Abstract
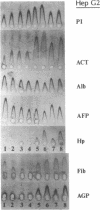
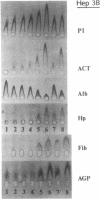
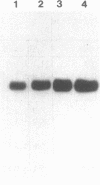
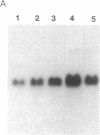
We explored the possible role of transforming growth factor beta 1 (TGF-beta), a cytokine that appears to be an important modulator of inflammation and tissue repair, in regulation of human plasma protein synthesis during the acute-phase response. In Hep 3B cells, TGF-beta led to increased secretion of the positive acute-phase proteins alpha 1-protease inhibitor and alpha 1-antichymotrypsin and decreased secretion of the negative acute-phase protein albumin. In Hep G2 cells, after incubation with TGF-beta, the same changes in secretion of alpha 1-protease inhibitor, alpha 1-antichymotrypsin, and albumin were observed, as well as decreased secretion of both the negative acute-phase protein alpha-fetoprotein and the positive acute-phase protein fibrinogen. In addition, TGF-beta modulated the effects of interleukin 6; these cytokines, in combination, were additive in inducing synthesis and secretion of alpha 1-protease inhibitor and alpha 1-antichymotrypsin and in decreasing secretion of albumin and alpha-fetoprotein. TGF-beta inhibited the induction of fibrinogen caused by interleukin 6. The effects on alpha 1-protease inhibitor were confirmed by metabolic labeling in Hep 3B cells and by demonstrating increased accumulation of specific mRNA in Hep G2 cells, and the effects on fibrinogen were confirmed in Hep 3B cells by studies of mRNA for the alpha chain of fibrinogen. TGF-beta had no effect on haptoglobin or alpha 1-acid glycoprotein secretion, either directly or in the presence of interleukin 6, which is capable of inducing these proteins. These studies demonstrate that TGF-beta can affect hepatic synthesis and secretion of a subset of acute-phase proteins, both directly and by modulating the effect of interleukin 6. The affected group of plasma proteins is distinct from those affected by other recognized acute-phase protein-inducing cytokines. These findings support the view that combinations of cytokines mediate the response of the hepatocyte to inflammatory stimuli.
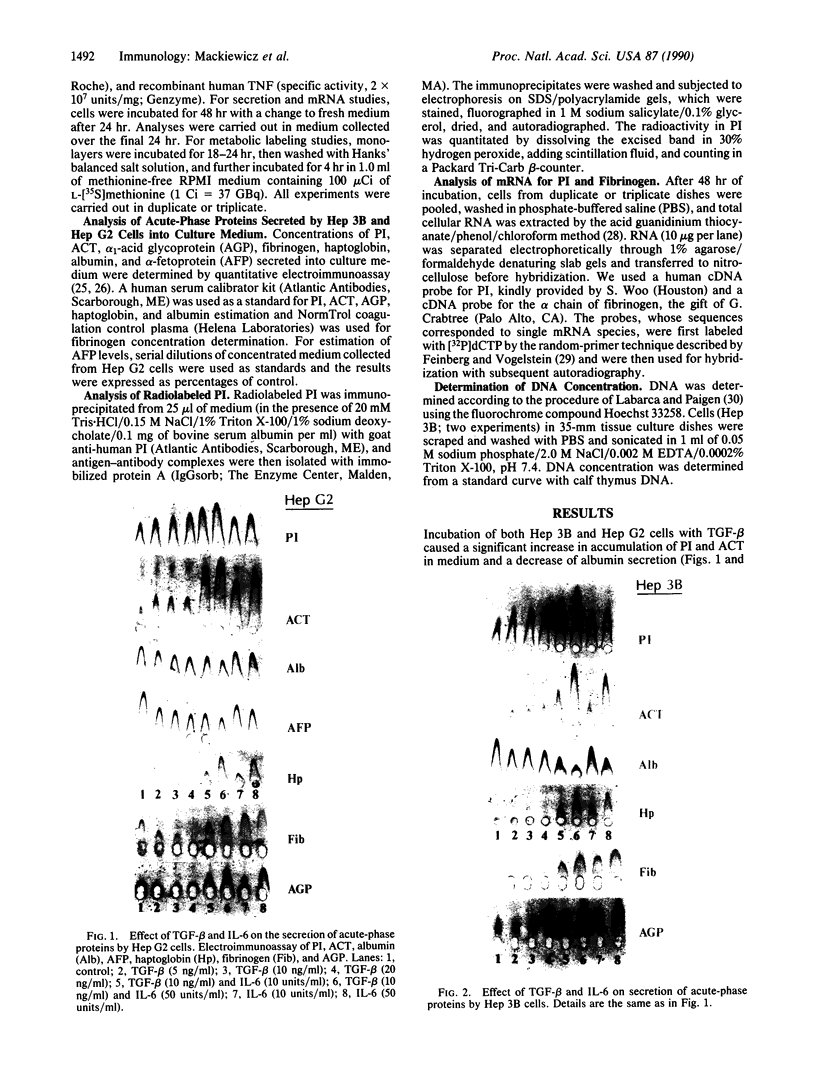
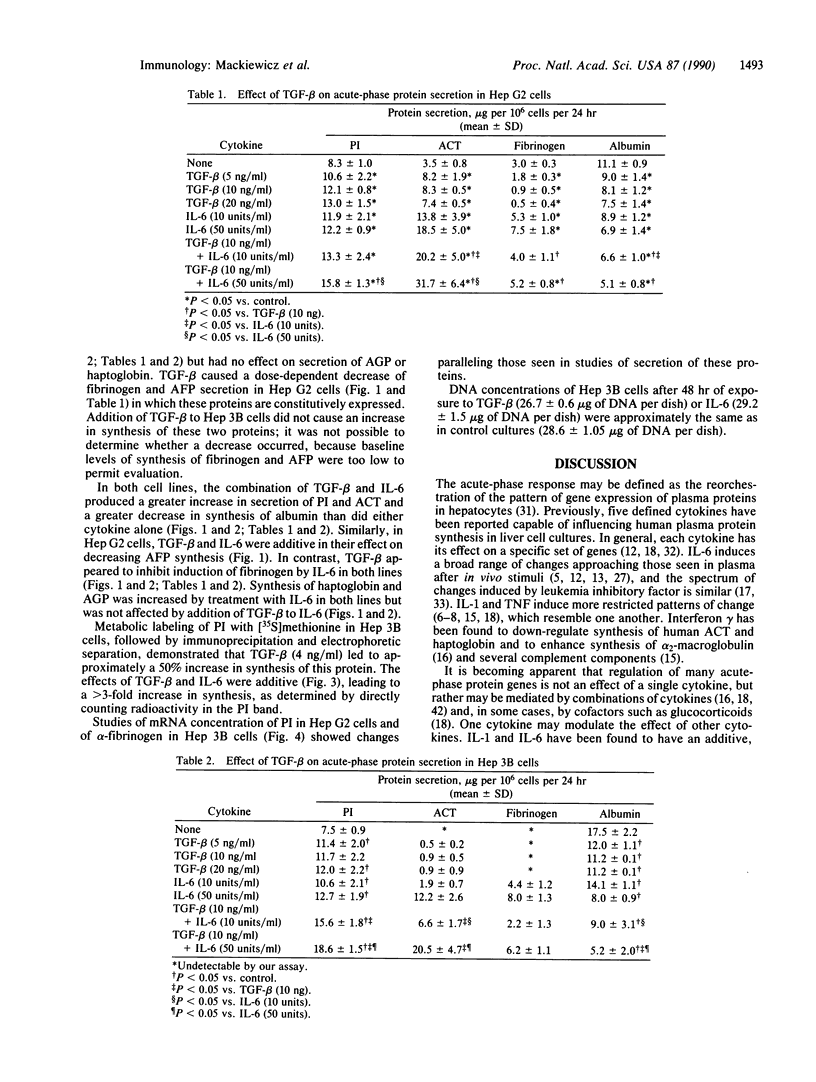
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Baumann H., Prowse K. R., Marinković S., Won K. A., Jahreis G. P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280-95, discussion 295-6. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Richards C., Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987 Dec 15;139(12):4122–4128. [PubMed] [Google Scholar]
- Baumann H., Won K. A., Jahreis G. P. Human hepatocyte-stimulating factor-III and interleukin-6 are structurally and immunologically distinct but regulate the production of the same acute phase plasma proteins. J Biol Chem. 1989 May 15;264(14):8046–8051. [PubMed] [Google Scholar]
- Baumann H., Wong G. G. Hepatocyte-stimulating factor III shares structural and functional identity with leukemia-inhibitory factor. J Immunol. 1989 Aug 15;143(4):1163–1167. [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Hirano T., Kishimoto T., Heinrich P. C. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett. 1988 May 23;232(2):347–350. doi: 10.1016/0014-5793(88)80766-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Revel M., Kelly J. H. Response of liver genes to acute phase mediators. Ann N Y Acad Sci. 1989;557:310–316. doi: 10.1111/j.1749-6632.1989.tb24023.x. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ganapathi M. K., May L. T., Schultz D., Brabenec A., Weinstein J., Sehgal P. B., Kushner I. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem Biophys Res Commun. 1988 Nov 30;157(1):271–277. doi: 10.1016/s0006-291x(88)80043-3. [DOI] [PubMed] [Google Scholar]
- Ganapathi M. K., Schultz D., Mackiewicz A., Samols D., Hu S. I., Brabenec A., Macintyre S. S., Kushner I. Heterogeneous nature of the acute phase response. Differential regulation of human serum amyloid A, C-reactive protein, and other acute phase proteins by cytokines in Hep 3B cells. J Immunol. 1988 Jul 15;141(2):564–569. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N. D., Liu T. Y. Biosynthesis of human C-reactive protein in cultured hepatoma cells is induced by a monocyte factor(s) other than interleukin-1. J Biol Chem. 1987 Feb 15;262(5):2363–2368. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Sweeney G. D., Regoeczi E., Sauder D. N. A simple bioassay for monocyte-derived hepatocyte stimulating factor: increased synthesis of alpha 2-macroglobulin and reduced synthesis of albumin by cultured rat hepatocytes. J Immunol Methods. 1985 Feb 11;76(2):317–327. doi: 10.1016/0022-1759(85)90309-6. [DOI] [PubMed] [Google Scholar]
- Kushner I., Ganapathi M., Schultz D. The acute phase response is mediated by heterogeneous mechanisms. Ann N Y Acad Sci. 1989;557:19–30. doi: 10.1111/j.1749-6632.1989.tb23996.x. [DOI] [PubMed] [Google Scholar]
- Kushner I., Mackiewicz A. Acute phase proteins as disease markers. Dis Markers. 1987 Mar;5(1):1–11. [PubMed] [Google Scholar]
- Kushner I. Postmodernism, the acute phase response, and interpretation of data. Ann N Y Acad Sci. 1989;557:240–242. doi: 10.1111/j.1749-6632.1989.tb24017.x. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Mackiewicz A., Ganapathi M. K., Schultz D., Kushner I. Monokines regulate glycosylation of acute-phase proteins. J Exp Med. 1987 Jul 1;166(1):253–258. doi: 10.1084/jem.166.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz A., Ganapathi M. K., Schultz D., Samols D., Reese J., Kushner I. Regulation of rabbit acute phase protein biosynthesis by monokines. Biochem J. 1988 Aug 1;253(3):851–857. doi: 10.1042/bj2530851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magielska-Zero D., Bereta J., Czuba-Pelech B., Pajdak W., Gauldie J., Koj A. Inhibitory effect of human recombinant interferon gamma on synthesis of acute phase proteins in human hepatoma Hep G2 cells stimulated by leukocyte cytokines, TNF alpha and IFN-beta 2/BSF-2/IL-6. Biochem Int. 1988 Jul;17(1):17–23. [PubMed] [Google Scholar]
- May L. T., Ghrayeb J., Santhanam U., Tatter S. B., Sthoeger Z., Helfgott D. C., Chiorazzi N., Grieninger G., Sehgal P. B. Synthesis and secretion of multiple forms of beta 2-interferon/B-cell differentiation factor 2/hepatocyte-stimulating factor by human fibroblasts and monocytes. J Biol Chem. 1988 Jun 5;263(16):7760–7766. [PubMed] [Google Scholar]
- McMahon J. B., Richards W. L., del Campo A. A., Song M. K., Thorgeirsson S. S. Differential effects of transforming growth factor-beta on proliferation of normal and malignant rat liver epithelial cells in culture. Cancer Res. 1986 Sep;46(9):4665–4671. [PubMed] [Google Scholar]
- Miossec P., Ziff M. Immune interferon enhances the production of interleukin 1 by human endothelial cells stimulated with lipopolysaccharide. J Immunol. 1986 Nov 1;137(9):2848–2852. [PubMed] [Google Scholar]
- Morrone G., Ciliberto G., Oliviero S., Arcone R., Dente L., Content J., Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988 Sep 5;263(25):12554–12558. [PubMed] [Google Scholar]
- Morrone G., Ciliberto G., Oliviero S., Arcone R., Dente L., Content J., Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988 Sep 5;263(25):12554–12558. [PubMed] [Google Scholar]
- Morrone G., Cortese R., Sorrentino V. Post-transcriptional control of negative acute phase genes by transforming growth factor beta. EMBO J. 1989 Dec 1;8(12):3767–3771. doi: 10.1002/j.1460-2075.1989.tb08553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., May L. T., Sehgal P. B. Interferon beta 2/interleukin 6 modulates synthesis of alpha 1-antitrypsin in human mononuclear phagocytes and in human hepatoma cells. J Clin Invest. 1989 Jul;84(1):138–144. doi: 10.1172/JCI114133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Transforming growth factor beta. Adv Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]