±.036 | 0.041 |
| RIC.L | 1.34±.048 | 1.32±.041 | 0.308 | 0.572±.057 | 0.530±.035 | 0.032 |
| ACR.L | 1.14±.055 | 1.12±.029 | 0.278 | 0.630±.041 | 0.594±.047 | 0.037 |
| PCR.L | 1.24±.055 | 1.21±.049 | 0.121 | 0.067±.058 | 0.63±.032 | 0.023 |
Data are expressed as mean ± standard deviation.
p-values were obtained using a Mann-Whitney U-test between the Sleepy and Non-sleepy groups.
sCC - splenium of Corpus Callosum; RIC - Retrolenticular part of internal capsule; ACR - Anterior corona radiata; PCR - Posterior corona radiata. L: left.
The correlations between the FA or MD values of specific fiber tracts and the clinical somnolence assessment scores are shown in Fig. 4. The FA value in the PCR.L (R = −0.520, p = 0.045) and the MD value in the sCC (R = 0.557, p = 0.024) correlated with the ESS scores (Figs. 4a, c). Additionally, the FA value in the RIC.L (R = −0.517, p = 0.048) and the MD value in the SS.L (R = 0.534, p = 0.036) exhibited correlation with the PVT results (Figs. 4b, d).
Fig. 4.
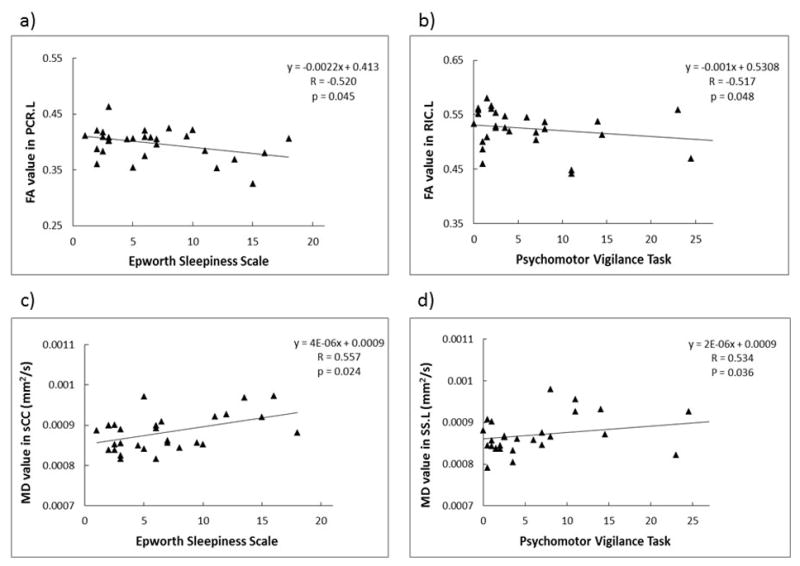
Correlation between the FA (a and b) or MD (c and d) values of the specific fiber tracts and the Epworth Sleepiness Scale (ESS; a and c) or the Psychomotor Vigilance Task (PVT; b and d) score. The linear regression results are indicated in the figure (R: Pearson correlation coefficient with BMI and age as covariates). MD is expressed in units of mm2/s. The abbreviations for the individual fiber tracts are defined in the caption of Fig. 1.
DISCUSSION
In this study, we have observed that the OSA patients with residual sleepiness showed significantly different MD and radial diffusivity in the whole brain WM analysis and altered FA and MD values in specific WM regions when compared to the non-sleepy participants with similar high levels of CPAP use. These differences, which have been largely attributed to an increase in radial diffusivity in patients with residual sleepiness, suggest that FA and MD can serve as sensitive imaging markers for the differing responses to CPAP treatment on OSA patients and provide new insights into the pathogenesis of this disorder. We have also demonstrated quantitative relationships between specific DTI metrics (i.e., FA and MD) and two commonly used scores (i.e., PVT and ESS) for clinical assessment of somnolence. These correlations illustrate the potential of using more objective, imaging-based quantitative markers to complement the conventional approach for assessing somnolence. Unlike a recent longitudinal study by Castronovo et al. 10, which shows that WM brain structures can change in OSA patients before, during, and after CPAP treatment, the present cross-sectional study focuses on a different question – why the OSA patients respond differently even with the same CPAP adherence. Our study is among the first effort to examine both global and regional WM differences between reversed and residual sleepiness in OSA patients after CPAP treatment using DTI with an FSL-based TBSS analysis.
The blood supply of deep WM comes from the perforating arteries, which are tiny branches (diameter ~ 100–400μm) of the pial artery. Because of the lack of anastomosis and effective collateral circulation, these tiny arteries are quite vulnerable to blood flow and pressure changes.27 The reduced cerebral blood flow and intermittent hypoxia in OSA patients, as demonstrated in other studies,28 can result in acute or chronic ischemic changes and in turn WM structural alterations. Using DTI, we observed widespread WM changes, revealed by the increased MD, in the OSA patients with residual sleepiness compared to those without sleepiness on treatment.
Our observation is consistent with the findings in several other studies showing the sensitivity of MD to tissue structural changes, as outlined in a review article.29 The radial diffusivity λ23, which measures water diffusion perpendicular to axons, is found to be the main contributor to the increased MD, indicating possible myelin damage30, 31 in the patients not responsive to CPAP treatment. Oligodendrocytes, the myelin-forming cells of the central nervous system, have very little regenerative capacity. An episode of intermittent hypoxia-ischemia can cause different degree of damage to these particularly vulnerable cells.32 Once the oligodendrocytes are severely damaged by hypoxia-ischemia, the OSA patients are expected to continue showing sleepiness despite persistent CPAP therapy. Thus, it is plausible that the WM structural changes revealed by MD and/or λ23 are a possible mechanism to hinder functional recovery of the OSA patients even after CPAP treatment.
In the whole-brain analysis, the areas with MD (or λ23) change were extensive comprising all three main fiber types: (a) connecting fibers within the ipsilateral hemisphere (frontal WM), (b) commissural fibers between bilateral hemisphere (corpus callosum), and (c) projecting fibers from cerebral cortex to subcortical structures (corona radiata). It is somewhat surprising that FA did not exhibit significant difference between the two patient groups, given the change in λ23 and lack of change in λ1. However, a decrease in FA was observed when the p-value threshold was relaxed to 0.07 (see the supplementary figure), indicating a trend towards significance. Furthermore, changes in FA values of specific fiber tracts were evident in the ROI-based regional analysis. The observed FA change in individual fiber tracts is consistent with the increased radial diffusivity λ23 and little change in axial diffusivity λ1. A greater change in radial diffusivity than axial diffusivity was also reported in another study that compared the OSA patients with healthy controls.20
It is worth noting that the regional FA and MD changes preferentially appeared on the left hemisphere, although the possible mechanism of injury is bilateral. Similar findings were reported by others.20 The reasons underlying the asymmetry are unclear, but are likely related to the relative perfusion difference between the left and right hemispheres. Asymmetrical injury to WM in the watershed areas shows a very high correlation with cerebrovascular insufficiency.33 In addition, it has been suggested that the left hemisphere may be more vulnerable than the right to detrimental influences, including hypoxia.34 In our study sample, all of the 29 participants are right-handed, which may also be a contributing factor to the observed asymmetry.
Clinically, the self-report ESS provides an assessment of daytime sleepiness, while the PVT measures deteriorated alertness, reduced ability to sustain attention, and declined psycho-motor skills that are typically related to sleep-deficit. Higher ESS scores (usually ≥10) and/or more PVT lapses represent greater sleepiness. In our study, the FA values (in PCR.L and RIC.L) negatively correlated with the ESS scores or PVT lapses, while the MD values (in sCC and SS.L) showed a positive correlation. This suggests that the WM damage can be a continuous process in OSA patients and such process may be captured by quantitative DTI metrics. Given the limited sample size, we did not intend to establish a threshold FA or MD value by which reversible sleepiness (i.e., non-sleepiness after CPAP therapy) can be distinguished from residual sleepiness. However, our study paves the way for developing such imaging-based quantitative markers to complement conventional clinical assessment of somnolence.
This study has several limitations. First, disease duration may vary among the OSA participants enrolled in this study, which may introduce confounding variables in our DTI observations. Second, our study design was cross-sectional. An expanded longitudinal study is expected to provide additional valuable information to test our hypothesis. Third, only male participants were included to reduce the heterogeneity of the sample and gender-related differences. The nature and pattern of injury may differ in female patients.35 Lastly, we provided the evidence suggesting that the compromised WM structures can be the cause for residual sleepiness even after CPAP treatment. However, another possibility that the compromised WM structures are a result of the CPAP treatment may also exist. This requires further investigation.
In conclusion, the results from this study supported our hypothesis that global and regional brain structural differences can be a possible mechanism to explain why OSA patients with high levels of CPAP use can have differing responses. Extensive WM alterations, as revealed by DTI metrics, have been observed in CPAP-treated OSA patients exhibiting daytime somnolence despite CPAP adherence. Changes in DTI metrics suggest compromised myelin sheath in patients not responding to CPAP treatment, and some of these metrics correlated with PVT lapses and ESS scores.
Supplementary Material
Fig. A. (supplementary figure) FA changes between the sleepy and non-sleepy groups when the statistical significance threshold was set at p < 0.07 using an FSL-TBSS analysis. Green: mean FA skeleton (threshold = 0.2). Blue: regions with decreased FA in the sleepy group when compared to the non-sleepy group.
Acknowledgments
The authors are grateful to Hagai Ganin for technical assistance and Drs. Qiang Zhang and Frederick C. Damen for helpful discussions.
Grant support: This work was supported in part by grants from TEVA Pharmaceuticals, Inc., (T. Weaver) and the NIH (grant number NIH-1S10RR028898; X. Joe Zhou).
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, Oxidative Stress, and Repair Capacity of the Vascular Endothelium in Obstructive Sleep Apnea. Circulation. 2008;117(17):2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 7.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. The Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 8.Douglas NJ. Systematic review of the efficacy of nasal CPAP. Thorax. 1998;53(5):414–5. doi: 10.1136/thx.53.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest. 1999;116(6):1545–9. doi: 10.1378/chest.116.6.1545. [DOI] [PubMed] [Google Scholar]
- 10.Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37(9):1465–75. doi: 10.5665/sleep.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meurice JC, Paquereau J, Neau JP, et al. Long-term evolution of daytime somnolence in patients with sleep apnea/hypopnea syndrome treated by continuous positive airway pressure. Sleep. 1997;20(12):1162–6. [PubMed] [Google Scholar]
- 12.Kingshott RN, Vennelle M, Hoy CJ, Engleman HM, Deary IJ, Douglas NJ. Predictors of improvements in daytime function outcomes with CPAP therapy. Am J Respir Crit Care Med. 2000;161(3 Pt 1):866–71. doi: 10.1164/ajrccm.161.3.9905053. [DOI] [PubMed] [Google Scholar]
- 13.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canessa N, Castronovo V, Cappa SF, et al. Obstructive Sleep Apnea: Brain Structural Changes and Neurocognitive Function before and after Treatment. Am J Respir Crit Care Med. 2011;183(10):1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 15.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Donoghue FJ, Wellard RM, Rochford PD, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. 2012;35:41–8. doi: 10.5665/sleep.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 19.Macey PM, Kumar R, Woo MA, et al. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–77. [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Pham TT, Macey PM, et al. Abnormal Myelin and Axonal Integrity in Recently Diagnosed Patients with Obstructive Sleep Apnea. Sleep. 2014;37(4):723–32. doi: 10.5665/sleep.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with Tract-Based Spatial Statistics. Nat Protoc. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B. 1995;57:289–300. [Google Scholar]
- 27.Brown WR, Thore CR. Review: Cerebral microvascular pathology in aging and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innes CR, Kelly PT, Hlavac M, Melzer TR, Jones RD. Decreased Regional Cerebral Perfusion in Moderate-Severe Obstructive Sleep Apnoea during Wakefulness. Sleep. 2015;38(5):699–706. doi: 10.5665/sleep.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bihan D. The ‘wet mind’: water and functional neuroimaging. Phys Med Biol. 2007;52(7):R57–90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- 30.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307:106–13. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mifsud G, Zammit C, Muscat R, et al. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther. 2014;20(7):603–12. doi: 10.1111/cns.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minkner K, Lovblad KO, Yilmaz H, et al. White matter lesions in watershed territories studied with MRI and parenchymography: a comparative study. Neuroradiology. 2005;47:425–30. doi: 10.1007/s00234-005-1358-8. [DOI] [PubMed] [Google Scholar]
- 34.Njiokiktjien Ch. Differences in vulnerability between the hemispheres in early childhood and adulthood. Fiziol Cheloveka. 2006;32(1):45–50. [PubMed] [Google Scholar]
- 35.Macey PM, Kumar R, Yan-Go FL, Woo MA, Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep. 2012;35:1603–13. doi: 10.5665/sleep.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. A. (supplementary figure) FA changes between the sleepy and non-sleepy groups when the statistical significance threshold was set at p < 0.07 using an FSL-TBSS analysis. Green: mean FA skeleton (threshold = 0.2). Blue: regions with decreased FA in the sleepy group when compared to the non-sleepy group.