Abstract
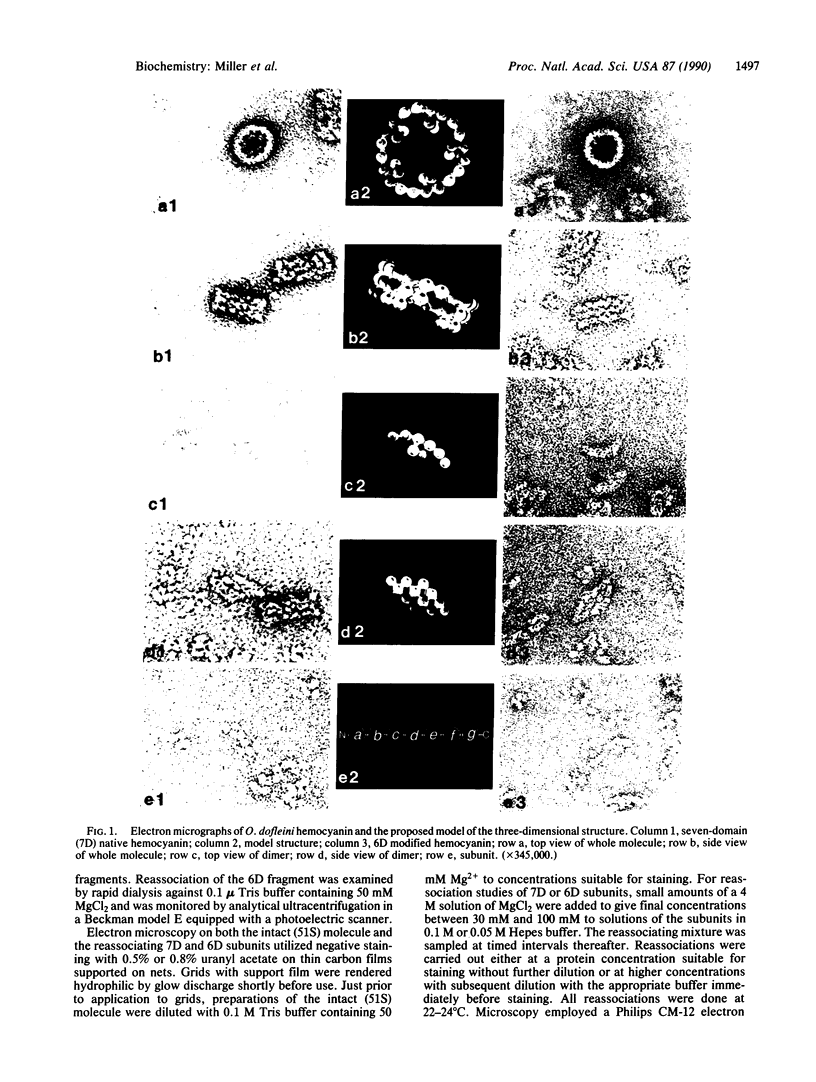
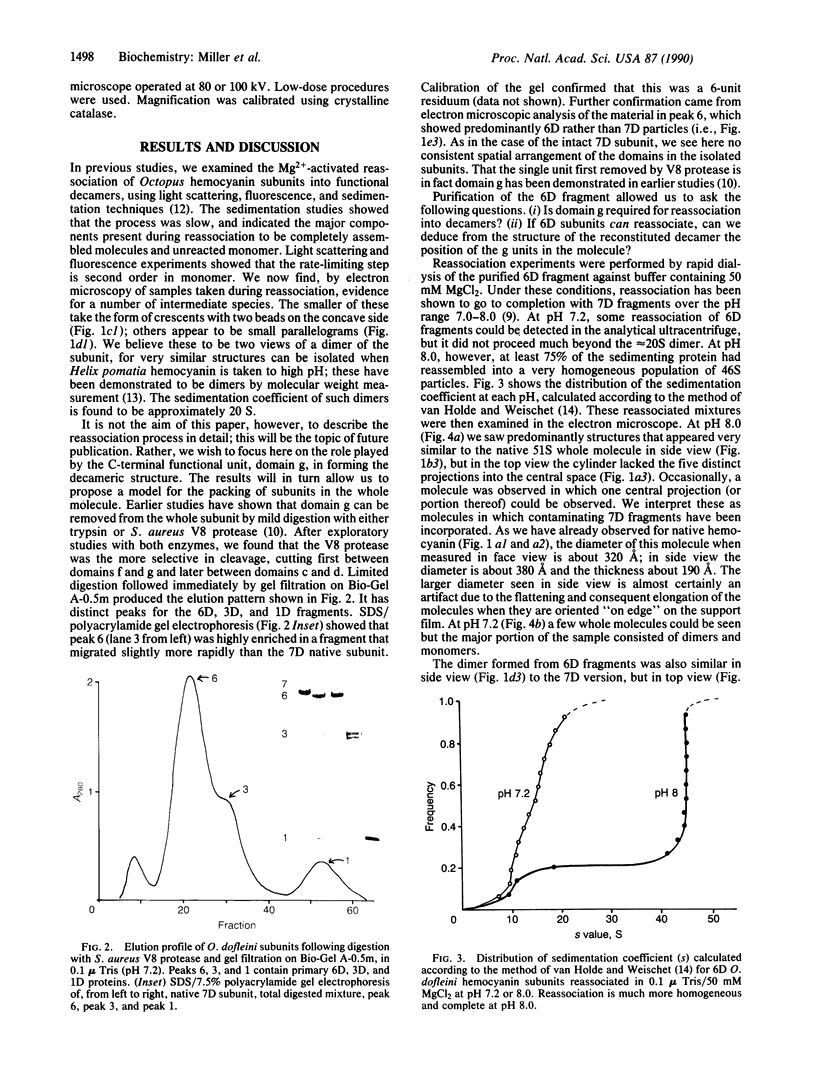
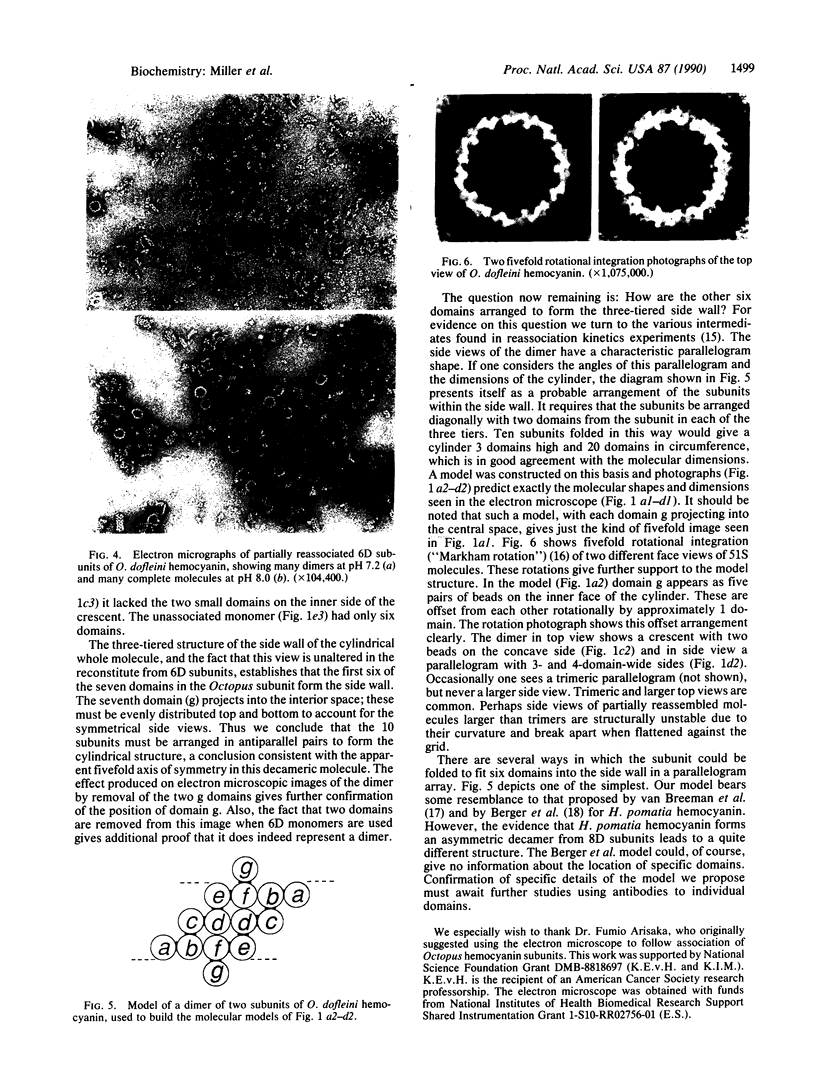
Native Octopus dofleini hemocyanin appears as a hollow cylinder in the electron microscope. It is composed of 10 polypeptide subunits, each folded into seven globular oxygen-binding domains. The native structure reassociates spontaneously from subunits in the presence of Mg2+ ions. We have selectively removed the C-terminal domain and purified the resulting six-domain subunits. Although these six-domain subunits do not associate efficiently at pH 7.2, they undergo nearly complete reassociation at pH 8.0. The resulting molecule looks like the native cylindrical whole molecule but lacks the usual fivefold protrusions into the central cavity. Partially reassociated mixtures show dimers of the subunit that have a characteristic parallelogram shape when lying flat on the electron microscope grid, and a "boat" form in side view. Removal of the C-terminal domain from monomers results in the removal of two characteristically placed domains in the dimers. These observations allow the development of a model for the arrangement of the subunits within the whole molecule. The model predicts exactly the views seen in the electron microscope of both whole molecule and dimeric intermediates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J., Pilz I., Witters R., Lontie R. Studies by small-angle X-ray scattering of the quaternary structure of the beta-haemocyanin of Helix pomatia. Eur J Biochem. 1977 Oct 17;80(1):79–82. doi: 10.1111/j.1432-1033.1977.tb11858.x. [DOI] [PubMed] [Google Scholar]
- Ellerton H. D., Ellerton N. F., Robinson H. A. Hemocyanin--a current perspective. Prog Biophys Mol Biol. 1983;41(3):143–248. doi: 10.1016/0079-6107(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Lang W. H. cDNA cloning of the Octopus dofleini hemocyanin: sequence of the carboxyl-terminal domain. Biochemistry. 1988 Sep 20;27(19):7276–7282. doi: 10.1021/bi00419a015. [DOI] [PubMed] [Google Scholar]
- Volbeda A., Hol W. G. Crystal structure of hexameric haemocyanin from Panulirus interruptus refined at 3.2 A resolution. J Mol Biol. 1989 Sep 20;209(2):249–279. doi: 10.1016/0022-2836(89)90276-3. [DOI] [PubMed] [Google Scholar]
- van Holde K. E., Miller K. I. Association-dissociation equilibria of Octopus hemocyanin. Biochemistry. 1985 Aug 13;24(17):4577–4582. doi: 10.1021/bi00338a014. [DOI] [PubMed] [Google Scholar]
- van Holde K. E., Miller K. I. Haemocyanins. Q Rev Biophys. 1982 Feb;15(1):1–129. doi: 10.1017/s0033583500002705. [DOI] [PubMed] [Google Scholar]