Abstract
Background
Patients with a new diagnosis of acute myeloid leukemia (AML) are at risk of experiencing a high symptom burden due to the disease and its treatment, which includes a long period of hospitalization.
Objective
To describe in this pilot work the multi-dimensional symptoms and health-related quality of life (HRQoL) experienced by patients with a new diagnosis of AML across induction chemotherapy.
Methods
A prospective, longitudinal descriptive study design was implemented to evaluate symptoms and health-related quality of life at the time of enrollment through six-weeks post-diagnosis and identify who might be most at risk of experiencing high symptom burden.
Results
A total of 19 participants were included in this analysis. Moderate to severe levels of distress were present in 25–50% of participants depending on timing in treatment. Females and those with a previous history of a psychological disorder reported higher symptom burden during treatment
Conclusions
Our findings indicate that adults with AML experience multiple distressing symptoms during the induction treatment.
Implications for Practice
Timely routine multidimensional assessment of symptoms in individuals undergoing induction chemotherapy treatment for AML is critical as they may be experiencing multiple concurrent symptoms. Additional research to advance symptom assessment and amelioration of distressing symptoms to improve HRQoL is needed in this unique population.
Introduction
Historically, the diagnosis of acute myeloid leukemia (AML) was associated with a poor prognosis, especially in older adults. However, improvements in the treatment and management of the disease have led to trends in increased overall survival in certain age groups.1 Today, approximately 60–80% of patients can expect an initial complete remission after treatment, with a higher probability of success occurring in those less than 60 years of age.2,3 However, AML is still considered a devastating disease given the high rates of leukemia relapse which occur within three years.4 Outcomes for patients who have relapsed are often more bleak.4,5 The relative five-year survival rates from 2004–2010 for adults with AML was 24.9%.6
The diagnosis of AML for patients and their families is inevitably a time of fear and uncertainty. Added to the emotional toll of this diagnosis is the need for aggressive treatment that requires lengthy hospitalizations for treatment-related complications such as life-threatening infections and bleeding.4 Induction chemotherapy for AML is often a stressful period when the patient does not yet know if there will be clinical benefit from the intensive treatment they have just undergone.7,8 These individuals may be at increased risk of experiencing a high symptom burden, decreased health-related quality of life (HRQoL), and psychological sequela such as cancer-related distress.
There is limited research focused on the symptom experience and HRQoL in patients receiving treatment for AML, which makes it difficult for clinicians to identify patients at high risk of adverse health outcomes. Recently a conceptual model was posited that illustrates the potential effect high levels of distress may have on symptoms and HRQoL in individuals undergoing treatment for acute leukemia.9 In this model, several antecedent factors including the dynamic characteristics of an individual are suggested to influence the degree of distress that one may experience during hospitalization and treatment.10,11 The degree of distress in turn impacts the HRQoL and other health outcomes for the individual.10–12 The ideas proposed in this conceptual model and those proposed by Lenz in the Theory of Unpleasant Symptoms13 which asserts symptoms are multidimensional, occur concurrently and ultimately negatively affect domains of HRQoL, provided a framework for the current study. The purpose of this study was to describe the symptoms experienced and begin to identify clinical characteristics that may predict who is most at risk of experiencing higher symptom burden and lower HRQoL during induction chemotherapy for AML.
Methods
Design and Sample
This pilot study reports the findings from data collected as part of a prospective longitudinal descriptive study that examined the symptom and HRQoL of adults with a new diagnosis of AML. This study was approved by the institutional review board (IRB) at a large university medical center. The inclusion criteria for this study included, adult patients (18 years of age or older) newly diagnosed with AML who were scheduled for induction chemotherapy treatment, and who were able to read and speak English were eligible to participate in the study. All study participants provided informed consent within 7 days of their diagnosis. Exclusion criterion was anyone who was experiencing a psychiatric or neurological disorders (assessed through clinical team members) that would prevent obtaining informed consent.
Procedures
Self-reported responses to the Memorial Symptom Assessment Scale (MSAS), Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu), Functional Assessment of Chronic Illness Therapy-Spiritual (FACT-Sp), Visual Analog Scale of depression of anxiety and the Distress Thermometer (DT) gathered every 14-days (+/− 2 days) for 6-weeks. Participants answered each of the questionnaires using pencil and paper. The average time it took for participants to complete all questionnaires was 20 minutes. Trained research assistants entered the participant responses directly into SPSS. All data entered were quality checked by a second research assistant.
Measures
Symptom experience was measured using the MSAS. The MSAS is a 32-item patient-rated instrument used to provide a multidimensional perspective on symptoms experienced by adults diagnosed with cancer.14 A 4-point Likert scale is used to measure the frequency and severity of each symptom; and a 5-point Likert scale is used to measure the degree of distress for each symptom. The dimensions (frequency, severity and distress) of symptoms are then scored as a composite of the average score reported for each symptom dimension. Psychometric testing of the MSAS has found the Cronbach’s alpha coefficient to be .835, .882, and .851 for all three of the subscales: psychological, physical, and distress, respectively.14 These findings support its use as reliable and valid when reporting symptom characteristics in people with cancer.14,15 In this study the Cronbach’s alpha coefficient for the subscales was .89 (psychological), .78 (physical) and .87 (distress).
HRQoL was measured using the FACT-Leu which is a 27-item scale designed to measure dimensions quality of life specific to leukemia including 17 physical symptoms and 10 emotional/social concerns.16,17 The FACT-Leu builds from the FACT-General, a well tested instrument for the measurement of HRQoL in individuals with a variety of chronic illnesses including cancer.18 The Cronbach’s alpha coefficient was found to range from .86 (time 1), .88 (time 2), and .87 time 3),17 supporting the validity and reliability of the FACT-Leu.17,18 The Cronbach’s alpha coefficient for this study was found to be .90 for FACT-Leu.
The spiritual domain of HRQoL was assessed using the FACIT-Sp. The FACIT-Sp is a 12-item subscale to the larger FACT core instrument, developed with the input of patients with cancer, therapists, and chaplains to assess the aspects of spirituality and faith that contributed to quality of life.19 The Cronbach’s alpha for the scale and subscales were found to be between 0.81 – 0.88 supporting the use of this measure to assess the spiritual domain of HRQoL.19,20
Anxiety and Depression Visual Analog Scale (VAS) are single-item measures of the minimal or maximal degree of the dimension of the subjective psychosocial measure of interest.21 The patient is asked: “What is your current level of distress/anxiety/depression?” and then is instructed to place a mark at the point on the line (0–10 centimeters) that best represents his or her current level of the dimension under investigation. These instruments were selected to reduce the burden for study participants. Convergent and divergent validity showed anxiety correlated with the SF-36 Emotional Role (r=−0.54, p<0.01); Social Function (r=−0.48, p<0.01) and Mental Health Subscales (r=−0.68, p<0.01).22 The Cronbach’s alpha for depression previously reported was 0.89.23 The psychometric testing of the VAS for a variety of symptoms including psychosocial measures found these instruments to be reliable and valid.21
Cancer-related distress was measured using the Distress Thermometer (DT). The DT is a screening tool developed for ease of use by health professionals in the clinical setting.24,25 The DT assesses the presence of distress over the past week on a 0–10 numeric rating scale. Validity testing of the DT with the Hospital Anxiety and Depression Scale (HADS) found significant (p<0.01) strong correlations between DT and HADS Anxiety (r=0.68), DT and HADS Depression (r=0.54), as well as DT and HADS total score (r=0.70).26 Similar correlation findings have been reported in other published studies.25 Psychometric testing comparing the DT to the Brief Symptom Inventory found the instrument to have good internal consistency reliability (Alpha=0.81).27 Overall the psychometric tests support the validity and reliability of the DT for rapid assessment and identification of cancer-related distress.
Sample Adequacy
The overarching goal of this study was to obtain estimates of the means and variances of all study measures to facilitate a power analysis for future clinical trials. van Belle proposed that a minimum of 12 observations should be used to calculate confidence intervals based on the t-statistic with n – 1 degrees of freedom.28 This rule is based on the fact that the half-width confidence interval for the mean decreases rapidly up to n = 12, at which point the decrease is less dramatic and the half-width curve begins to asymptotically decrease. Thus, the sample size of 19 was adequate for estimation.
Statistical Analysis
First, descriptive analyses were conducted to characterize the sample demographics, clinical and symptom data. Second, using the model building approach proposed by Hosmer and Lemeshow,29 a mixed effects linear model30 was fit to determine best subset of predictors of VAS (depression, anxiety & distress), MSAS (MSAS-GDI & TMSAS) and FACT (LeuTotal, GTotal & LeuTOI). In the first stage of the model building process, a base model was selected that represented the design of the data collection: fixed effects included week (1, 2, 4 & 6), and a random effect for subject. In the second stage, each potential predictor was fit individually with the base model and, if the p-value was .25 or less, that predictor was used in the next stage. Potential predictors included age (years), gender (female or male), education level (≥college, < college), ICU stay (yes, no), palliative or behavioral health consult (yes, no), history of psychological treatment (present, absent) and length of hospital stay (days). In the third stage, all potential regressors (p < .25) were put in a multiple variable model. This initial model was further refined by sequentially removing variables from the model with the highest p-values (backward stepwise) until all remaining factors had a p-value of .10 or less. At this stage, all pairwise interactions were added. Again using a backward stepwise approach, all interaction terms with p-values greater than .10 were removed. Due to the small sample size, an alpha of .10 was chosen to reduce the Type II error. This model was considered the final prediction model. SAS v 9.2 and JMP v 11.1 was used for the analyses.
Results
Patient characteristics
The study cohort consisted of 19 participants newly diagnosed with AML. Refusal rate was 23%. The median age was 57.0 years (28–79 years). Participant demographics and baseline characteristics are presented in Table 1. Four participants (21%) had an unfavorable-risk karyotype, 13 (68%) had an intermediate-risk karyotype, and 4 (21%) had a favorable-risk karyotype.
Table 1.
Demographics
| Mean | Std Dev | |
|---|---|---|
| Age (years) | 55.47 | 12.54 |
| Length of Stay (days) | 58.58 | 17.73 |
|
| ||
| N | % | |
|
| ||
| Gender | ||
| male | 9 | 47% |
| female | 10 | 53% |
| Race | ||
| white | 16 | 84% |
| black | 3 | 16% |
| Marital Status | ||
| married | 16 | 84% |
| not married | 3 | 16% |
| Education Level | ||
| < college | 12 | 63% |
| >= college | 7 | 37% |
| Employment Status | ||
| employed | 6 | 32% |
| medical leave or disabled | 6 | 32% |
| unemployed or retired | 7 | 37% |
| Cytogenetic Risk | ||
| favorable | 2 | 11% |
| intermediate | 13 | 68% |
| poor | 4 | 21% |
| Psych History | ||
| yes | 3 | 16% |
| no | 16 | 84% |
| ICU Stay | ||
| yes | 7 | 37% |
| no | 12 | 63% |
| Palliative Care Consult | ||
| yes | 5 | 26% |
| no | 14 | 74% |
| Behavioral/Psych Consult | ||
| yes | 7 | 37% |
| no | 12 | 63% |
The majority of participants were white (84%) and female (53%). Most participants were married (84%) and had graduated from high school but had not entered college (63%). Three participants reported past psychiatric history. The average length of stay in the hospital for induction therapy was 58 days (34–96 days). During their hospital stay, some of the participants were transferred to the ICU (37%) and most did not receive a palliative care (74%) or behavioral/psychiatric consultation (63%).
Symptom and health-related quality of life experience
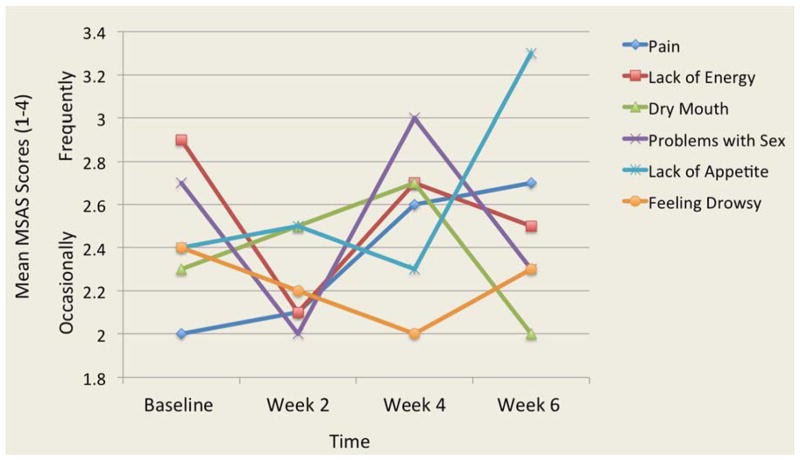
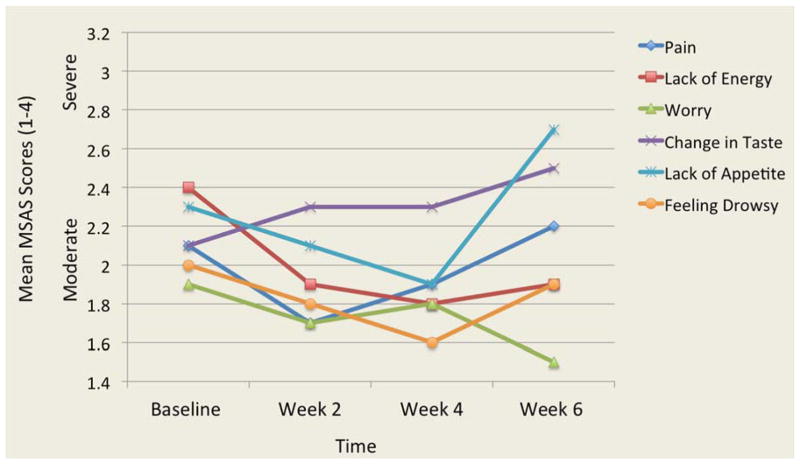
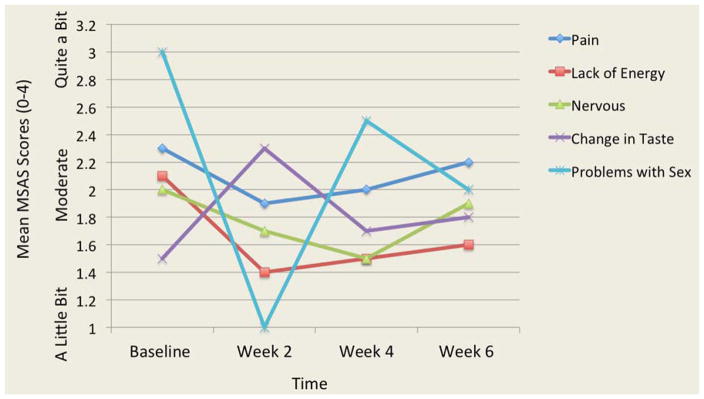
The analysis of 32 symptoms from the MSAS showed that pain and lack of energy were two symptoms that were the most frequent, severe and distressing. Two other symptoms were both frequent and severe: lack of appetite and feeling drowsy. Three participants reported frequent and distressing problems with sex. Overall, the frequency, severity and distress of symptoms varied over time. Figures 1, 2, and 3 illustrate the most frequent, severe and distressing symptom trajectories across time reported on the MSAS, respectively.
Figure 1.
Mean Scores of the Most Frequent Symptoms Reported Over Time
MSAS: Memorial Symptom Assessment Scale
Figure 2.
Mean Scores of the Most Severe Symptoms Over Time
MSAS: Memorial Symptom Assessment Scale
Figure 3.
Mean Scores of the Most Distressing Symptoms Over Time
MSAS: Memorial Symptom Assessment Scale
The final model for MSAS Global Distress Index (MSAS-GDI) included effects for week and psychological consult. The MSAS-GDI scores were fairly constant across the weeks (p = .33); however, those participants with a psychological consult had higher scores (1.8 ± .16 vs. 1.4 ± .17, p = .08) as compared to those that did not receive a consult. Similarly, the final model for Total Symptom Score (TMSAS) included effects from week and gender. The TMSAS scores were constant across the weeks (p = .81); however, females had significantly higher scores (1.9 ± .12 vs. 1.54 ± .12, p = .02) versus males (Table 2).
Table 2.
Mean Symptom and Quality of Life Subscales Scores
| Subscale Totals; mean (SD) | Baseline | Week 2 | Week 4 | Week 6 | p value |
|---|---|---|---|---|---|
| Total MSAS | 1.74 (0.37) | 1.78 (0.47) | 1.62 (0.61) | 1.75 (0.61) | .81 |
| MSAS GDI | 1.76 (0.54) | 1.48 (0.64) | 1.47 (0.87) | 1.67 (0.72) | .33 |
| FACT-G Overall HRQoL | 70.66 (12.66) | 70.66 (12.66) | 71.90 (17.74) | 75.62 (17.20) | .09 |
| FACTLeu-Overall HRQoL | 112.39 (22.11) | 112.39 (22.11) | 117.87 (29.39) | 119.70 (24.74) | .58 |
Abbreviations: FACT-G, Functional Assessment of Cancer General; FACT-Leu, Functional Assessment of Cancer Leukemia; HRQoL, Health related quality of life; MSAS, Memorial Symptom Assessment Scale; MSAS GDI, Memorial Symptom Assessment Scale Global Distress Scale.
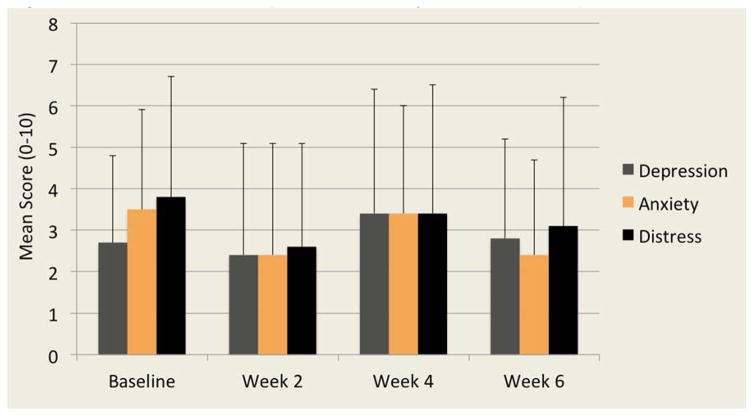
Observed psychological symptom scores for depression, anxiety and distress showed higher mean scores observed in week one and four across the study sample (Figure 4). At week one, 42% and 48% of participants reported moderate to severe distress and anxiety respectively. This increased to 50% of participants reporting moderate to severe depression, anxiety and distress at week four. The presence of moderate to severe psychological symptoms decreased slightly at week six with 31–38% reporting high levels of depression, anxiety and distress.
Figure 4.
Mean Scores of Depression, Anxiety and Distress Reported Over Time Characteristics and Predictors of Symptoms and Quality of Life in AML
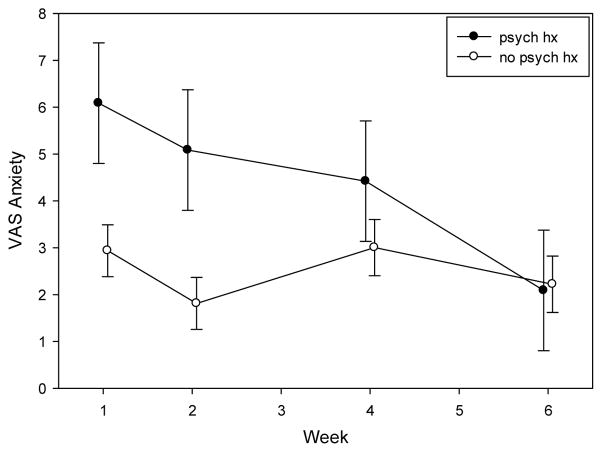
The final model for anxiety included effects for palliative or behavioral health consult, psychological history and week by psychological history interaction. Those participants that received a consult for either palliative or behavioral health were found to report more anxiety (4.2 ± .62 vs. 2.7 ± .70, p = .07) when compared to those that did not receive a palliative or behavioral health consult. Study participants who had reported a past psychological history were found to have higher anxiety at week 1 (p = .03) and week 2 (p = .02). However anxiety levels in this group with a past psychological diagnosis decreased in weeks 4 (p = .32) and 6 (p = .92) to levels similar to those observed in study participants without a psychological history (Figure 5).
Figure 5.
Least Square Means of Anxiety levels by psychologic history across weeks
The final model for VAS depression included effects for week and psychological consult. The depression scores were fairly constant across the weeks (p = .61). However, those participants who received a psychological consult reported higher depression scores (3.5 ± .60 vs. 1.9 ± .64, p = .08) when compared to those participants that did not receive a psychological consult.
The final model for VAS distress included effects for week, education level, and psychological consults. Reported distress scores by participants remained fairly constant across the study weeks (p = .31). However, those participants who received psychological consult during the study reported higher distress scores (4.2 ± .69 vs. 2.4 ± .68, p = .08) when compared to those that did not receive a psychological consult. The participant’s level of education was also a predictor of distress scores, with those who had a college degree reporting higher levels of distress (4.2 ± .77 vs. 2.4 ± .61, p = .08) when compared to those who did not have a college degree.
Mean scores reported by participants for the FACT-Leukemia (FACT-LEU) were found to be lowest at baseline (mean = 112; SD ± 22) and highest at week 2 and 6 (mean = 119 ± 24). Similarly, mean FACT-Global Score (FACT-G) were lowest at baseline (mean= 70 ± 13) and highest at week 2 and 6 (mean = 76 ± 14 and 76 ± 17 respectively).
The final model for FACT-LEU included effects for week and psychological consult. The FACT-LEU scores were fairly constant across the study weeks (p = .57). However, those participants’s who had received a psychological consult reported lower FACT-LEU scores (108.4 ± 5.3 vs. 127 ± 5.6, p = .02) when compared to those that did not receive a psychological consult. The final model for the FACT-G included effects for week and psychological consult. The FACT-G scores were marginally higher at weeks two and six of the study (p = .08) and those participants who had received a psychological consult reported lower FACT-G scores (66.1 ± 3.4 vs. 82.4 ± 3.5, p = .003) when compared to those participants that did not receive a psychological consult.
Finally, age, ICU stay and length of hospital stay were not predictors in any of the 7 models fit. However, the fact that these variables were not included in any of the models may be due to the small sample size, even though an alpha of .10 was used to reduce the probability of a Type ll error.
Discussion
The aim of this prospective pilot study was to describe the symptoms experienced as well as to identify clinical characteristics that may predict the adults with AML undergoing induction treatment who may be most at risk of experiencing higher symptom burden and lower. While this study enrolled slightly more females than males, the age distribution is reflective of epidemiological models of the disease distribution. Overall, this pilot study demonstrates that patients undergoing induction chemotherapy treatment for the AML experience negative symptom sequela during treatment.
The mean MSAS scores found in this study were higher than other studies examining hematologic cancers31 as well as in solid tumors.32 The most frequently identified and bothersome symptoms found in this study (pain and lack of energy) have also been identified in other studies.33 Another symptom identified by several participants to be both frequent and distressing by a small sample of participants was sexual dysfunction. The presence of sexual dysfunction has been explored previously and was found, as in this study, to be an issue for a small group of individuals, thus warranting individualized assessment and appropriate referral as necessary.34
In this study both age and gender were found to be predictive for experiencing higher symptom burden. For example, gender was found be a predictive of developing a high symptom burden, where females were found to have higher total MSAS scores. This finding is similar to other studies that have found that females report higher symptom scores.35 In other cancer populations it has been found that women are at a higher risk of experiencing an emetic response to certain chemotherapy agents and increased opioid-related side effects when compared to men.35 However, it is unclear whether these explanations translate to the acute leukemia population.
Interestingly, age was not found to be a significant predictor of symptom burden. While, to our knowledge no other studies have examined age in the AML population, age as a predictor has been examined in other populations. For example, in studies examining age, symptom burden and quality of life in adults with solid tumors, those of younger age were found to have higher symptom burden and lower quality of life.32,35 Also, studies that have examined symptom burden in young adults have found that this age group reports a higher symptom burden and lower quality of life when compared with younger and older age groups.36 This risk for a high symptom burden in young adults has been thought to be associated with disruption in normal developmental stages, limited life experience which leads to underdeveloped problem-solving and or coping skills.37
The influence of a psychological condition on patients is an important consideration in healthcare. In this study we found a history of a psychological condition such as general anxiety disorders, depression or bipolar to significantly predict whether participants reported higher levels of anxiety early in treatment. Interestingly, this study also found that the anxiety levels of participants in this group returned by week 6 to a level equivalent to participants who did not have a history of a psychological illness. A difference in reported anxiety levels was not found between those who responded to chemotherapy treatment (obtained remission on first induction) compared with those who required additional induction chemotherapy treatments to achieve remission. These findings illustrate that while patients with a psychological history may have high psychological burden early in diagnosis and chemotherapy treatment, their anxiety may normalize over time.
Examining the trajectory of HRQoL in the AML population, this study found that mean scores for both the FACT-G and FACT-LEU are lower than the HRQoL scores reported in other studies that also used the FACT.17,32,37,38 Specifically, higher HRQoL scores have been reported in studies with hematologic malignancies that included AML17,38 and other solid tumors.32,39 While the HRQoL reported in this study was generally low, the results did show a trend of improving HRQoL over the six weeks. The general trend for HRQoL to improve over time may illustrate the participant’s innate resilience and adaptability to a new life-threatening illness.
In this study participants who reported lower HRQoL scores and higher depression, anxiety, and distress scores were more likely to receive a palliative care/behavioral health consultation. Additionally, the number of participants who received a palliative care or behavioral health consult was quite high compared with findings from other studies.33,40 One explanation for this is that the study was conducted at an institution that has a nationally recognized palliative care program, and thus providers may be better equipped to identify and make referrals appropriate referrals to supportive services.
Limitations
Care should be taken when interpreting the statistical models in this manuscript due to the lack of adjustment for multiplicity and the relatively small sample (typical for AML studies). However, in spite of the small sample, this study supports findings from a small number of cross-sectional studies that individuals with AML experience a high symptom burden especially when compared to other oncology populations. Additionally, HRQoL levels are lower across time when compared with other studies and populations. These findings suggest that further research is necessary to develop targeted interventions aimed at improving the symptom burden and HRQoL needs during treatment for adults with AML.
Conclusions
This study is one of the first to explore the symptoms and psychosocial needs in a homogenous sample of adults newly diagnosed with AML across the initial 6-weeks of induction chemotherapy treatment. Results from this study suggest that individuals undergoing induction chemotherapy experience multiple concurrent symptoms that are often distressing. These individuals also experienced a decreased HRQoL that is not only lower than in the general population, but also lower than individuals with other solid and hematologic malignancies. Findings from this study suggest the importance of multidimensional symptom assessments by clinicians at key points in treatment. For example, this study illustrates that individuals undergoing induction chemotherapy treatment for AML, may be at higher risk of experiencing increased symptom burden, including psychological distress at weeks 1, 4 and 6 of treatment. This knowledge can assist the clinician to provide individualized patient-centered care to this unique population. However, more research is necessary to further evaluate the specific symptom and symptom management needs of this patient population and to explore novel personalized interventions to manage the negative consequences associated with AML and its treatment.
Acknowledgments
This study was supported by research funding from Sigma Theta Tau International Small Grants as well as the National Institute of Nursing Research Post-doctoral Training Grant, The Interdisciplinary Training of Nurse Scientists in Cancer Survivorship (T32NRO11092, at the University of Pittsburgh School of Nursing).
The authors would like to thank the following individuals for their assistance with this study: Ann Welsh, RN, OCN (participant identification); Yvonne Shedlock, RN, Christine Bayer, and Natalie Tucker (data collection and entry); Sally Russell and Erica Gregory (database management).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Pulte D, Gondos A, Brenner H. Expected long-term survival of patients diagnosed with acute myeloblastic leukemia during 2006–2010. Ann Oncol. 2010;21(2):335–341. doi: 10.1093/annonc/mdp309. [DOI] [PubMed] [Google Scholar]
- 2.Dohner H, Weisdorf D, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 3.Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98(2):222–229. doi: 10.3324/haematol.2012.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Chevallier P, Labopin M, Turlure P, et al. A new leukemia prognostic scoring system for refractory/relapsed adult acute myelogeneous leukaemia patients: A GOELAMS study. Leukemia. 2011;25(6):939–944. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1976–2011. Bethesda, MD: 2014. [Google Scholar]
- 7.Efficace F, Kemmler G, Vignetti M, Mandelli F, Molica S, Holzner B. Health-related quality of life assessment and reported outcomes in leukaemia randomised controlled trials – A systematic review to evaluate the added value in supporting clinical decision making. Eur J Cancer. 2008;44(11):1497–1506. doi: 10.1016/j.ejca.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Danhauer SC, Russell GB, Tedeschi RG, et al. A longitudinal investigation of posttraumatic growth in adult patients undergoing treatment for acute leukemia. J Clin Psychol Med Settings. 2013;20(1):13–24. doi: 10.1007/s10880-012-9304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albrecht TA, Rosenzweig M. Distress in patients with acute leukemia: A concept analysis. Cancer Nurs. 2013 doi: 10.1097/NCC.0b013e31829193ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado-Guay MO, Parsons HA, Li Z, Palmer LJ, Bruera E. Symptom distress, interventions, and outcomes of intensive care unit cancer patients referred to a palliative care consult team. Cancer. 2009;115(2):437–445. doi: 10.1002/cncr.24017. [DOI] [PubMed] [Google Scholar]
- 11.Morselli M, Bandieri E, Zanin R, et al. Pain and emotional distress in leukemia patients at diagnosis. Leuk Res. 2010;34(2):e67–e68. doi: 10.1016/j.leukres.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Rodin G, Yuen D, Mischitelle A, et al. Traumatic stress in acute leukemia. Psychooncology. 2011;22(2):299–307. doi: 10.1002/pon.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. Adv Nurs Sci. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 15.Molassiotis A, Wengström Y, Kearney N. Symptom Cluster Patterns During the First Year After Diagnosis with Cancer. J Pain Symptom Manage. 2010;39(5):847–858. doi: 10.1016/j.jpainsymman.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Webster K, Chivington K, Shonk C, Eremenco S, Yount S, Hahn E. Measuring quality of life (QOL) among patients with leukemia: The Functional Assessment of Cancer Therapy- Leukemia (FACT-Leu) Qual Life Res. 2002;11(7):678. [Google Scholar]
- 17.Cella D, Jensen S, Webster K, et al. Measuring health-related quality of life in leukemia: The Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) questionnaire. Value Heal. 2012;15(8):1051–1058. doi: 10.1016/j.jval.2012.08.2210. [DOI] [PubMed] [Google Scholar]
- 18.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 19.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp) Ann Behav Med. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 20.Murphy PE, Canada AL, Fitchett G, et al. An examination of the 3-factor model and structural invariance across racial/ethnic groups for the FACIT-Sp: A report from the American Cancer Society’s Study of Cancer survivors-II (SCS-II) Psychooncology. 2010;19(3):264–272. doi: 10.1002/pon.1559. [DOI] [PubMed] [Google Scholar]
- 21.Hasson D, Arnetz B. Validation and findings comparing VAS vs. Lickert Scales for psychosocial measurements. Int Electron J Health Educ. 2005;8:178–192. [Google Scholar]
- 22.Williams VS, Morlock RJ, Feltner D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Heal Qual Life Outcomes. 2010;8:57. doi: 10.1186/1477-7525-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lees N, Lloyd-Williams M. Assessing depression in palliative care patients using the visual analogue scale: A pilot study. Eur J Cancer Care (Engl) 1999;8(4):220–223. doi: 10.1046/j.1365-2354.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 24.Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Distress Management. 2012. [Google Scholar]
- 25.McLaren Baken D, Woolley C. Validation of the distress thermometer, impact thermometer and combinations of these in screening for distress. Psychooncology. 2011;20:609–614. doi: 10.1002/pon. [DOI] [PubMed] [Google Scholar]
- 26.Patrick-Miller LJ, Broccoli JK, Levine E. Validation of the distress thermometer: A single item screen to detect clinically significant psychological distress in ambulatory oncology patients. Am Soc Clin Oncol. 2004;22(14S):6024. [Google Scholar]
- 27.Hoffman B, Zevon M, D’Arrigo M, Cecchini T. Screening for distress in cancer patients: The NCCN rapid-screening measure. Psychooncology. 2004;13:792–799. doi: 10.1002/pon.796. [DOI] [PubMed] [Google Scholar]
- 28.van Belle G. Statistics rules of thumb. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 29.Hosmer D, Lemeshow S. Applied Logistic Regression. John Wiley & Sons; 2000. [Google Scholar]
- 30.Brown H, Prescott R. Statistics in Practice in Applied Mixed Methods in Medicine. 2. Chichester, UK: John Wiley & Sons; 2006. [Google Scholar]
- 31.Manitta V, Zordan R, Cole-Sinclair M, Nandurkar H, Philip J. The symptom burden of patients with hematological malignancy: A cross-sectional observational study. J Pain Symptom Manage. 2011;42(3):432–442. doi: 10.1016/j.jpainsymman.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Deshields TL, Potter P, Olsen S, Liu J. The persistence of symptom burden: Symptom experience and quality of life of cancer patients across one year. Support Care Cancer. 2014;22(4):1089–1096. doi: 10.1007/s00520-013-2049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann C, Yuen D, Mischitelle A, et al. Symptom burden and supportive care in patients with acute leukemia. Leuk Res. 2013;37(7):731–736. doi: 10.1016/j.leukres.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGrath PD. The impact on sexuality after diagnosis and treatment for a hematologic malignancy: Findings from Australia. Oncol Nurs Forum. 2012;39(6):595–600. doi: 10.1188/12.ONF.595-600. [DOI] [PubMed] [Google Scholar]
- 35.Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19(3):417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 36.Evan EE, Zeltzer LK. Psychosocial dimensions of cancer in adolescents and young adults. Cancer. 2006;107(7S):1663–1671. doi: 10.1002/cncr.22107. [DOI] [PubMed] [Google Scholar]
- 37.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90(12):2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yost KJ, Thompson Ca, Eton DT, et al. The Functional Assessment of Cancer Therapy - General (FACT-G) is valid for monitoring quality of life in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2012;54(February):1–8. doi: 10.3109/10428194.2012.711830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28(2):192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 40.Manitta VJ, Philip JAM, Cole-Sinclair MF. Palliative care and the hemato-oncological patient: Can we live together? A review of the literature. J Palliat Med. 2010;13(8):1021–1025. doi: 10.1089/jpm.2009.0267. [DOI] [PubMed] [Google Scholar]