Abstract
Background and Aim
Early transjugular intrahepatic portosystemic shunt (TIPS) used as preventive therapy prior to recurrent bleeding has been recommended in patients presenting with acute esophageal variceal bleeding (EVB) who are at high risk of further bleeding and death. We investigated the impact of early TIPS on outcomes of U.S. patients hospitalized with EVB from 2000 to 2010.
Methods
The Nationwide Inpatient Sample database was queried to identify patients with EVB and decompensated cirrhosis (because early TIPS is recommended only in high-risk patients). The primary outcome was in-hospital death, and secondary outcomes included rebleeding and hepatic encephalopathy. Early preventive TIPS was defined by placement within 3 days of hospitalization for acute EVB after one session of endoscopic therapy. Rescue TIPS was defined as TIPS after two interventions for EVB.
Results
The study included 142,539 patients. From 2000 to 2010, the age-adjusted in-hospital mortality rate decreased 37.2% from 656 per 100,000 to 412 per 100,000 (p <0.01), while early and rescue TIPS increased (0.22% to 0.70%; p<0.01 and 1.1% to 6.1%; p<0.01). On multivariate analysis, as compared to no TIPS, early TIPS was associated with decreased inpatient mortality (RR=0.87; 95% CI, 0.84–0.90) and rebleeding (RR=0.56; 95% CI, 0.45–0.71) without an increase in hepatic encephalopathy (RR=1.01; 95% CI, 0.93–1.11).
Conclusion
Early preventive TIPS in patients with EVB and decompensated cirrhosis was associated with significant in-hospital reductions in rebleeding and mortality without a significant increase in encephalopathy in “real-world” U.S. clinical practice.
Keywords: Esophageal Varices, Upper Gastrointestinal Bleeding, Liver Disease, Cirrhosis, Transjugular Intra-hepatic Portosystemic Shunt (TIPS)
INTRODUCTION
Esophageal variceal bleeding (EVB) is a severe complication of portal hypertension with significant morbidity and mortality. The greatest risk of rebleeding in patients presenting with acute EVB occurs within the first 48–72 hours, and over 50% of rebleeding episodes occur within the first 10 days.1 The current approach for acute EVB combines endoscopic band ligation and pharmacologic treatment as first-line therapy.2–6 However, 10–20% of patients will have persistent or recurrent bleeding necessitating alternative “rescue” treatment with transjugular intrahepatic portosystemic shunt (TIPS).7
Early TIPS (within 72 hours of presentation) as preventive therapy prior to recurrent bleeding has also been studied in patients presenting with acute EVB. Small, randomized trials of early TIPS report benefit in patients at high risk for further bleeding and death, such as those with a hepatic venous pressure gradient >20 mmHg or with Child-Pugh class C cirrhosis (Child-Pugh score 10–13) or Child-Pugh class B cirrhosis with active bleeding.8,9 However, population-based studies of the impact and timing of TIPS on outcomes of patients with EVB are not available. We used a United States (U.S.) nationwide database to determine the impact of early TIPS when utilized for prevention of rebleeding and of rescue TIPS used to treat persistent or recurrent bleeding in hospitalized patients with decompensated cirrhosis and EVB. We hypothesized that early TIPS would be associated with decreased mortality and rebleeding during hospitalization of decompensated cirrhotics with acute EVB.
METHODS
Data Source and Extraction
The Nationwide Inpatient Sample (NIS) database was queried to identify patients with EVB in the U.S. from 2000 to 2010.10 The NIS is part of the Healthcare Cost and Utilization Project, sponsored by the Agency for Healthcare Research and Quality. The NIS is a database of hospital inpatient stays derived from billing data submitted by hospitals to statewide data organizations across the U.S. Inpatient data includes clinical and resource use information typically available from discharge abstracts. Each discharge is coded with a principal diagnosis for that specific hospitalization in addition to the potential for 14 secondary diagnoses and 15 associated procedures. The NIS is the largest U.S. inpatient care database, encompassing hospitals from a total of 46 states, which comprise 97% of the U.S. population.
Inclusion and Exclusion Criteria
Patients were identified by hospitalizations related to EVB through appropriate International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes. Patients were initially included with a primary diagnosis of esophageal varices with bleeding or patients who had a secondary diagnosis of bleeding varices but a primary diagnosis of cirrhosis. We used previously defined criteria for decompensated liver disease by using the Baveno V classification of cirrhosis severity.2
The Baveno five-stage classification system has been devised as follows: 1) compensated cirrhosis with no varices; 2) compensated cirrhosis with varices; 3) upper gastrointestinal bleeding without ascites; 4) ascites (and/or hepatocellular carcinoma, encephalopathy, or jaundice) without bleeding; and 5) ascites and bleeding (with/without hepatocellular carcinoma, hepatic encephalopathy, or jaundice).11,12 Only patients with decompensated liver disease as defined by Baveno V classification (Stages 3 and 5) were included in our analyses.
Clinical Characteristics and Covariates
Demographic information was collected, including gender, age, and race/ethnicity. Additional characteristics extracted included etiology of liver disease as well as disease complications: hepatorenal syndrome, hepatocellular carcinoma, ascites, sepsis, encephalopathy, or development of spontaneous bacterial peritonitis. Procedures examined included esophagogastroduodenoscopy (EGD) and endoscopic therapy for variceal bleeding, balloon tamponade, interventional radiology, surgery, and TIPS occurring during hospital stay. The Elixhauser comorbidity index was calculated based upon the Agency for Healthcare Research and Quality comorbidity measures obtained from the NIS.13 The original index contained 30 comprehensive categories of comorbidity; however the list of specific ICD diagnoses was expanded to 31 categories in 2012.13,14 An Elixhauser comorbidity index of >2 is associated with adverse outcomes and can be used to predict in-hospital mortality.15
Early TIPS was defined by placement within 3 days of hospitalization for acute EVB after one EGD with endoscopic therapy. This definition was chosen to simulate recommended practice in which initial endoscopy to diagnose EVB and provide endoscopic therapy prior to the early TIPS is recommended.6,13 Rescue TIPS, used to treat patients with persistent or recurrent bleeding, was defined by two or more EGD’s with endoscopic therapy or one EGD plus a non-endoscopic intervention for control of variceal bleeding (i.e., balloon tamponade, interventional radiology, or surgery) prior to TIPS. Rebleeding was defined as at least 2 blood transfusions following initial endoscopic or non-endoscopic intervention for control of variceal bleeding or repeat endoscopic or non-endoscopic intervention for control of variceal bleeding. Additional hospitalization data included day of admission (weekday or weekend), route of admission, mean length of stay, hospitalization charges, and primary payor source. Further details are highlighted in Appendix 1.
Measured Outcomes
The primary outcome measure was in-hospital death. Secondary outcomes included rebleeding, hepatic encephalopathy, sepsis, length of hospital stay, and hospitalization cost.
Data Synthesis and Statistical Analysis
Data were analyzed using the Stata 13.0 software package (Stata Corp LP, College Station, TX). Healthcare Cost and Utilization Project NIS data are provided in a 2-stage cluster design incorporating clustering at the hospital level and discharge level. The project provides weighting of discharges based on the hospital type and volume of discharges relative to the sampling region. Quantitative variables were expressed as means ± standard deviation (SD). Two-way chi-square analyses were performed on categorical variables. The age-adjusted mortality rate was calculated for each year of study by summing the product of age-specific mortality rates by the age-specific weights. For trends in overall population, the total number of cases were standardized per 100,000 based upon total population derived from the U.S. census data for specific year (2000 to 2010).16 The weights used in the age adjustment of the data were the proportion of the year 2000 standard U.S. population within each age group.17
Secular trends in mortality rates were observed in linear Poisson regression models. The models were used to observe the effect of the period of diagnosis (independent variable) on the in-hospital mortality rate (dependent variable), while controlling for other independent variables. Risk estimates and 95% confidence intervals (CI’s) were calculated for all independent variables in the final model. Poisson regression with robust (Huber–White) standard errors was also used to determine incident risk ratios (RR) for predictors of in-hospital mortality. Prior to our analysis we tested the Poisson models for over-dispersion using a Pearson goodness-of-fit test. Models were not over dispersed and were appropriate for our analyses.
We also hypothesized that improved care and incorporation of guidelines for variceal bleeding may have improved survival and would be reflected in decreasing mortality risk ratios from year to year. Therefore, we created interaction terms between each diagnosis and year of discharge (EVB 2000, EVB 2001, and so forth). To validate our results in patients with a Child-Pugh Score of <14, we carried out a sensitivity multivariate analysis limited to patients without ascites because the maximum Child-Pugh score for patients without ascites is 13.
RESULTS
Demographic and Clinical Characteristics
A total of 142,539 patients with a discharge diagnosis of EVB were included in our study. Demographic and baseline characteristics are shown in Table 1. A majority of the study population was White (65%) and male (68%). The mean age was 58 (SD 0.6) years. The average Elixhauser comorbidity index of patients was 2.9 (SD 1.7). Early TIPS was performed in 0.5% (n=713) of the population studied. The primary method or route of admission was through the emergency department (n=97,924 or 68.7%) with a mean hospital stay of 5.5 days. The mean hospitalization cost accrued for one visit was $35,453 with the largest proportion of patients covered by Medicare (n=60,722 or 42.6%). Elixhauser comorbities of included patients is shown in Supplemental Table 1.
Table 1.
Demographic and Clinical Characteristics of Hospitalized Patients with Cirrhosis and Acute Esophageal Variceal Bleeding in the U.S. from 2000 to 2010 (n = 142,539).
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 97212 | 68.2 |
| Female | 45327 | 31.8 |
| Age, years | ||
| <40 | 8695 | 6.1 |
| 41–50 | 30931 | 21.7 |
| 51–60 | 46325 | 32.5 |
| 61–70 | 28650 | 20.1 |
| 71–80 | 19243 | 13.5 |
| >80 | 8695 | 6.1 |
| Race/ethnicity | ||
| White | 92650 | 65 |
| Black | 15252 | 10.7 |
| Hispanic | 25942 | 18.2 |
| Other race | 8695 | 6.1 |
| Liver complications | ||
| Hepatorenal syndrome | 4133 | 4.2 |
| Hepatocellular carcinoma | 5416 | 5.6 |
| Ascites | 47180 | 48.6 |
| Sepsis | 7269 | 7.4 |
| Encephalopathy | 29078 | 30.1 |
| Spontaneous bacterial peritonitis |
3991 | 4.1 |
| Comorbidities | ||
| Elixhauser comorbidity index (SD) |
2.9 (1.7) | |
| Procedures | ||
| EGD | 124864 | 87.6 |
| EGD ≤1 day from admission |
117595 | 82.5 |
| Balloon tamponade | 855 | 0.6 |
| Non-TIPS IR procedure | 161 | 0.1 |
| Surgery | 98 | 0.07 |
| TIPS | ||
| Early TIPS | 713 | 0.5 |
| Rescue TIPS | 5844 | 4.1 |
| Hospitalization data | ||
| Admitted on weekend | 30646 | 21.5 |
| Routine admission | 35777 | 25.1 |
| Admitted through emergency room |
97924 | 68.7 |
| Admitted from outside hospital |
6129 | 4.3 |
| Admitted from another facility |
2423 | 1.7 |
| Admitted through court/law | 285 | 0.2 |
| Primary payor | ||
| Private insurance | 32927 | 23.1 |
| Medicaid | 30646 | 21.5 |
| Medicare | 60722 | 42.6 |
| Other payment source | 6129 | 4.3 |
| Self-pay | 10690 | 7.5 |
| No charge | 1283 | 0.9 |
IR – interventional radiology
Trends in Variceal Bleeding
From 2000 to 2010, the number of patients with EVB increased by approximately 2-fold [n=7,839 (2.8 per 100,000) to n=16,391 (5.3 per 100,000)] with a gradual increase each year during this period (Supplemental Table 2).18 During the same period, a decline in overall in-hospital age-adjusted mortality rate was seen among patients with EVB [decreased 37.2% from 656 per 100,000 to 412 per 100,000 (p <0.01); Figure 1]. The age-adjusted mortality rates were consistently higher in males as compared to females during the study period and both sexes demonstrated decreasing mortality rates over the study period. Age-adjusted in-hospital mortality rate for males decreased from 749 per 100,000 to 505 per 100,000 from 2000 to 2010. Similarly, the age-adjusted in-hospital mortality rate for females decreased from 557 per 100,000 in to 313 per 100,000 over the study period (Figure 1).
Figure 1.
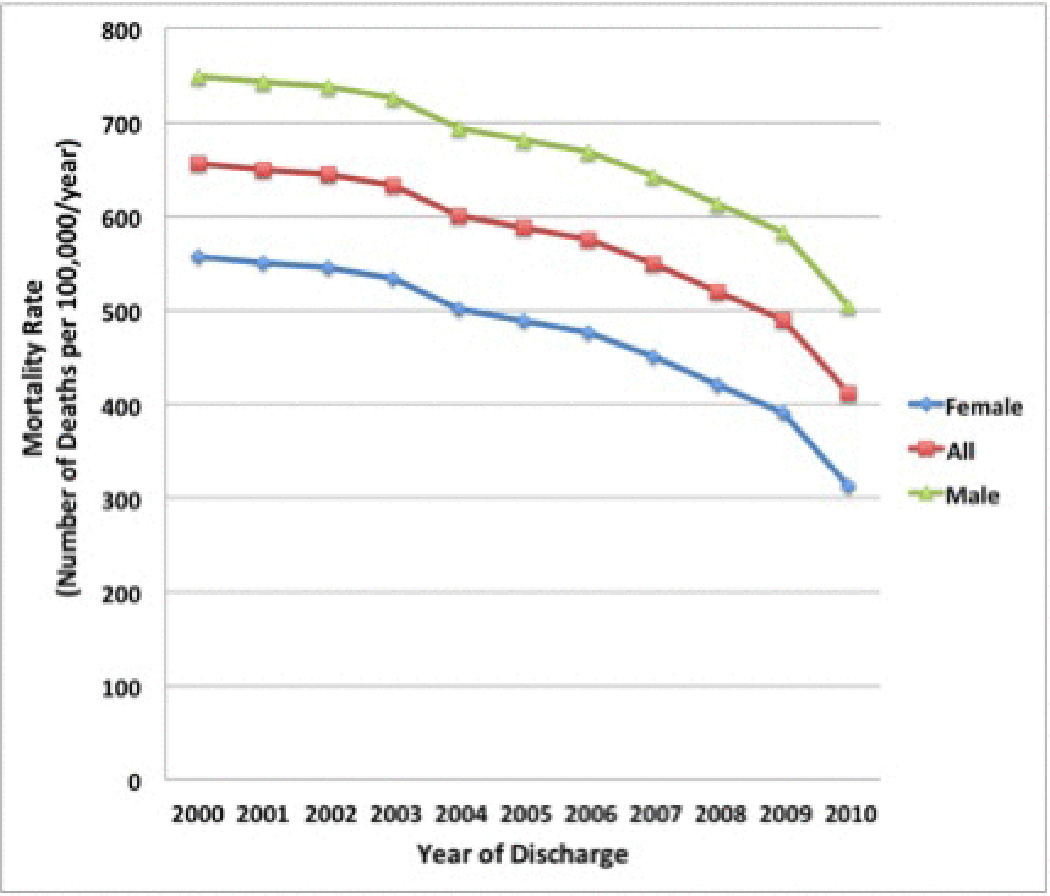
In-hospital age-adjusted mortality rate among patients with cirrhosis and acute esophageal variceal bleeding in the U.S. from 2000 to 2010. From 2000 to 2010, the overall age-adjusted in-hospital mortality rate decreased 37.2% from 656 per 100,000 to 412 per 100,000 (p <0.01).
Use of TIPS
Utilization of TIPS (early and rescue) significantly increased over the study period [(0.22% to 0.70%; p<0.01 and 1.1% to 6.1%; p<0.01, respectively); Figure 2]. Linear univariate poisson regression analysis showed a significant inverse association of TIPS utilization and the mortality rate ratio of EVB from 2000 to 2010 (RR 0.88; 95% CI: 0.83–0.92). Overall, early TIPS had a lower rate of in-hospital death compared to patient with no TIPS and rescue TIPS, [(1.5% versus 5.6%; p <0.01 and 1.5% versus 8.1%; p< 0.01, respectively) Table 2]. Early TIPS also showed a lower rate of rebleeding when compared to no TIPS and rescue TIPS (0.5% versus 15.4%; p <0.01 and 0.5% versus 2.2%; p <0.01, respectively). When comparing in-hospital rate of encephalopathy, early TIPS was similar to no TIPS and rescue TIPS (30.1% versus 27.3%; p=0.19 and 30.1% versus 29.5%; p=0.15, respectively).
Figure 2.
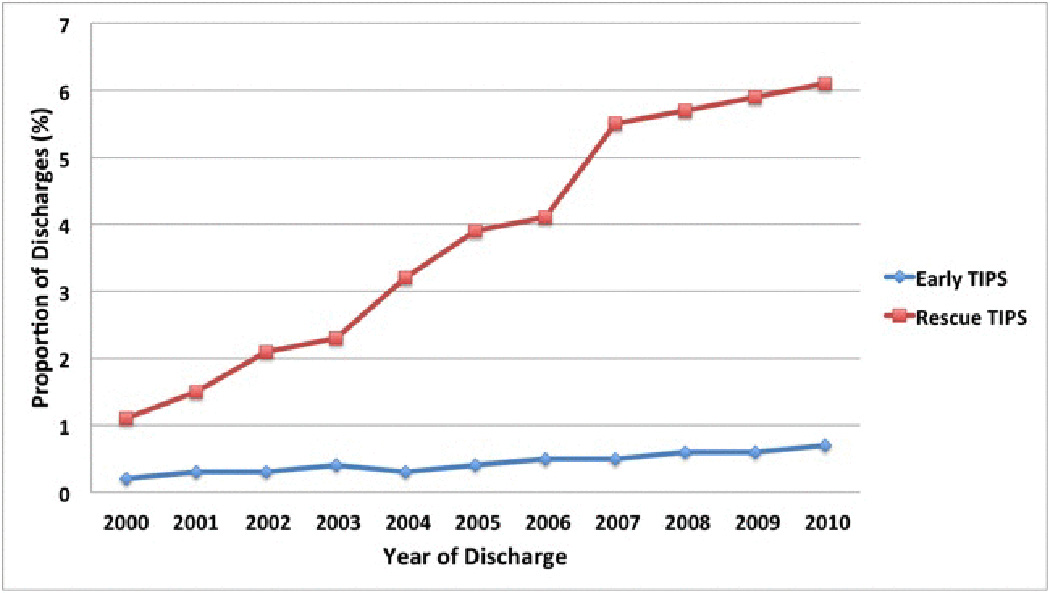
Proportion of discharges who had a TIPS procedure among patients with cirrhosis and acute esophageal variceal bleeding in the U.S. from 2000 to 2010. Utilization of TIPS (early and rescue) significantly increased over the study period (0.22% to 0.70%; p<0.01 and 1.1% to 6.1%; p<0.01, respectively).
Table 2.
Multivariable Regression Analysis for Outcomes with Early TIPS vs. No TIPS or Rescue TIPS in Patients with Cirrhosis and Acute Esophageal Variceal Bleeding (n = 142,539).
| Outcome | P value | RRa (95% CI) |
P value | ||
|---|---|---|---|---|---|
|
Early TIPS versus No TIPS |
Early TIPS | No TIPS | |||
| In-hospital death | 1.5% | 5.6% | <0.01 | 0.87 (0.84, 0.90) |
<0.01 |
| In-hospital rebleeding | 0.5% | 15.4% | <0.01 | 0.56 (0.45, 0.71) |
<0.01 |
| Hepatic encephalopathy | 30.1% | 27.3% | 0.19 | 1.01 (0.93, 1.11) |
0.62 |
| Sepsis | 7.5% | 7.1% | 0.38 | 1.28 (0.75, 2.19) |
0.40 |
| Mean length of stay (days) | 5.1 | 4.7 | 0.05 | 0.6 (−0.3 to 1.0)b |
0.18 |
| Mean hospitalization cost (U.S. $) |
42,789 | 34,154 | <0.01 | 8,226.68 (3,807.33 to 18,137.42)b |
<0.01 |
|
Early TIPS versus Rescue TIPS |
Early TIPS |
Rescue TIPS |
|||
| In-hospital death | 1.5% | 8.1% | <0.01 | 0.85 (0.82, 0.88) |
<0.01 |
| In-hospital rebleeding | 0.5% | 2.2% | <0.01 | 0.57 (0.50, 0.64) |
<0.01 |
| Hepatic encephalopathy | 30.1% | 29.5% | 0.15 | 0.97 (0.95, 1.02) |
0.22 |
| Sepsis | 7.5% | 7.3% | 0.22 | 0.83 (0.66, 1.04) |
0.32 |
| Mean length of stay (days) | 5.1 | 9.6 | <0.01 | −4.6 (−5.1 to −4.3)b |
<0.01 |
| Mean hospitalization cost (U.S. $) |
42,789 | 58,982 | <0.01 | −7,288.37 (− 8,922.45 to − 6,697.62)b |
<0.01 |
Adjusted for age, ethnicity, sex, Elixhauser Comorbidities, and Baveno V stage (i.e. presence or absence ascites)
Adjusted absolute difference (95% CI)
Multivariate Analysis
A multivariate logistic regression analysis was also performed with variables controlled for age, ethnicity, sex, Elixhauser comorbidities, and Baveno V stage (Table 2). On multivariate analysis, as compared to no TIPS and rescue TIPS, early TIPS was associated with decreased inpatient mortality (aRR 0.87; 95% CI, 0.84–0.90 and aRR 0.85; 95% CI, 0.82–0.88, respectively). A significant decrease in inpatient rebleeding rates was also noted in the early TIPS group compared to no TIPS and rescue TIPS (aRR 0.56; 95% CI, 0.45–0.71 and aRR 0.57; 95% CI, 0.50–0.64, respectively). Patients who had early TIPS had a decrease in hospital stay by 4.6 days (95% CI, 4.3–5.1; p<0.01) compared to rescue TIPS. Early TIPS was also associated with a decrease in hospitalization costs by $7,288.37 (95% CI, $6,697.62–$8,922.45) compared to rescue TIPS. Although there was no significant difference in length of hospital stay for early TIPS compared to no TIPS (adjusted difference of 0.6 days; 95% CI, −0.3 to 1.0; p=0.18), patients who had early TIPS accrued a higher hospitalization cost than those with no TIPS (absolute difference of $8,226.68, 95% CI $3,807.33 to $18,137.42).
Early TIPS was not associated with higher rates of hepatic encephalopathy or sepsis compared to no TIPS or rescue TIPS (Table 2). A sensitivity analysis limited to patients without ascites revealed similar results (Supplemental Table 3): compared to no TIPS and rescue TIPS, early TIPS was associated with decreased inpatient mortality (aRR 0.80; 95% CI, 0.82–0.92 and aRR 0.81; 95% CI, 0.79–0.89, respectively). A significant decrease in inpatient rebleeding rates was also noted in our sensitivity analysis in the early TIPS group compared to no TIPS and rescue TIPS (aRR 0.49; 95% CI, 0.44–0.77 and aRR 0.52; 95% CI, 0.49–0.69, respectively).
DISCUSSION
This U.S. nationwide database study showed that early TIPS was associated with decreased rebleeding, inpatient mortality, and length of hospital stay amongst inpatients with decompensated cirrhosis and acute EVB. Sensitivity analysis with early TIPS performed for EVB in decompensated cirrhotics without ascites (which guarantees all patients had Child-Pugh Score <14) also showed similar results. A significant decrease in inpatient mortality among decompensated cirrhotics with EVB in the U.S. was also seen from 2000 to 2010, with a concurrent increase in the utilization of TIPS.
Previous small, randomized trials have supported the use of early TIPS as a treatment for prevention of rebleeding and death in selected patients with acute EVB. Cello et al showed no significant difference in outcomes during initial hospitalization, and EVB was significantly less frequent in the early TIPS group (mean follow-up 570 days).19 Other outcomes measured were not significantly different. Monescillo and colleagues demonstrated significantly improved inpatient mortality and control of acute bleeding with early TIPS in a randomized trial of 52 patients with hepatic venous pressure gradients ≥ 20mm Hg; however, TIPS was again not compared to current standard therapy, ligation, but rather to sclerotherapy.8 More recently, García-Pagán et al compared early TIPS to combined medical therapy and endoscopic ligation and showed significantly lower rates of uncontrolled bleeding/rebleeding and mortality at 6 weeks and 1 year [1-year actuarial survival of 61% in the pharmacotherapy-ligation group versus 86% in the early TIPS group (p<0.001)].9 A subsequent retrospective observational study of 75 patients with the same criteria in the same centers after completion of the randomized trial reported a benefit of early TIPS in control of bleeding or rebleeding at 2 years (but not 6 weeks) and a trend to a mortality benefit over 2 years.20
Thus, randomized trials indicate a benefit of early TIPS in prevention of long-term further bleeding. Results, however, are mixed regarding the short-term benefit of early TIPS, with one study reporting benefit during the initial hospitalization13, and two others not documenting this.14,23 Nevertheless, these studies were small and only one compared early TIPS to the current standard of care. Furthermore, these studies were generally performed at centers with expertise in the management of cirrhotic patients with variceal bleeding. To our knowledge, no population-based studies have assessed the impact of early TIPS on EVB outcomes in clinical practice.
We found a steady rise in the annual number of patients with cirrhosis and acute EVB in the U.S. from 2000 to 2010. This occurred despite evidence-based recommendations to employ medical therapy and/or ligation for primary and secondary prevention of variceal bleeding.3,18 This increase is likely secondary to the maturation of the hepatitis C epidemic and the rise in non-alcoholic fatty liver disease (NAFLD). Previous data extracted from the NIS database has shown a growing number of patients with cirrhosis from 2002 to 2010 (discharge diagnosis n=71,899 to n=99,252, respectively).21 Despite the increase in EVB over the 10-year study period, we did find a nearly 40% decrease in hospital mortality in patients with EVB. This presumably is due at least in part to improved management of EVB, including use of TIPS, but also may relate to improvements in overall management of cirrhosis.
A previous study by Schmidt and colleagues demonstrated a steady decline in inpatient mortality among hospitalized cirrhotic patients in the U.S. from 9.1% in 2002 to 5.4% in 2010.21 In addition to the increase in use of early TIPS, a much larger absolute increase in the use of rescue TIPS was identified from 2000 to 2010. This group represents a high-risk population with further bleeding in the hospital and, as expected, has a higher mortality than those with no TIPS or early TIPS. However, the marked increase in rescue TIPS from 2000 to 2010 also may account for at least a portion of the improvement in mortality, as TIPS replaced no therapy or therapies that are less effective and/or more morbid for treatment of further bleeding after hospital admission (i.e., balloon tamponade, surgery).
Our study had several limitations. Laboratory data to compute the Child-Pugh score or MELD score in our cohort of patients cannot be obtained from the NIS database. The Child-Pugh Score and MELD score have been shown to predict outcomes in patients with EVB.17,22,23 However, we were able to use the Baveno classification for cirrhosis severity to select decompensated patients and based on the absence of ascites we were also able to identify a subgroup of decompensated patients who had a Child-Pugh Score of <14. This notion that patients at high risk of rebleeding should receive consideration for early TIPS was again demonstrated in retrospective studies and more recent guidelines including the updated Baveno VI report.9,20,24,25 However, while early TIPS with polytetrafluoroethylene (PTFE) covered stents have demonstrated impressive results (improved survival 86% with early TIPS vs 61% with band ligation and pharmacotherapy, at 1 year)9, the NIS database does not allow for the ability to differentiate between different stent types. While PTFE stents are more common in traditional U.S. practice today, an inability to differentiate between different stent types remains another limitation to our study.
Furthermore, the diagnosis of EVB and identification of comorbidities are dependent on the accuracy of coding procedures. While large datasets based upon insurance reimbursement may be affected due to the several assumptions in the analysis, a recent study demonstrated administrative data can identify patients with cirrhosis with high accuracy.26 Additionally, previous studies have defined a combination of codes for EVB to improve the sensitivity, and we adapted the codes from these studies.21,27 Although, we found no significant increase in hepatic encephalopathy in patients with early TIPS compared to no TIPS or rescue TIPS, this does not confirm that there was no difference in incident hepatic encephalopathy with use of early TIPS because the NIS database does not differentiate between outcomes that develop during an inpatient stay versus those present prior to hospitalization. Nevertheless, recent randomized trials in high-risk patients also failed to show an increase in hepatic encephalopathy with early TIPS as compared to endoscopic and medical therapy13,14, possibly because a reduction in rebleeding also reduced subsequent hepatic decompensation. Moreover, additional management questions are unable to be answered by our analysis including changes in pharmacotherapy standard of care over time (i.e. type and duration of antibiotics, choice of vasoactive agent, and the transition from sclerotherapy to band ligation).
In addition, our definition of early TIPS, requiring TIPS after initial endoscopy with endoscopic therapy, was chosen to simulate the recommended practice of performing initial diagnostic and therapeutic endoscopy prior to early TIPS. However, because NIS is not a medical records database, we cannot rule out that some early TIPS cases were mis-categorized because the hospitalization for EVB represented a second or third episode of rebleeding after prior hospitalizations or because patients had clinical evidence of persistent bleeding after their initial endoscopy, which led to rescue TIPS rather than early preventive TIPS. Given the nature of the dataset, we cannot rule out that patients were admitted for their first variceal bleed as compared to a recurrent bleed. Also, because TIPS is not readily performed at all hospitals, the results of this study may not be generalizable.
Despite these limitations, our study has several strengths and clinical implications. All of the patients included in this database were admitted with EVB and had decompensated cirrhosis. The sampling of EVB patients spanning the U.S. population over a decade is also an advantage over smaller, single-centered studies. Furthermore, the use of data from a nationwide population-based sample minimizes the possible biases that may be seen in single-center studies and provides strong data for generalizing the observations from this study to clinical practice in the U.S.
In conclusion, this population-based study from the U.S. demonstrates the significant impact of early TIPS on EVB outcomes in patients with decompensated cirrhosis. The early use of TIPS as a preventive treatment was associated with significant short-term reductions in rebleeding and mortality. The small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and EVB. Early use of TIPS, together with patient and physician education on current guidelines and treatment protocols, should continue to be a priority to improve patient outcomes.
Supplementary Material
Acknowledgments
Financial Support: Supported by NIH T32 DK007017 (BN)
Footnotes
Author Contributions – Study concept and design: BN, TRM, LL; acquisition and analysis of data: BN; Interpretation of data: BN, TRM, LL; Initial draft: BN; Critical revision of manuscript: TRM, LL. All authors approved the final draft submitted.
Potential competing interests: None
REFERENCES
- 1.de Franchis R, Primignani M. Why do varices bleed? Gastroenterol Clin North Am. 1992;21(1):85. [PubMed] [Google Scholar]
- 2.de Franchis R, Baveno VF. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of hepatology. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD Practice Guidelines Committee of American Association for Study of Liver D, Practice Parameters Committee of American College of G. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. The American journal of gastroenterology. 2007;102:2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 4.Hwang JH, Shergill AK, Acosta RD, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointestinal endoscopy. 2014;80:221–227. doi: 10.1016/j.gie.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Runyon BA, Committee APG. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 6.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatology international. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Pagan JC, Reverter E, Abraldes JG, Bosch J. Acute variceal bleeding. Seminars in respiratory and critical care medicine. 2012;33:46–54. doi: 10.1055/s-0032-1301734. [DOI] [PubMed] [Google Scholar]
- 8.Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793–801. doi: 10.1002/hep.20386. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. The New England journal of medicine. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 10.HCUP Nationwide Inpatient Survery. [Accessed 30, July 2015];2002–2010 www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 11.Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 12.D'Amico G. Stages Classification of Cirrhosis: Where Do We Stand? In: de Franchis R, editor. Portal Hypertension V: Proceedings of the Fifth Baveno International Consensus Workshop. Fifth. Oxford, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Garland A, Fransoo R, Olafson K, Ramsey C, Yogendran M, Chateau D, McGowan K. The Epidemiology and Outcomes of Critical Illness in Manitoba. Winnipeg, MB: Manitoba Centre for Health Policy; 2012. [Google Scholar]
- 15.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Medical care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 16.United States Census Bureau; [Accessed 14, October 2015]. Population Estimates. https://www.census.gov/popest/data/intercensal/national/nat2010.html. [Google Scholar]
- 17.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 1998;47:1–16. 20. [PubMed] [Google Scholar]
- 18.Jamal MM, Samarasena JB, Hashemzadeh M. Decreasing in-hospital mortality for oesophageal variceal hemorrhage in the USA. European journal of gastroenterology & hepatology. 2008;20:947–955. doi: 10.1097/MEG.0b013e32830280c7. [DOI] [PubMed] [Google Scholar]
- 19.Cello JP, Ring EJ, Olcott EW, et al. Endoscopic sclerotherapy compared with percutaneous transjugular intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal hemorrhage. A randomized, controlled trial. Annals of internal medicine. 1997;126:858–865. doi: 10.7326/0003-4819-126-11-199706010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Pagan JC, Di Pascoli M, Caca K, et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. Journal of hepatology. 2013;58:45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt ML, Barritt AS, Orman ES, Hayashi PH. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology. 2015;148:967–977. e2. doi: 10.1053/j.gastro.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asrani SK, Kamath PS. Prediction of early mortality after variceal bleeding: score one more for MELD. Gastroenterology. 2014;146:337–339. doi: 10.1053/j.gastro.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Reverter E, Tandon P, Augustin S, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412–419. e3. doi: 10.1053/j.gastro.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 24.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. Journal of hepatology. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680–1704. doi: 10.1136/gutjnl-2015-309262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ananthakrishnan AN, McGinley EL, Saeian K. Higher hospital volume predicts endoscopy but not the in-hospital mortality rate in patients with acute variceal hemorrhage. Gastrointestinal endoscopy. 2009;69:221–229. doi: 10.1016/j.gie.2008.04.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


