Abstract
Introduction
Anti-diabetes medication regimen adherence is a clinical challenge in elderly patients with type 2 diabetes (T2D) and other comorbidities associated with aging. Glucagon-like peptide-1 receptor agonists (GLP-1RA) therapies such as exenatide once weekly (QW), exenatide twice daily (BID), and liraglutide once daily (QD) are an increasingly used class of drugs with proven efficacy and tolerability. Real-world evidence on adherence to GLP-1RAs in elderly or disabled patients is limited. To further the understanding of this drug class, the current study examined medication adherence in Medicare patients aged ≥65 years with T2D initiating a GLP-1RA.
Methods
This retrospective cohort study used medical and pharmacy claims between 2010 and 2013 for Medicare members in a United States health plan diagnosed with T2D who were new initiators of either exenatide QW (n = 537), exenatide BID (n = 923), or liraglutide QD (n = 3,673). Included patients were between the ages of 65 and 89 and were continuously enrolled for 6 months pre- and post-index. Medication adherence was examined during the post-index period using proportion of days covered (PDC) ≥80% and ≥90%.
Results
A significantly higher percentage of patients receiving exenatide QW had a PDC ≥80% (43.2%) versus exenatide BID (39.0%, P < 0.01) and liraglutide QD (35.0%, P < 0.001). The patients receiving exenatide QW were significantly more likely to reach a PDC of ≥90% (37.2%, P < 0.001) than those initiating exenatide BID (20.6%) or liraglutide QD (23.3%).
Conclusions
While results from this retrospective study suggest room for improvement in adherence to GLP-1RAs, medication adherence rates for patients initiating therapy with exenatide QW were higher than patients initiating therapy with exenatide BID or liraglutide QD. Further research is needed to validate these findings in other T2D patient populations.
Funding: AstraZeneca Pharmaceuticals.
Keywords: Adherence, Diabetes, Exenatide BID, Exenatide QW, GLP-1RA, Liraglutide QD, Medicare
Introduction
Diabetes currently affects 29.1 million, or 9.3%, of the United States (US) population, and a nearly equal percentage of individuals worldwide [1]. Type 2 diabetes (T2D) accounts for approximately 90–95% of all diabetes cases and is associated with significant morbidity and mortality, is the leading cause of kidney failure, heart disease, and stroke, and is the seventh leading cause of death in the US. The high prevalence of the disease in the US has led to continually rising costs [2]. In fact, the American Diabetes Association estimated that diabetes costs in the US are approximately US$245 billion annually [2].
More than one-quarter or 25% of adults 65 years and older have diabetes, and approximately 95% of these patients have T2D [3]. As these patients grow older, the social economic impact and personal burden of their disease will increase [4, 5]. A study examining this growing burden in the elderly found that from 1994 to 2004 the incidence and prevalence of diabetes increased by 23% and 62%, respectively; as expected, increases in comorbid complications such as congestive heart failure, hypertension and renal events were also found [1, 4]. While elderly patients with diabetes are at risk for comorbid conditions similar to their younger counterparts, they are also at a high risk for other geriatric comorbid conditions such as cognitive impairment and depression [5, 6]. As the size of the elderly population is expected to grow in the future, the prevalence of diabetes in the US and worldwide will also likely continue to rise in parallel [7].
Because of the increased risk of geriatric-related comorbid conditions, treating T2D in elderly patients is at times difficult [8, 9]. As with all patients with diabetes, one of the main treatment objectives is managing glucose control [10]. As initial therapy, increased exercise and dietary changes may be recommended; however, in elderly patients with T2D, lifestyle changes may not be sufficient [11]. There are oral anti-diabetes treatments available such as metformin, sulfonylureas, alpha glucosidase inhibitors, sodium-glucose transporter-2 inhibitors, dipeptidyl-peptidase-4 inhibitors (DPP-4i), or thiazolidinediones (TZDs). These therapies have shown beneficial effects, but monotherapy often does not have sustained benefits. Because of the progression of diabetes, especially in the older adult population, polypharmacy may be required and can carry an increased risk for adverse events [11].
In recent years, incretin-based therapy, such as treatment with glucagon-like peptide-1 receptor agonists (GLP-1RAs), has attracted interest as a novel therapeutic alternative for all patients with T2D, particularly elderly patients with T2D. GLP-1RAs such as Bydureon® [exenatide extended-release (QW) for injectable suspension], Byetta® [exenatide twice-daily (BID) injection], and Victoza® [liraglutide (QD) (rDNA origin) injection] are indicated as adjunct therapy to diet and exercise to improve glycemic control in adults with T2D [12–14]. These glucose-lowering therapies improve insulin secretion and sensitivity, reduce glycosylated hemoglobin levels, suppress inappropriate glucagon secretion, slow gastric emptying, and reduce food intake by increasing satiety [8, 9, 15, 16]. GLP-1RA therapies could also be associated with a lower risk of cardiovascular disease events and hospitalizations [17].
Adherence to these therapies is an important modifiable factor when considering the treatment complexity of this disease, particularly in elderly patients (age 65 and older). Currently, there is limited real-world data regarding adherence to GLP-1RAs for Medicare patients with T2D; however, there is some evidence in commercially insured patients [18, 19]. One 6-month retrospective database study of adult patients with T2D from a large commercial health plan suggested that patients initiating treatment with exenatide QW were more likely to be adherent than patients initiating treatment with exenatide BID and liraglutide QD [20]. Another study examining the same commercial database found that patients receiving exenatide QW had lower all-cause medical costs compared with liraglutide QD 6 months following treatment initiation [18].
The value of exenatide QW in comparison to other GLP-1RAs can be established based on comparative efficacy data. Because older adult patients with diabetes are at a higher risk for increased resource utilization such as hospitalizations [21], understanding adherence to these newer therapies in an aging population is crucial for reducing morbidity, mortality, and healthcare costs, as well as informing clinicians on disease management.
Methods
Data Source
Data for this study were procured from the Humana administrative claims database. Medical and pharmacy claims with a service date between July 1, 2009 and August 31, 2014 for the Medicare population were extracted. The data sources for this study included patient enrollment and medical and pharmacy claims. Enrollment data included patient demographics such as age, gender, and geographical region. Medical claims data included diagnosis codes based on the International Classification of Diseases, Ninth Revision, Clinical Modifications (ICD-9-CM) associated with medical encounters and financial information. Pharmacy claims included fill dates for prescriptions, national drug codes (NDC), and drug cost data. The study protocol was submitted to and approved by an institutional review board (IRB), Schulman Associates IRB, prior to study initiation. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Patient and Cohort Selection
Three cohorts of patients were identified based on medical diagnosis and the type of GLP-1RA they were prescribed, exenatide QW, exenatide BID, or liraglutide QD, between January 1, 2010 and February 28, 2014. The first fill date for a GLP-1RA was considered the index date. All the identified patients were required to be treatment-naïve, and new initiators to GLP-1RA therapy were defined as no evidence of any GLP-1RA prescription during the 6-month baseline period. The liraglutide QD cohort was further split based on the dosage patients received at index, which was either 1.2 mg or 1.8 mg.
Patients were required to have ≥1 medical claim for T2D during the observation period, defined as ≥1 inpatient or ≥2 outpatient medical claims with an ICD-9-CM of 250.x0 or 250.x2 at any time during the observation period. Patients were also required to have been enrolled for both medical and pharmacy benefits and continuously enrolled for 6 months pre- and post-index. Finally, patients were required to be between the ages of 65 and 89, calculated at the index date. Patients were excluded from any of the cohorts if they had a diagnosis of pregnancy, gestational diabetes, or type 1 diabetes (T1D) during the observation period, pharmacy claims for ≥2 GLP-1RAs on the index date, or any GLP-1RA utilization during the baseline period. Five cohorts were created to be used in further analyses: exenatide QW, exenatide BID, liraglutide QD (all patients treated with liraglutide QD at index, regardless of dosage), liraglutide QD 1.2 mg and liraglutide QD 1.8 mg.
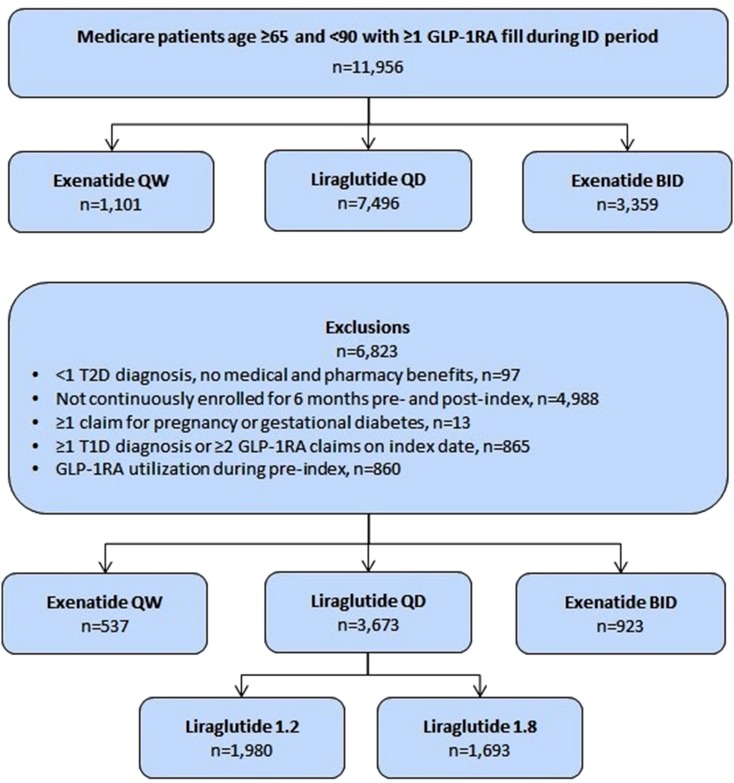
Figure 1 displays the selection criteria used to identify the study cohorts and the final attrition counts for each. A total of 11,956 patients had ≥1 claims during the identification period for one of the GLP-1RAs. After applying all inclusion and exclusion criteria, a total of 5133 patients were included and divided into the final study cohorts. Final sample sizes for each cohort were as follows: exenatide QW = 537, exenatide BID = 923, liraglutide QD = 3673, liraglutide QD 1.2 mg = 1980 and liraglutide QD 1.8 mg = 1693.
Fig. 1.
Attrition counts for final GLP-1RA cohorts. Final counts attained by applying all inclusion and exclusion criteria. BID Twice daily, GLP-1RA glucagon-like peptide-1 receptor agonist, QD once daily, QW once weekly, T1D type 1 diabetes, T2D type 2 diabetes
Study Measures
Demographic and Clinical Characteristics
Demographic and clinical characteristics were evaluated for patients in all cohorts for the 6-month baseline period. Demographic characteristics included age, gender, race/ethnicity, geographical region, as well as plan type [e.g., health maintenance organization (HMO) and preferred provider organization (PPO)], prescriber specialty, and the number of patients who filled their index prescription at a retail pharmacy. Several clinical characteristics and indices of general health and resource use were also examined. The clinical characteristics consisted of the Deyo–Charlson Comorbidity Index (DCCI) and 8 additional comorbid conditions: microvascular complications of diabetes, cardiovascular disease, dyslipidemia, hypertension, hypoglycemia, obesity, renal impairment, and endocrinologist visits. Indices of general health and resource use consisted of mean total baseline healthcare costs and the number of inpatient admissions during the baseline period. All-cause hospitalizations consisted of inpatient stays.
Other anti-diabetes medication classes taken prior to the initiation of any GLP-1RA were tracked and assessed. The 10 medication classes were alpha-glucose inhibitors, biguanides, DPP-4i, dopamine receptor agonists, meglitinides, sulfonylureas, TZD, insulins, amylin analogues, and fixed dose combinations. The mean number of other anti-diabetes medication classes taken by patients in each cohort was also recorded.
Index Dose and Refill Rates
Index dose was evaluated using the specific GLP-1RA being prescribed to ensure the dose was captured and used the quantity and duration commonly available on pharmacy claims. A weekly index dose was calculated for the exenatide QW cohort and a daily index dose was calculated for the exenatide BID and liraglutide QD cohorts and sub-cohorts. For each calculation, the Drug Strength and Package Size were determined from the NDC on the claim for each of the GLP-1RAs. The Days’ supply and metric quantity on the index GLP-1RA claim were also recorded. The following are the computations used to determine the index dose for each of the cohorts:
exenatide QW:
exenatide BID:
liraglutide QD:
The refill rate was computed as the number of times the patients obtained a refill for the index therapy. A 90-day fill was counted as 3 refills (3 × 30 days). An original fill consisting of a 90-day fill was counted as 2 refills (original fill for 30 days + 2 × 30 day refills). Given the length of the post-index period, the maximum number of refills was right-censored at a total of 5 refills. Index dose, daily dose, and refill rates were examined for the 6-month period after the index date.
GLP-1RA Medication Adherence
Adherence was measured using the proportion of days covered (PDC) measure. The Pharmacy Quality Alliance considers patients adherent to their therapy if they have fills for the target therapies for 80% of the examined post-index period. Adherence results were calculated for patients with T2D who reached a PDC of ≥80% or a PDC of ≥90% [19]. This measure was computed by chaining all the fill dates for the target therapy and counting the number of days for which the appropriate dose of the therapy was available during the post-index period. The Leslie method was used to calculate PDC [22]. The mean PDC was also calculated for each of the cohorts in the study.
Post-Index Hospitalization
All-cause hospitalizations were identified during the post-index period using claims data. A hospitalization consisted of a claim associated with a hospital as a place of service and lasting for at least one overnight stay as measured by admit and discharge dates. Both elements are required to be considered a valid hospitalization.
Statistical Analyses
All analyses were conducted using SAS v.9.3. The a priori alpha level for analyses was set at 0.05, and subjected to the Holm adjustment to compensate for each pair of contrasts, and all statistical tests were two-tailed unless otherwise specified. For the demographic and clinical characteristics, means and standard deviations were calculated for continuous variables. For any categorical variables, counts and percentages were calculated. For the demographic and clinical characteristics, comparisons between the cohorts were conducted using t tests for all continuous variable data and Chi square tests for all categorical data.
To minimize the risk of selection bias for the cohorts before computing any outcome measures, inverse propensity treatment score weighting (IPTW) was employed [23]. This method uses propensity scores, but is designed to maintain the sample size of each cohort. A propensity score is a conditional probability of each patient receiving a particular treatment based on pretreatment variables. The inverse propensity treatment weight is then generated in two steps. First, the inverse of the propensity score is calculated for all individuals (1/propensity score), then the weight is adjusted to size of each cohort. The success of the matching is determined by re-testing for differences among the same co-variates tested prior to matching. Successful matching is determined by establishing that the cohorts do not differ. The resulting IPT weights are then carried forward for all remaining analyses. Age, gender, DCCI and other comorbid conditions, geographic regions and other anti-diabetes medication classes were used in matching. However, prior to matching the liraglutide QD, liraglutide QD sub-cohorts and the exenatide BID cohorts to the exenatide QW cohort, the existence of significant differences between the cohort pairings on the clinical and demographic characteristics were tested using two-sample Chi square tests for the categorical variables and two sample t tests for the continuous variables.
Additionally, the index treatment of other anti-diabetes medication classes was computed for each cohort. The exenatide BID, liraglutide QD, liraglutide QD 1.2 mg and liraglutide QD 1.8 mg cohorts were then contrasted with the exenatide QW cohort to further describe potential differences between these cohorts. To calculate the adherence rate, a logistic model was generated to determine which factors may be associated with variation in adherence rates.
A series of logistic regression models were performed to predict the likelihood of patients being adherent to their GLP-1RA or being hospitalized following the initiation of a GLP-1RA. The first regression model was generated to determine which patients were more likely to be adherent during the 6-month follow-up period. A second logistic regression model was generated to determine the risk of hospitalization (all-cause inpatient stays) for patients during the 6-month follow-up period. Both models included the following covariates: age, gender, geographical region, race/ethnicity, plan type, prescriber specialty, DCCI overall score, the eight additional comorbid conditions listed above, and all T2D treatments. The exenatide QW cohort was used as the reference group for both logistic regression models. All resulting statistical tests from these analyses consisted of logistic regression estimates, significance level and a likelihood score (% likelihood adherent or hospitalization).
Furthermore, a series of statistical tests comparing the matched cohorts were performed. For continuous variables other than patient counts, a series of pairwise t tests were performed to determine if there were significant differences between the cohorts. For categorical variables, Chi square tests (Fischer’s exact test) for two samples were performed on the matched data.
Results
GLP-1RA Cohort Populations
All analyses for the demographic and clinical characteristics were completed prior to the IPTW adjustment. Table 1 reports the unadjusted results for all demographic and clinical characteristics for all cohorts.
Table 1.
Unadjusted baseline demographic and clinical characteristics for GLP-1RA cohorts
| Exenatide QW | Liraglutide QD | Exenatide BID | Liraglutide 1.2 mg | Liraglutide 1.8 mg | |
|---|---|---|---|---|---|
| Total sample size, n | 537 | 3673 | 923 | 1980 | 1693 |
| Gender, male (%) | 49.50 | 46.60 | 44.10 | 45.70 | 47.70 |
| Mean age, years (SD) | 71 (5) | 71a (4) | 70a (4) | 71a (4) | 70a (4) |
| Plan type | |||||
| HMO (%) | 50.30 | 43.10 | 42.40 | 42.1 | 44.2 |
| POS (%) | 1.30 | 2.40 | 2.00 | 2.3 | 2.4 |
| PPO (%) | 40.00 | 43.40 | 38.10 | 44.6 | 42.0 |
| FFS (%) | 8.40 | 11.10 | 13.80 | 10.9 | 11.3 |
| Race | |||||
| White (%) | 85.5 | 85.0 | 82.9 | 85.6 | 84.4 |
| Black (%) | 8.0 | 10.2 | 8.3 | 10.3 | 10.1 |
| Hispanic (%) | 3.9 | 1.2 | 0.1 | 1.0 | 1.4 |
| Others (%) | 2.6 | 3.6 | 8.7 | 3.2 | 4.1 |
| Geographical region | |||||
| South (%) | 70.4 | 65.8 | 56.7 | 64.9 | 66.8 |
| Midwest (%) | 20.7 | 21.0 | 25.0 | 21.6 | 20.3 |
| Northeast (%) | 2.2 | 2.1 | 4.6 | 1.7 | 2.5 |
| West (%) | 6.7 | 11.1 | 13.8 | 11.8 | 10.4 |
| Prescriber specialty | |||||
| Endocrinologist (%) | 4.1 | 3.6 | 5.0 | 3.0 | 4.4 |
| General practitioner (%) | 37.8 | 41.1 | 38.2 | 43.8 | 37.9 |
| Internal specialist (%) | 40.6 | 36.4 | 40.0 | 35.4 | 37.7 |
| Unknown (%) | 17.5 | 18.8 | 16.4 | 17.8 | 20.0 |
| Index filled at retail pharmacy (%) | 81.2 | 68.9a | 73.8a | 71.5a | 65.9a |
| Comorbidities | |||||
| Microvascular complications of diabetes (%)b | 40.0 | 36.4 | 34.5a | 35.2 | 37.9 |
| Cardiovascular disease (%) | 34.3 | 31.6 | 32.2 | 32.0 | 31.2 |
| Dyslipidemia (%) | 89.8 | 89.4 | 83.6a | 88.6 | 90.3 |
| Hypertension (%) | 90.5 | 88.6 | 85.6a | 88.7 | 88.4 |
| Hypoglycemia (%) | 1.3 | 1.1 | 0.9 | 0.9 | 1.2 |
| Obesity (%) | 23.5 | 22.5 | 19.0a | 21.6 | 23.6 |
| Renal impairment (%) | 26.1 | 25.6 | 23.3 | 26.3 | 24.8 |
| Endocrinologist visit (%) | 21.4 | 19.2 | 19.5a | 16.6a | 22.2 |
| Indices of general health status and resource use | |||||
| Deyo–Charlson Comorbidity Index (DCCI) | 1.8 (1.6) | 1.7 (1.5) | 1.6 (1.6) | 1.7 (1.6) | 1.7 (1.5) |
| Total healthcare costs at baseline | $6007 ($6415) | $5412 ($6646) | $5404 ($7772) | $5275 ($6077) | $5573 ($6887) |
| Inpatient admission at baseline | 0.18 (0.5) | 0.18 (0.6) | 0.15 (0.5) | 0.19 (0.6) | 0.16 (0.5) |
| Baseline anti-diabetes medications | |||||
| Alpha-glucosidase inhibitors (%) | 0.6 | 0.8 | 1.1 | 0.7 | 0.9 |
| Biguanides (%) | 60.1 | 60.8 | 58.8 | 60.8 | 60.9 |
| DPP-4i (%) | 28.3 | 17.5a | 11.8a | 17.8a | 17.2a |
| Dopamine receptor agonists (%) | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 |
| Meglitinides (%) | 1.9 | 1.6 | 1.8 | 2.0 | 1.1 |
| Sulfonylureas (%) | 50.3 | 54.5 | 53.1 | 56.4a | 52.3 |
| TZDs (%) | 11.9 | 11.5 | 19.0a | 12.6 | 10.2 |
| Insulins (%) | 25.0 | 24.7 | 16.7a | 23.6 | 26.0 |
| Amylin analog (%) | 0.4 | 0.1 | 0.2 | 0.2 | 0.1 |
| Fixed-dose combination medications (%) | 15.5 | 10.7a | 7.8a | 11.0a | 10.4a |
| Number of other anti-diabetes medication classes, mean (SD) | 2.10 (1.1) | 1.93a (1.0) | 1.80 (1.1) | 1.96a (1.0) | 1.89a (1.1) |
BID Twice daily, DCCI Deyo Charlson comorbidity index, DPP-4i depeptidyl peptidase-4 inhibitor, FFS fee for service, HMO health maintenance organization, POS point of service, PPO preferred provider organization, QD once daily, QW once weekly, TZD thiazolidinedione
aDenotes a significant difference from exenatide QW cohort
bMicrovascular complications of diabetes includes diabetic nephropathy, diabetic retinopathy, and diabetic peripheral neuropathy; cardiovascular disease included atherosclerosis, stroke, myocardial infarction, unstable angina pectoris, heart failure, percutaneous coronary intervention, and coronary artery bypass graft
Baseline Characteristics
When examining the seven different demographic characteristics listed above, the average age was 70 years for patients in each cohort, with the exception of the exenatide QW cohort where the average age was 71. The percentage of males in each cohort ranged between 44% (exenatide BID) and 49% (exenatide QW). Most patients in each cohort were covered by an HMO or PPO plan, with significantly more exenatide QW patients (50.3%) on an HMO plan. Of the geographical regions served by the health plan, the largest representation was in the South for all five cohorts, with the next largest representation in the Midwest. Very small percentages of patients in all cohorts were found in the West and Northeast. Most of the patients in each cohort were receiving prescriptions for a GLP-1RA from their general practitioner or an internal specialist, and very few received GLP-1RA prescriptions from an endocrinologist. More than 70% of the patients in each cohort had their index therapy filled at a retail pharmacy, with 81% of patients in the exenatide QW cohort having their index GLP-1RA filled at a retail pharmacy.
Clinical Characteristics
The overall mean DCCI scores were low (below 2.0) for all cohorts with the mean score for the exenatide QW cohort being the highest at 1.8, and the mean DCCI score for the exenatide BID cohort being the lowest at 1.6. When examining the additional comorbid conditions, a large percentage of patients in each of the five cohorts had a diagnosis of dyslipidemia (between 84% and 90%) or hypertension (between 86% and 91%). Very few patients in any of the cohorts had a claim for hypoglycemia (1% or less). When looking at all conditions, the exenatide BID cohort had significantly fewer comorbid conditions than the exenatide QW cohort, and the patients in the exenatide QW cohort had similar counts of comorbid conditions as the liraglutide QD and liraglutide QD sub-cohorts. The general health and resource use results showed that the exenatide QW cohort had higher costs at baseline ($6007) than all other cohorts and more inpatient admissions at baseline (0.18) than the exenatide BID (0.15) and liraglutide QD 1.8 mg (0.16) cohorts.
After adjusting using the IPTW methods discussed in the “Statistical Analyses” section and re-computing the statistical tests for the demographic and clinical characteristics, all failed to generate any significant differences between the cohorts. The results for all remaining analyses were based on IPTW-adjusted analyses.
Baseline Anti-diabetes Medication Use
The baseline use of anti-diabetes medications showed that larger percentages of patients in each cohort were prescribed biguanides (approximately 60%) and sulfonylureas (between 50% and 56%). Very few patients in any of the cohorts had claims for dopamine receptor agonists (<1%), amylin analogues (<1%) or meglitinides (<2%). Those patients who initiated on exenatide QW had greater utilization of DPP-4i (28.3%) and fixed-dose combination medications (15.5%) than all other cohorts. The exenatide QW patients also had more insulin use, but used fewer TZDs than patients initiating therapy with exenatide BID. The number of other anti-diabetes medication classes prescribed to patients in each cohort was approximately two. However, the exenatide QW cohort had slightly more prescribed classes than any of the other cohorts.
Dosage and Refill Rates
Given the variations between products, determining the dosage for each of the cohorts provided some insight on how these products are used in an aged population. Table 2 shows the mean index dose based on the index fill, the daily dose calculation, and the rate of refills for each cohort.
Table 2.
Mean index dose, daily dose and refill rates for each GLP-1RA cohort: IPTW adjusted
| Exenatide QW | Liraglutide | Exenatide BID | Liraglutide 1.2 mg | Liraglutide 1.8 mg | |
|---|---|---|---|---|---|
| n = 537 | n = 3373 | n = 923 | n = 1980 | n = 1693 | |
| Index dose, mean (SD) | 2.0 mg (0.15) | 1.5 mg (0.41) | 15.2 mcg (5.05) | 1.3 mg (0.35) | 1.7 mg (0.35) |
| Range | [0.5–2.5] | [0.3–2.5] | [5.0–22.0] | [0.3–2.5] | [0.6–2.5] |
| Daily dose, mean (SD) | 2.0 mg (0.17) | 1.5 mg (0.38) | 15.9 mcg (4.70) | 1.3 mg (0.34) | 1.7 mg (0.32) |
| Range | [1.3–2.5] | [0.4–2.5] | [5.0–22.0] | [0.4–2.5] | [0.6–2.5] |
| Refill rate, mean (SD) | 3.1 (1.98)a | 3.0 (1.82) | 2.7 (1.89) | 3.1 (1.80)b | 2.7 (1.81) |
| Range | [0.0–5.0] | [0.0–5.0] | [0.0–5.0] | [0.0–5.0] | [0.0–5.0] |
BID Twice daily, GLP-1RA glucagon-like peptide-1 receptor agonist, IPTW inverse propensity treatment weighting, mcg micrograms, mg milligrams, SD standard deviation, QW = once weekly
aDenotes significantly different from Exenatide BID cohort
bDenotes significantly different from Liraglutide 1.8 mg cohort
The results presented in Table 2 show that the index dose approximated the recommended dosing for each cohort. For the overall liraglutide QD cohort, the mean index dose of 1.5 represented the weighted mean of the liraglutide QD 1.2 mg and liraglutide QD 1.8 mg cohorts. The index dose of 15.2 for the exenatide BID cohort represented an accurate initial dosage since the therapy has a recommended dosage of 10 or 20 mcg. The index dosages found for each liraglutide QD sub-cohort were also very close approximations of the recommended dosage for these two cohorts.
Whereas the index dose represented the initial dose of the target drug, the daily dose represented the average of the calculated daily dose for the entirety of the post-index period. As seen in Table 2, results for the daily dose calculations were similar to those for index dose.
When examining refill rates, the exenatide QW cohort had more refills on average (3.1) than the exenatide BID (2.7) or liraglutide QD 1.8 mg (2.7) cohorts. However, the exenatide QW cohort had similar refill rates as the overall liraglutide QD (3.0) and liraglutide QD 1.2 mg (3.1) cohorts. Within the liraglutide QD sub-cohorts, the liraglutide QD 1.2 mg cohort had higher refill rates than the liraglutide QD 1.8 mg cohort.
Adherence
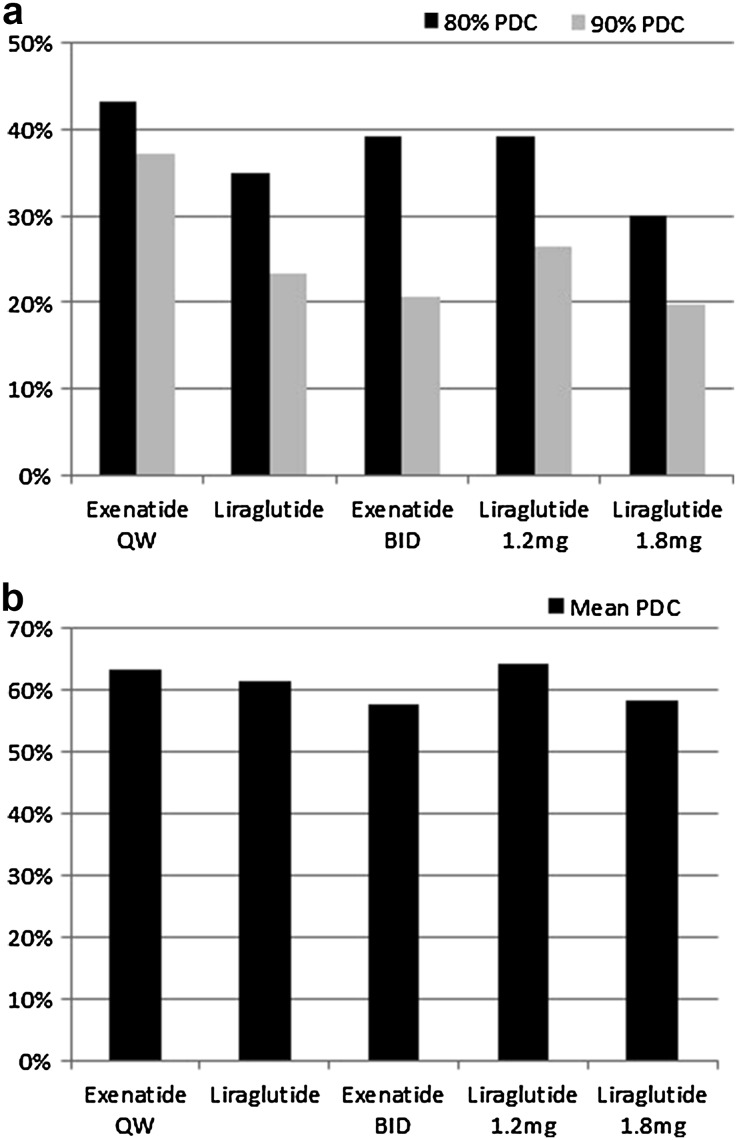
Adherence to the index therapy was computed using the PDC. Adherence was measured for patients who had a PDC of 80% or 90% or greater. Figure 2a displays the percentage of patients in each cohort who reached the 80% and 90% PDC, while Fig. 2b shows the mean PDC for each cohort. When examining patients who reached an 80% PDC or better, exenatide QW patients were significantly more adherent to their medication (43.2%) than patients initiating on liraglutide QD (35.0%; P < 0.001), exenatide BID (39.0%; P < 0.01) or liraglutide QD 1.8 mg (30.0%; P < 0.001). While exenatide QW patients had a slightly higher PDC at 80% than liraglutide QD 1.2 mg patients (39.3%), there was no significant difference between groups (P < 0.10).
Fig. 2.
a Adherence as a function of GLP-1RA type and dose form. Adherence measured using PDC (proportion of days covered) at 80% and 90% for all GLP-1RAs and doses for liraglutide QD. BID Twice daily, mg milligrams, PDC proportion of days covered, QD once daily, QW once weekly. b Mean adherence as a function of GLP-1RA type and dose form
The percentage of patients in each cohort who reached a 90% PDC was smaller than those who reached an 80% PDC (Fig. 2a). The proportion of patients who reached a 90% or better adherence rate was significantly higher among patients who initiated treatment with exenatide QW (37.24%; P < 0.001) than patients who initiated on liraglutide QD (23.31%), exenatide BID (20.6%), liraglutide QD 1.2 mg (26.36%) or liraglutide QD 1.8 mg (19.73%).
Figure 2b shows the mean PDC for exenatide QW patients (63.5%) was significantly higher than the exenatide BID (57.7%; P < 0.01) and the liraglutide QD 1.8 mg (58.3%) patients. However, the mean PDC was similar for the exenatide QW patients and liraglutide QD (61.5%) and liraglutide 1.2 mg (64.2%) patients.
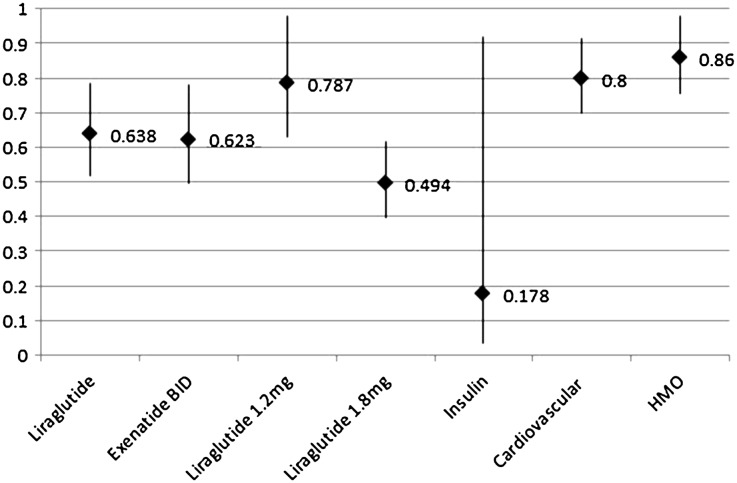
When looking at the probability of one cohort achieving an adherence rate of ≥80%, Fig. 3 indicates that patients initiating on exenatide BID and liraglutide QD showed a lower probability of reaching a PDC of 80% or better than patients who initiated on exenatide QW. It appears that the liraglutide QD 1.8 mg cohort drove the reduced probability. The liraglutide QD 1.2 mg cohort also had lower probabilities of adherence than the exenatide QW patients. Figure 3 also shows the contributing factors that could have contributed to lower probabilities of adherence for these older patients. The logistic regression estimate for these factors is converted into a likelihood percentage of being adherent. The presence of baseline cardiovascular comorbidities, insulin use, and health plan type (HMO vs PPO) were associated with significantly lower adherence rates in the liraglutide QD and exenatide BID cohorts. The other factors included in the model did not reach significance.
Fig. 3.
Multivariate logistic regression adjusted odds of adherence during 6-month follow-up period. Contributing factors that could lead to lower probabilities of adherence for older patients with T2D. Adherence measured using PDC (proportion of days covered). Exenatide QW was used as the reference point. BID Twice daily, HMO health maintenance organization, QD = once daily; QW = once weekly
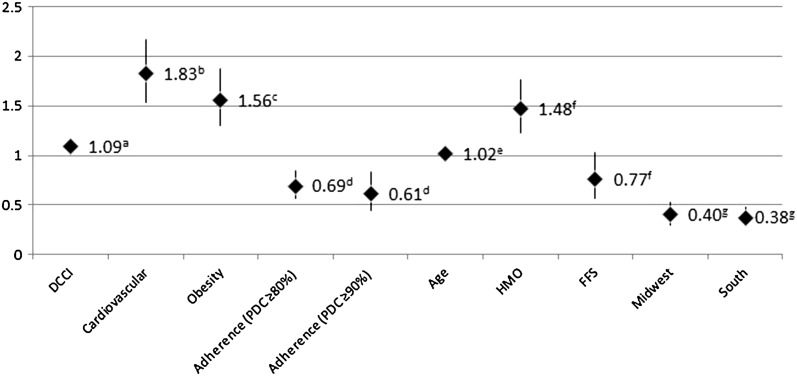
Figure 4 shows the probabilities of different patient characteristics that could have led to a greater chance of hospitalization (all-cause hospitalization). As with adherence, the likelihood of an all-cause hospitalization came from the logistic regression coefficients. An increase in the DCCI score, presence of baseline cardiovascular comorbidities, obesity, and increases in age were all significantly associated with a higher risk of being hospitalized in the 6 months post-index period (all P values less than 0.05). An increase of 1 point for the DCCI increased the risk of hospitalization by 9.4%, while a 5-year age increase was associated with a nearly 10% increase risk of all-cause hospitalization. However, for those patients who were adherent to their GLP-1RA therapies, at a PDC of ≥80% and ≥90%, the risk for all-cause hospitalization was significantly reduced. Other factors included in the model such as plan type (HMO or FFS) or geographical region did not reach significance.
Fig. 4.
Multivariate logistic regression adjusted odds of hospitalization during the 6-month follow-up period. Patient characteristics that could lead to a greater chance of hospitalization. Examined all cause hospitalization using exenatide QW as the reference point. a P < 0.01 Deyo charlson comorbidity index (DCCI) at baseline; b P < 0.0001 vs. no cardiovascular comorbidities at baseline; c P < 0.0001 vs. no obesity at baseline; d P < 0.001 vs. non-adherence; e P < 0.05 being an HMO or fee for service (FFS) member vs. PPO member; g P < 0.05 vs West geographical region
Discussion
The results of this study indicated that medication adherence was different for patients based on the individual GLP-1RA therapy selected at treatment initiation. The GLP-1RA-naïve exenatide QW cohort patients achieved a higher adherence rate, as measured by PDC, than patients in the other two GLP-1RA-naïve treatment cohorts. This pattern was similar for the two levels of PDC measured, which were ≥80% and ≥90%. Index and daily dosing results also showed that patients in all GLP-1RA cohorts received the recommended dosage based on their prescribing label. This also held true for the two liraglutide sub-cohorts (1.2 mg and 1.8 mg). These dosing results are consistent with other studies that show the appropriate treatment is being dispensed to patients on GLP-1RAs [24].
Exenatide and liraglutide were compared, as well as three dosage regimens (BID, QD, and QW). The results showed that exenatide QW had higher adherence rates (≥80% and ≥90%) than either liraglutide QD or exenatide BID suggesting that the dosage regimen for GLP-1RAs may be an important factor contributing to higher adherence. It should be noted that the liraglutide 1.2 mg and 1.8 mg cohorts generated different adherence rates, suggesting that tolerance to the drug may be an important factor in achieving adherence to GLP-1RAs. Other contributing factors that were found to be associated with adherence in the elderly population included baseline cardiovascular comorbidities, insulin use, and health plan type (HMO vs. PPO). A better understanding of factors and its association with adherence may allow for a more personalized approach and aid in achieving therapeutic targets.
Because these are newer treatments for patients with T2D, information regarding adherence is sparse, particularly for older adults; however, there are a few previous studies that evaluated medication adherence for exenatide BID and liraglutide QD [25, 26]. Some of this evidence shows that there are no differences in adherence between these two treatments, while one study shows a significant difference in adherence rates [25, 26]. For example, Pelletier and colleagues [25] found similar medication adherence rates using a PDC calculation between exenatide BID and liraglutide QD in the 6 months following initiation [25]. In another retrospective study, Malmenas and colleagues [26] found that patients initiating therapy with exenatide BID had significantly higher rates of adherence than those patients initiating on liraglutide QD 1.8 mg [26].
Furthermore, a study by Johnston and colleagues [19] examined patient adherence rates between exenatide QW, exenatide BID, liraglutide QD, and the liraglutide sub-cohorts [19]. Their results showed that patients who initiated with exenatide QW had higher adherence rates when compared to other GLP-1RAs. The results of the current study are consistent with Johnston et al., and expand on existing literature by examining how these medications are being used by an older population with T2D.
One possible reason for the better adherence rates observed for exenatide QW among older patients with T2D could be due to the once-a-week dosage regimen. Exenatide BID and liraglutide QD require twice- or once-daily injections, respectively. Treatments for diabetes require that individuals have the ability to perform self-care tasks such as weight management and exercise, as well as the self-administration of medications. Because older adults are at increased risk for cognitive and physical limitations, this could have a greater impact on self-care tasks and administration of the designated GLP-1RA as prescribed, and therefore lead to lower adherence [27–29]. Cognitive function is an interesting discussion as it pertains to adherence and to overall diabetes care in elderly patients. There are so many factors that could influence adherence, and it could be inferred from the current findings (and others about once-weekly administration) that once-weekly administration and predictable dosing regimen may support other types of non-pharmaceutical patient care, particularly in the elderly population. As a visiting nurse, understanding the adherence of different therapies within class could potentially further support personalized, targeted diabetes care. Assessing for cognitive dysfunction and depression along with perceived self-efficacy, coupled with the understanding of adherence, could further help to identify vulnerable patients and assist with personalized care in elderly patients with T2D. While non-adherence to the GLP-1RAs may not be as significant for younger diabetes patients, the dosage regimens for these treatments could become a problem for those with memory issues or mild physical impairments which may limit their ability to perform these tasks. Only having to remember a once-weekly injection such as exenatide QW may help aid older patients with T2D with their adherence to their GLP-1RA. The treatment regimen and dosing frequency should be considered when making treatment decisions for older patients.
Medication adherence is a growing concern among clinicians, healthcare systems, and other stakeholders (e.g., payers) because of mounting evidence that non-adherence is prevalent and associated with adverse outcomes such as more frequent hospitalizations and higher costs of medical care [30]. Medication non-adherence is likely to grow as the US population ages and as patients take more medications to treat chronic conditions [31]. Therefore, understanding the barriers to medication adherence, particularly in the elderly population, will be essential [32]. The emphasis on performance measures that reward quality based on the attainment of treatment targets such as blood pressure, or outcomes such as 1-year mortality after hospitalization for conditions such as acute myocardial infarction, reinforces the importance of longitudinal medication adherence. In particular, the Centers for medicare and medicaid services (CMS) utilize several quality measures to assess medication adherence in patients with T2D [33]. Barriers for optimal adherence to treatments for T2D could be due to regimen complexity. Studies examining the association between anti-diabetes medication regimen complexity and anti-diabetes medication adherence have shown that regimens with less frequent dosing are associated with increased adherence [34, 35]. However, adherence to treatments after a certain period of time is similar for orals and injectables, so lack of adherence may not be solely based on regimen complexity. Previous work has shown that GLP-1RAs have better adherence rates than insulin but lower rates than oral treatments. Counseling patients on the use and benefits of GLP-1RAs could also be key in promoting lifestyle modifications and adherence to these treatments [36].
Medication adherence also has an impact on resource utilization in older patients, not only for diabetes but for the other comorbidities. As these patients age, they will require or seek out more healthcare services and require more hospitalizations [37]. Based on these results, the specific GLP-1RA a patient received did not have an impact on hospitalizations; however, adherence to their GLP-1RA therapy resulted in a significant reduction in risk of hospitalization. Because of the increasing resource use, it is important to understand the relationship of adherence with resource utilization and also other factors that may contribute to increased health care resource use. In this analysis, we also identified different patient characteristics that were associated with a greater chance of hospitalization, which may provide further understanding in identifying high-risk patients with diabetes and supporting patient-centered, personalized care.
Limitations
This study has several important limitations that should be considered when interpreting the current findings. Administrative claims data are subject to potential coding error and are not collected for research purposes. Such errors may introduce measurement error with respect to ICD-9-CM–based variables. Administrative claims data also have certain limitations regarding the type and amount of data available. The representations of provider specialties are not always captured well on medical claims, therefore making it difficult to examine how a visit with a diabetes educator or nurse or who prescribed a specialty treatment could influence outcomes. Claims data can also constrain the ability to look at social conditions that might influence outcomes. Not all data points are available for patients, such as HbA1c levels, which could be used to further show the importance of an outcome.
Findings from the study may not be generalizable to the entire US population, including the populations of individuals who are uninsured or those who have insurance coverage through Medicaid or the military. Most plans are regional in nature and therefore will show larger patient populations in certain areas of the United States. The database used for this study has broader coverage in the South and Midwest than the West or Northeast which caused the disparity in geographic representation. Although we classified patients treated with liraglutide into 1.2 mg or 1.8 mg on the basis of days supplied and metric quantity recorded on the pharmacy claims, liraglutide is delivered in a self-adjustable prefilled dosing pen, and it is therefore possible that patients may have self-administered more or less liraglutide than would be indicated for given prescription’s days supplied and metric quantity. Despite our attempt to control for confounding through the use of multivariable logistic regression, observational analyses such as the present study may be subject to residual confounding due to unmeasured variables such as disease severity. Finally, if physicians selectively prescribe a once-weekly regimen because of anticipated medication non-adherence, such channeling bias may have led to worse than expected adherence among exenatide QW patients.
Conclusion
Medication adherence is an important factor in the treatment of chronic conditions like diabetes. It is particularly important for older patients diagnosed with diabetes in order to prevent disease complications. GLP-1RA therapies have shown good efficacy results in patients with diabetes, yet there is limited patient adherence and dosing frequency impact information, particularly for elderly patients with diabetes. Further research should be pursued to determine the characteristics of the patients with high and low medication adherence to determine the most appropriate treatment plan.
Acknowledgements
We would like to thank Mary Costantino and Neelam Davis, employees of Comprehensive Health Insights, Inc, for their editorial assistance. This study, publication charges and open access fee was funded by AstraZeneca Pharmaceuticals. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The data was presented in part at the American Diabetes Association 75th Scientific Session, 5–9 June, 2015, Boston, MA, USA.
Disclosures
Authors were either employed by AstraZeneca Pharmaceuticals or received consulting fees from AstraZeneca Pharmaceuticals. H. Nguyen was an employee of AstraZeneca Pharmaceuticals during the time the study was conducted. R. Dufour is an employee of Comprehensive Health Insights, Inc. A. Caldwell-Tarr is an employee of Comprehensive Health Insights, Inc. R. Dufour had full access to all of the data in this study and takes complete responsibility for the integrity of the data. All authors take complete responsibility for the accuracy of the data analysis.
Compliance with Ethics Guidelines
This study was approved by an institutional review board (IRB), Schulman Associates, before the start of the study. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. It is a private database that is accessible through fee subscription.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/7D47F0604CA496AA.
References
- 1.Center for disease control and prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2011. http://www.cdc.gov/DIABETES/pubs/factsheet11.htm.
- 2.ADA. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-46. (Epub 2013/03/08). [DOI] [PMC free article] [PubMed]
- 3.Centers for disease control and prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United Sates, 2014. Atlanta, GA: U.S. Department of Health and Humana Services; 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- 4.Sloan FA, Bethel MA, Ruiz D, Jr., Shea AM, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med. 2008;168(2):192–9 (discussion 9. Epub 2008/01/30). [DOI] [PubMed]
- 5.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chau D, Edelman SV. Clinical management of diabetes in the elderly. Clin Diabetes. 2001;19(4):4. doi: 10.2337/diaclin.19.4.172. [DOI] [Google Scholar]
- 7.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 8.Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A. 2001;56(1):M5–M13. doi: 10.1093/gerona/56.1.M5. [DOI] [PubMed] [Google Scholar]
- 9.Abbatecola AM, Maggi S, Paolisso G. New approaches to treating type 2 diabetes mellitus in the elderly: role of incretin therapies. Drugs Aging. 2008;25(11):913–925. doi: 10.2165/0002512-200825110-00002. [DOI] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 11.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 12.Amylin Pharmaceuticals, Inc. Bydureon Package Insert. http://druginserts.com/lib/rx/meds/bydureon-2/ Accessed 29 Aug 2014.
- 13.Amylin Pharmaceuticals, Inc. Byetta Package Insert. http://druginserts.com/lib/rx/meds/byetta/page/4/. Accessed 29 Aug 2014.
- 14.Novo Nordisk. Victoza Package Insert. http://www.novo-pi.com/victoza.pdf. Accessed 29 Aug 2014.
- 15.Holst JJ, Vilsboll T. Combining GLP-1 receptor agonists with insulin: therapeutic rationales and clinical findings. Diabetes Obes Metab. 2013;15(1):3–14. doi: 10.1111/j.1463-1326.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 16.Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectrum. 2004;17:8. doi: 10.2337/diaspect.17.3.183. [DOI] [Google Scholar]
- 17.Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–95. doi: 10.2337/dc10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston SS, Nguyen H, Cappell K, Nelson JK, Chu BC, Kalsekar I. Retrospective study comparing healthcare costs and utilization between commercially insured patients with type 2 diabetes mellitus who are newly initiating exenatide once weekly or liraglutide in the United States. J Med Econ. 2015;18(9):666–677. doi: 10.3111/13696998.2015.1039539. [DOI] [PubMed] [Google Scholar]
- 19.Johnston SS, Nguyen H, Felber E, Cappell K, Nelson JK, Chu BC, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31(11):1119–1133. doi: 10.1007/s12325-014-0166-0. [DOI] [PubMed] [Google Scholar]
- 20.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 21.CDC. Distribution of first-listed diagnoses among hospital discharges with diabetes as any-listed diagnosis, adults aged 18 years and older, United States, 2010. 2010 [cited 2015 November 5]. http://www.cdc.gov/diabetes/statistics/hosp/adulttable1.htm.
- 22.Leslie RS. Using Arrays to Calculate Medication Utilization. SAS Global Forum Orlando, CA; 2007.
- 23.Leslie RS, editor. Using Propensity Scores to Adjust for Treatment Selection Bias. Proceedings of Western Users of SAS Software 2008 Educational Forum and Conference, Universal City, CA; 2008.
- 24.Miller LA, Burudpakdee C, Zagar A, Bhosle M, Reaney M, Schabert VF, et al. Exenatide BID and liraglutide QD treatment patterns among type 2 diabetes patients in Germany. J Med Econ. 2012;15(4):746–757. doi: 10.3111/13696998.2012.679756. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier EM, Pawaskar M, Smith PJ, Best JH, Chapman RH. Economic outcomes of exenatide vs liraglutide in type 2 diabetes patients in the United States: results from a retrospective claims database analysis. J Med Econ. 2012;15(6):1039–1050. doi: 10.3111/13696998.2012.688903. [DOI] [PubMed] [Google Scholar]
- 26.Malmenas M, Bouchard JR, Langer J. Retrospective real-world adherence in patients with type 2 diabetes initiating once-daily liraglutide 1.8 mg or twice-daily exenatide 10 mug. Clin Ther. 2013;35(6):795–807. doi: 10.1016/j.clinthera.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–539. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelberg HK, Shallenberger E, Wei JY. Medication management capacity in highly functioning community-living older adults: detection of early deficits. J Am Geriatr Soc. 1999;47(5):592–596. doi: 10.1111/j.1532-5415.1999.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdelhafiz AH, Sinclair AJ. Management of type 2 diabetes in older people. Diabetes Ther. 2013;4(1):13–26. doi: 10.1007/s13300-013-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Wei W, Miao R, Xie L, Baser O. Real-world outcomes of US employees with type 2 diabetes mellitus treated with insulin glargine or neutral protamine Hagedorn insulin: a comparative retrospective database study. BMJ Open. 2013;3(4) (Epub 2013/05/02). [DOI] [PMC free article] [PubMed]
- 32.Yap AF, Thirumoorthy T, Kwan YH. Systematic review of the barriers affecting medication adherence in older adults. Geriatr Gerontol Int. 2015. (Epub 2015/10/21). [DOI] [PubMed]
- 33.Centers for Medicare & Medicaid Services. Quality Measures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html. Accessed 30 Apr 2016.
- 34.Zhang L, Zakharyan A, Stockl KM, Harada AS, Curtis BS, Solow BK. Mail-order pharmacy use and medication adherence among Medicare Part D beneficiaries with diabetes. J Med Econ. 2011;14(5):562–567. doi: 10.3111/13696998.2011.598200. [DOI] [PubMed] [Google Scholar]
- 35.Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31(3):213–224. doi: 10.1007/s10865-007-9147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalra S, Kalra B. Counselling patients for GLP-1 analogue therapy: comparing GLP-1 analogue with insulin counselling. N Am J Med Sci. 2012;4(12):638–640. doi: 10.4103/1947-2714.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available. It is a private database that is accessible through fee subscription.