Norepinephrine (NE) is released throughout the brain in many behavioral contexts, but its impacts on information processing are not well understood. We studied the impact of NE on chemosensory tuning in the mouse accessory olfactory bulb (AOB). Electrophysiological recordings from AOB neurons in ex vivo preparations revealed that NE, on balance, inhibited mitral cell responses to chemosensory cues. However, NE’s effects were heterogeneous, indicating that NE signaling reshapes AOB output in a cell- and stimulus-specific manner.
Keywords: accessory olfactory bulb, accessory olfactory system, norepinephrine, information processing, chemical senses
Abstract
Norepinephrine (NE) release has been linked to experience-dependent plasticity in many model systems and brain regions. Among these is the rodent accessory olfactory system (AOS), which is crucial for detecting and processing socially relevant environmental cues. The accessory olfactory bulb (AOB), the first site of chemosensory information processing in the AOS, receives dense centrifugal innervation by noradrenergic fibers originating in the locus coeruleus. Although NE release has been linked to behavioral plasticity through its actions in the AOB, the impacts of noradrenergic modulation on AOB information processing have not been thoroughly studied. We made extracellular single-unit recordings of AOB principal neurons in ex vivo preparations of the early AOS taken from adult male mice. We analyzed the impacts of bath-applied NE (10 μM) on spontaneous and stimulus-driven activity. In the presence of NE, we observed overall suppression of stimulus-driven neuronal activity with limited impact on spontaneous activity. NE-associated response suppression in the AOB came in two forms: one that was strong and immediate (21%) and one other that involved gradual, stimulus-dependent monotonic response suppression (47%). NE-associated changes in spontaneous activity were more modest, with an overall increase in spontaneous spike frequency observed in 25% of neurons. Neurons with increased spontaneous activity demonstrated a net decrease in chemosensory discriminability. These results reveal that noradrenergic signaling in the AOB causes cell-specific changes in chemosensory tuning, even among similar projection neurons.
NEW & NOTEWORTHY Norepinephrine (NE) is released throughout the brain in many behavioral contexts, but its impacts on information processing are not well understood. We studied the impact of NE on chemosensory tuning in the mouse accessory olfactory bulb (AOB). Electrophysiological recordings from AOB neurons in ex vivo preparations revealed that NE, on balance, inhibited mitral cell responses to chemosensory cues. However, NE’s effects were heterogeneous, indicating that NE signaling reshapes AOB output in a cell- and stimulus-specific manner.
norepinephrine (NE) and other neuromodulators strongly influence neuronal function throughout the brain. NE is released in response to states of arousal and novelty, and contributes to region-specific forms of neural plasticity (reviewed in Bouret and Sara 2005). Such impacts are seen even at early stages of sensory processing, indicating that centrifugal noradrenergic signaling influences sensory perception and behavioral responses to sensory stimuli (Bennett et al. 1998; Bouret and Sara 2002; Devilbiss and Waterhouse 2004; Hirata et al. 2006; Linster and Fontanini 2014). In the mammalian main and accessory olfactory bulbs (MOB and AOB, respectively), NE is released by centrifugal fibers originating in the locus coeruleus (LC; Fallon and Moore 1978; McLean et al. 1989; Rosser and Keverne 1985). In the AOB, the first and only dedicated neural circuit in the accessory olfactory system (AOS), NE release occurs during social encounters, including mating, and is critical for the expression of certain forms of pheromone-mediated social learning (Brennan et al. 1990; Brennan 2009; Brennan and Binns 2005; Bruce 1959; Linster et al. 2011; Matsuoka et al. 2004; Otsuka et al. 2001; Rosser and Keverne 1985). Because it directly links the sensory periphery to the limbic system, the AOB is an attractive system in which to investigate the influence of noradrenergic signaling on information processing and behavior.
The mouse AOS detects and interprets information about social chemosignals, including pheromones (intraspecies social cues) and kairomones (cross-species cues that benefit the detector; reviewed in Liberles 2014). In the natural environment, mice encounter AOS ligands in blends of socially-informative cues, which typically originate in animal excretions such as urine (Chamero et al. 2007; Fu et al. 2015; Leinders-Zufall et al. 2004; Nodari et al. 2008), tears (Kimoto et al. 2005), or feces (Doyle et al. 2016). Certain environmental nonvolatiles elicit innate behaviors, including courtship/mating (Fu et al. 2015; Haga-Yamanaka et al. 2014; Roberts et al. 2010), territorial aggression (Chamero et al. 2007; Hattori et al. 2016; Kaur et al. 2014), and predator avoidance (Papes et al. 2010). The strong behavioral influence of the AOS is well appreciated, but the circuit mechanisms linking sensory detection to behavior remain weakly understood.
The behavioral impacts of NE release in the AOB can be profound. For example, in female mice, chemosensory cues from a recently mated male drive AOB activity coincidentally with centrifugal noradrenergic drive from the LC. The coincidence of peripheral sensory input and centrifugal neuromodulation can generate a stable memory of the mated male’s chemosignals, which prevents subsequent exposure to the mated male or its excretions from triggering pregnancy termination (Brennan and Binns 2005; Brennan et al. 1995; Brennan and Keverne 1997; Rosser and Keverne 1985). Despite its importance, the capacity of noradrenergic signaling to shape information processing has not been thoroughly investigated.
Several studies have provided insights into cellular and synaptic changes that accompany neuromodulatory signaling via NE, acetylcholine, and oxytocin receptors (Araneda and Firestein 2006; Fang et al. 2008; Kaba and Keverne 1988; Smith and Araneda 2010; Smith et al. 2009; Smith et al. 2015). A common conclusion of each of these inquiries is that neuromodulation changes the balance of excitation/inhibition between the principal neurons of the AOB, called mitral cells (MCs), and local GABAergic interneurons (Brennan 2009; Brennan and Keverne 1997; Kaba and Keverne 1988).
AOB MCs and interneurons communicate via a dense network of reciprocal, dendrodendritic synapses (Jia et al. 1999; Larriva-Sahd 2008; Taniguchi and Kaba 2001). At these synapses, glutamate release by MC dendrites excites terminals of local GABAergic interneurons that can, in turn, release GABA back onto the same MC dendrite (Jia et al. 1999; Rall et al. 1966; Taniguchi and Kaba 2001). Targeted investigation into the impacts of NE on AOB MCs and interneurons revealed opposing effects on MC and interneuron excitability (Araneda and Firestein 2006; Brennan 2009; Leszkowicz et al. 2012; Otsuka et al. 2001). In the MOB, NE’s effects are also variable, with some studies showing an NE-driven increase in MC activity (Hayar et al. 2001; Jahr and Nicoll 1982; Jiang et al. 1996; Trombley and Shepherd 1992), other studies showing a decrease (Shea et al. 2008; Zimnik et al. 2013), and yet other studies showing mixed effects (Nai et al. 2009). One hypothesis emerging from the AOB studies is that noradrenergic activation depolarizes internal granule cells (IGCs), comprising the largest AOB interneuron population, resulting in increased GABAergic inhibition of MCs (Araneda and Firestein 2006; Smith et al. 2009). However, it remains unclear how NE impacts MC activity in the context of chemosensory processing. For example, it is not known whether NE causes widespread, nonselective suppression of MC activity, or whether MC suppression is selective for the cues present during NE release.
In this article, we report the results of a targeted investigation into the impacts of NE on MC chemosensory tuning. We utilized ex vivo preparations of the functionally connected vomeronasal organ (VNO) and AOB (Meeks and Holy 2009; Meeks et al. 2010). Single-unit electrophysiological recordings of MCs during naturalistic chemosensory stimulation of the VNO revealed heterogeneous effects of NE on MC spontaneous and stimulus-driven activity. We found that the net impact of noradrenergic signaling in the AOB was a reduction in the stimulus-evoked MC activity and chemosensory discriminability. These results support the hypothesis that NE sculpts MC activity via inhibition, but the nonuniformity of MC responses to NE may help to reconcile seemingly contradictory models of pheromone learning.
MATERIALS AND METHODS
Animals.
All procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. Mouse urine and feces were collected from male and female BALB/c mice aged 6–12 wk. All electrophysiological experiments were conducted on C57Bl/6J and B6D2F1 male mice aged 6–10 wk.
Urine and feces collection and chemosignal extraction.
Male and female urine and feces were collected from 20 female and 10 male BALB/c mice over ~2 mo. Mice were suspended in a wire-bottomed cage over liquid nitrogen for 3–8 h per day. At the end of collection, frozen urine and feces were collected and stored at −80°C until extraction. Domestic cat urine was purchased from BioreclamationIVT (Westbury, NY). According to the vendor, cat urine was collected in a manner such that it came in contact with cat feces before collection.
Urine was processed as previously described (Meeks et al. 2010; Nodari et al. 2008). In brief, the urine was thawed, pooled, and centrifuged at 80 g for 2 min. The supernatant was removed and filtered through a 0.22-μm filter, aliquoted, and stored at −80°C. Freshly unfrozen mouse and cat urine were diluted 1:100 in Ringer saline before experiments.
Mouse fecal extracts were prepared as described previously (Doyle et al. 2016). Feces were diluted 1:10 in dH2O (wt/vol), homogenized, and left overnight at 4°C on an orbital shaker. The fecal slurry was then homogenized and centrifuged twice at 2,400 g for 10 min and at 2,800 g for 30 min. The supernatant was filtered through a 0.22-μm filter, aliquoted, and stored at −80°C. Freshly unfrozen feces extracts were diluted 1:300 in Ringer saline before experiments.
Reagents and solutions.
Ex vivo preparations were superfused with artificial cerebrospinal fluid (aCSF) containing (in mM) 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 3 myo-inositol, 2 sodium pyruvate, and 0.4 sodium ascorbate. For the duration of ex vivo experiments, the peripheral sensory epithelium was continuously perfused with a solution of Ringer saline containing (in mM) 115 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 25 NaHCO3, 10 HEPES, and 10 glucose. All stimuli were diluted into this Ringer saline.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Corticosterone 21-sulfate (Q1570), hydrocortisone 21-sulfate (Q3910), epitestosterone sulfate (A6940), testosterone sulfate (A7010), and 17α-estradiol sulfate (E0893) were purchased from Steraloids (Newport, RI). Q1570 was prepared in water, whereas all other sulfated sterols were dissolved in methanol to a concentration of 20 mM. Steroids were diluted to 10 μM in Ringer immediately before each experiment. Sulfated androgens and estrogens were omitted from some batteries.
Pharmacology.
NE (Abcam, Cambridge, MA) was prepared in water to a concentration of 10 mM and diluted to a concentration of 10 μM in aCSF for experiments. The rate of superfusion was 6–10 ml/min, and the aCSF stream was aimed directly at the AOB surface. This physical setup encourages convective exchange at the AOB surface and was previously shown to allow for the pharmacological disruption of GABAergic signaling at drug concentrations comparable to those used in brain slice experiments (Meeks and Holy 2009). These ex vivo conditions are similar, if not improved in comparison, to in vivo pharmacological experiments that were effective at disrupting NE signaling in the olfactory bulb (Eckmeier and Shea 2014). After completion of 3 trials of the stimulus battery to establish baseline responses, NE was washed in for 10 min without stimulus delivery. The 10-min wash-in is similar to the time courses utilized in brain slices and ex vivo preparations (Araneda and Firestein 2006; Meeks and Holy 2009; Smith et al. 2009). After the wash-in period, a second stimulus battery was delivered to the VNO that contained the same stimuli in a different order.
Because these experiments lasted up to 90 min, it was important to control for potential run-down (or run-up) of spontaneous activity and/or stimulus responses over time. Control recordings were performed using the exact same wash-in procedures used to deliver NE to the tissue, but NE was omitted from the superfusing aCSF. Statistical tests of NE effects were compared with values for these controls (see Data analysis and statistics).
Bath application of NE could theoretically lead to unintended activation of adrenergic receptors in the VNO. This is unlikely, however, because our setup allows continuous, independent perfusion of the VNO with a drug-free Ringer saline solution such that 1–2 VNO volumes are exchanged per second, exiting the VNO into the bath (i.e., generating a constant stream of drug-free saline that starts inside the VNO and exits into the bath). Additionally, the VNO remains inside its outer bony capsule, which serves as an additional barrier for NE diffusion into the VNO from the bath. Combined, these aspects of the ex vivo approach prevent bath-applied pharmacological reagents from passively diffusing into the VNO.
Ex vivo preparations.
Ex vivo preparations were performed as previously described (Doyle et al. 2014; Meeks and Holy 2009). Mice were anesthetized with isoflurane and decapitated into ice-cold aCSF containing an additional 9 mM MgCl2 to limit excitotoxic damage. Half of the mouse skull from the snout to the olfactory bulbs was dissected out and adhered to a small plastic plank with Vetbond surgical glue (3M, St. Paul, MN). The plastic plank was inserted into custom-built dissection chamber and superfused with room temperature aCSF. A secondary microdissection was performed to expose the vomeronasal nerves and accessory olfactory bulb. A 0.0045-in. internal diameter polyimide cannula was placed into the VNO with a steady stream of fresh Ringer saline at a rate of ~0.2 ml/min, driven by a pressurized stimulus delivery system (Automate Scientific, Berkeley, CA). The cannula was subsequently used for the delivery of stimulus batteries to the VNO. Batteries consisted of stimuli delivered in a random, interleaved order produced by custom software written in MATLAB (The MathWorks, Natick, MA). Stimuli were delivered 3 times per battery for 3 s, with a 12-s wait period between stimuli.
Electrophysiology.
Extracellular recordings were made with glass electrodes with a resistance of 1.5–6 MΩ as previously described (Meeks et al. 2010; Meeks and Holy 2009). Electrodes were filled with aCSF and advanced into the AOB by a micromanipulator, with distances measured by a micrometer (Siskiyou Corporation, Grants Pass, OR). All recordings were made from neurons in the AOB external cellular layer (between 125 and 375 μm from the AOB surface) that produced large, positive-going spikes, consistent with MCs (Meeks et al. 2010; Meeks and Holy 2009). Signals were amplified through a Cornerstone BVC-700A amplifier (Dagan, Minneapolis, MN), converted to digital signals by a data acquisition card (National Instruments, Austin, TX), and saved with custom LabVIEW software. Recordings were sorted for single-unit activity in custom MATLAB programs per previously described reports (Meeks et al. 2010; Meeks and Holy 2009). Cells that responded to at least one stimulus and that had separable waveforms lasting throughout the entire baseline and NE application period and were considered for analysis.
Data analysis and statistics.
All data analysis was performed with custom MATLAB software. Electrophysiological responses to VNO stimulation were analyzed during a 3-s window. Because subtle differences in cannula placement and pneumatic pressure across experiments resulted in small shifts in the time delay between stimulus valve switching and the onset of neuronal activity in the AOB, we chose the onset of the 3-s averaging window on the basis of the spike timing statistics. Specifically, the window was chosen at the time point at which the instantaneous spike frequency (as measured by interspike intervals, or ISIs), increased beyond the 95% confidence interval expected from measurements of the cell’s spontaneous activity. Spontaneous activity for each cell was calculated by determining the spontaneous rate in a defined 3-s window before each stimulus onset. Statistical significance for spontaneous rate changes between baseline and test conditions was determined using Mann-Whitney-Wilcoxon tests.
The change in spike firing rate (ΔR) was calculated by subtracting the baseline firing rate from the average firing rate during the 3-s window. We restricted our analysis to strong and reproducible responses by setting our threshold for significance to include only those ΔR values that exceeded 2 Hz and were statistically significant (P < 0.05) compared with responses to control Ringer saline (unpaired, 2-tailed Student’s t-test). Cells that did not demonstrate responses exceeding these criteria were excluded from analysis (32/55 total cells). ΔR ratios were calculated by calculating the mean change in ΔR between the control and test conditions, and then dividing by the mean ΔR in the baseline condition. To avoid divide-by-zero errors in the ratio calculations, all ΔR values less than 1 Hz were replaced with a value of 1 Hz. Within-cell statistical significance was evaluated using paired Student’s t-tests. Across-population differences were evaluated using Mann-Whitney-Wilcoxon tests.
Stimulus response suppression and enhancement were categorized as either monotonic or nonmonotonic. Stimulus suppression was categorized as monotonic if the response to each stimulus repeat during a single battery was decreased compared with the previous repeat. Monotonic enhancement exhibited the reverse trend (activity to each stimulus repeat was increased compared with the previous stimulus). Stimulus responses were categorized as undergoing nonmonotonic suppression or enhancement if the responses did not undergo monotonic suppression or enhancement but did have significantly different average responses between the two batteries, as determined using an unpaired, two-tailed Student’s t-test. Per-cell modulation was categorized as being enhanced or suppressed if it experienced monotonic or nonmonotonic changes to any of the stimuli to which it responded. Any cell that showed enhancement to one stimulus and suppression to another was considered to have a mixed response.
The discriminability of a response to baseline firing was determined using a discriminability index (d′; Davison and Katz 2007; Gale and Perkel 2010). The sensitivity index was calculated using the following formula:
Where ΔRs represents the mean firing response to a given stimulus and ΔRc represents the mean firing response to the paired Ringer control. σs2 and σc2 are the across-trial variances of ΔRs and ΔRc, respectively.
A shuffle test was used to determine whether response enhancement or suppression was statistically different than expected from controls (in which cells experienced the same procedures as in NE conditions, but no NE was applied; see Pharmacology). For this test, the percentage of cells or stimulus responses showing enhancement, suppression, etc., was calculated from the control recordings. These control percentages were used as the basis for the generation of 100,000 simulated data sets (containing the same number of observations as our experimental data) in which each simulated cell was assigned the identity of enhanced, suppressed, or unchanged on the basis of the control percentages. Random assignment into each category was achieved using MATLAB’s uniform distribution random number generator (“rand”). These simulated data sets established a distribution of values for the amount of enhancement and suppression expected in the absence of NE. We calculated the statistical probability of observing increased levels of enhancement or suppression in NE conditions compared with controls using the following formula:
Where Nsim≥obs is the number of simulated sets in which the number of cells or stimuli showing suppression or enhancement exceeded observations, and Nsim is the total number of simulated sets (North et al. 2002). If the observations indicated a decrease in enhancement or suppression, we used the following formula:
Where Nsim≤obs is the number of simulated sets in which the number of cells or stimuli showing suppression or enhancement was less than observations. These results were effectively equivalent to those expected from a binomial distribution (MATLAB’s “binocdf” function). Shuffle test and binomial P values are reported for each comparison.
RESULTS
Studying neuromodulation and sensory tuning in the AOS ex vivo preparation.
A major limitation of slice-based electrophysiological studies of neuromodulation is a lack of capacity to investigate neuronal responses to naturalistic stimuli. The ex vivo preparation of the AOS retains the functional connections between the peripheral sensory neurons in the VNO and their downstream targets in the AOB. This preparation also avoids severing intrinsic fibers of the AOB, leaving it physically intact. In these experiments, chemosensory stimuli were delivered to the VNO through a thin cannula while extracellular single-unit electrophysiological recordings of AOB MCs were made (Fig. 1A). This reduced AOS preparation is uniquely suited for examining the effects of neuromodulators on stimulus tuning because it allows pharmacological agents to be applied via the rapidly circulating bath (Fig. 1A) while the periphery is stimulated with defined concentrations of chemosignals (e.g., pure sulfated steroids) and/or chemosignal blends (e.g., dilute mouse urine).
Fig. 1.
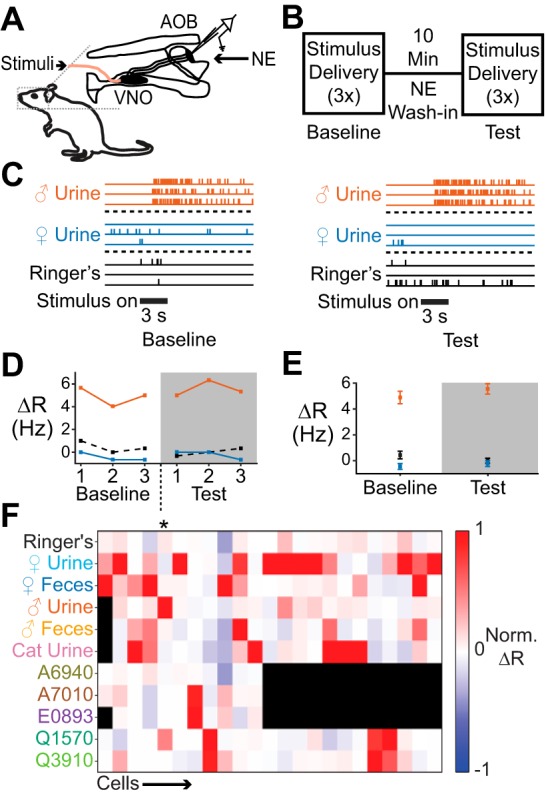
Studying stimulus tuning and modulation in the AOS ex vivo preparation. A: diagram of the ex vivo preparation of the mouse AOS. Stimuli were delivered to the VNO while single-unit electrophysiological activity was recorded in the AOB. B: experimental overview. Three rounds of randomized stimulus trials were conducted before (baseline) and during (test) NE application. C: raster plot of stimulus-driven single-unit responses in the AOB in baseline and test periods in a control experiment (not exposed to NE). Response magnitude and stimulus selectivity remained constant over time. D: changes in firing rate (ΔR) across stimulus responses for the same cell shown in C. Numbers on x-axis refer to the stimulus repeat within each stimulus battery. E: average ΔR per stimulus battery for the cell represented in C and D. Error bars reflect SE. F: heat map representation of stimulus responsiveness of 23 cells. Each row is a different stimulus; each column is a different cell. Black indicates stimuli that were not delivered to that particular cell. Asterisk indicates the cell shown in C–E. Norm. ΔR, normalized ΔR.
We delivered a panel of known AOS chemosignals to the VNO in three blocks of randomized, interleaved trials to the ex vivo preparation before (“baseline”) and during (“test”) 10 μM NE delivery to the AOB (Fig. 1B). These experiments required sustained isolation of spikes from a single neuron over relatively long time periods (~1 h per cell). We therefore performed parallel control experiments in which we repeated the exact same procedures but excluded NE from the “test” superfusate. This approach allowed us to compare the spontaneous and stimulus-evoked activity in AOB neurons that were exposed to NE to neurons that underwent the same recording procedures for the same length of time (n = 11 cells control; n = 12 cells NE).
As in previous studies, we encountered MCs demonstrating specific response profiles across stimuli (“tuning curves”) and the tuning of a given MC remained constant over the time course of these experiments (Fig. 1, C–E; Doyle et al. 2016; Meeks et al. 2010; Meeks and Holy 2009). MCs responded to chemosignal blends (e.g., dilute mouse urine and/or feces) and/or pure chemosignals (sulfated sterols) with a high degree of selectivity (Fig. 1F). The majority of cells responded to a single stimulus, with only a few cells in control and experimental groups responding to three or more stimuli. The across-trial reliability of the neuronal responses added confidence that this ex vivo approach would provide an appropriate platform in which to monitor the impacts of noradrenergic signaling on AOB function.
NE increases spontaneous activity and decreases response discrimination in some AOB neurons.
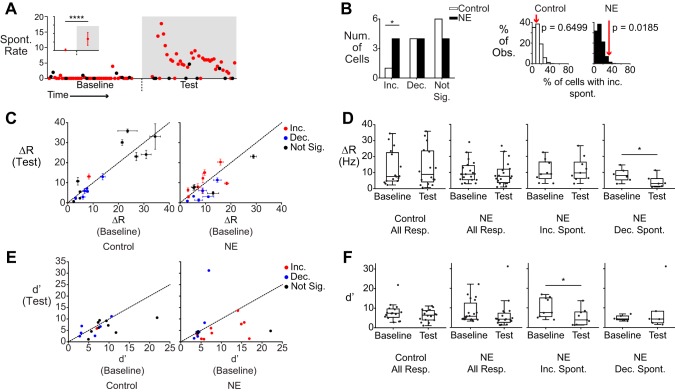
NE has been shown to increase the spontaneous activity of inhibitory interneurons in the AOB in vitro, leading to a widespread decrease in MC spontaneous activity (Araneda and Firestein 2006; Smith et al. 2009). In our experiments, we found that the population of NE-exposed cells exhibited more varied changes in spontaneous activity between the baseline and test period (Fig. 2A). Across the population, the control group showed a small but significant decrease in spontaneous activity during the test period compared with the baseline (baseline = 1.71 ± 0.13, test = 1.28 ± 0.13, means ± SE, P = 0.008, Mann-Whitney-Wilcoxon test). In contrast, NE-exposed cells showed a small but significant increase in spontaneous activity in the test period (baseline = 1.36 ± 0.16, test = 2.24 ± 0.20, P = 0.032, Mann-Whitney-Wilcoxon test). To evaluate the likelihood of observing a specific amount of spontaneous rate suppression or enhancement, we compared observed results with 1) the binomial distribution and 2) a shuffled population based on control recordings (see materials and methods).
Fig. 2.
Spontaneous activity is increased in a subpopulation of AOB neurons. A: scatter plot of spontaneous activity in an AOB neuron that showed increased spontaneous activity (Spont. Rate) in the NE period (****P = 1.25 × 10−16). Red circles indicate the unstimulated firing rate (measured just before a stimulus onset). Black circles indicate firing rate measurements that happened to follow strong stimulus responses (which may not fully recover to baseline before the subsequent trial). Inset shows the mean and SD of the measured spontaneous rate for this cell. B: the number of cells in control (n = 11) and NE-treated (n = 12) conditions that exhibited significantly changed or unchanged spontaneous activity (Num., number; Inc., increased; Dec., decreased; Not Sig., no change). There were significantly more cells that exhibited increased spontaneous activity (P = 0.0185 compared with a shuffled population). At right are histograms relating the observed prevalence (% Obs.) of spontaneous rate increases (red arrows) to shuffle test expectations (which are based on control recordings). C: scatter plot of stimulus-evoked ΔR, with changes in spontaneous activity indicated by color (blue, decreased; red, increased; black, no change). Error bars represent SE. D: box plots showing mean stimulus responses (Resp.) for control and NE-exposed cells (left 2 graphs), and breakdowns based on spontaneous activity changes (right 2 graphs). *P = 0.01 (paired Student’s t-test). E: scatter plots of the sensitivity index (d′) for control (n = 16) and NE-treated (n = 19) conditions. Colors reflect the same parameters as C. F: box plots showing d′ values for all stimuli in control and NE-treated conditions, broken down by categories as in D. *P = 0.0133 (paired Student’s t-test).
We observed a significantly higher incidence of increased spontaneous activity in NE conditions (Fig. 2B; P = 0.0185 shuffle test, P = 0.003 binomial test, n = 12). Importantly, many cells exposed to NE did not demonstrate any change in spontaneous activity (4/12 cells). A potential explanation for these results is that pharmacological agents in the superfusate may not uniformly penetrate the full depth of the AOB. This could result in recording depth-dependent differences in the effect of NE on spontaneous firing. To determine whether this was the case, we tested for correlations between recording depth and spontaneous firing rate changes. We found that spontaneous activity was not correlated with the depth of the recording electrode (r2 = 0.008, P = 0.787, n = 12 cells), indicating that variable effects of NE are not explained by variable NE penetration into the ex vivo preparation. These results indicate that NE does not produce uniform, unidirectional changes in spontaneous activity in all AOB MCs and that, as a whole, MC spontaneous activity is increased by exposure to NE.
NE has been hypothesized to promote suppression of stimulus-driven activity in addition to any effects on spontaneous activity (Brennan 2009). Across the population, ΔR responses in baseline and test periods were consistent in both control and NE conditions (Fig. 2C). However, when grouped on the basis of the cell’s change in spontaneous firing rate, we found that cells with decreased spontaneous activity in NE conditions showed decreased stimulus-evoked ΔR compared with baseline (Fig. 2, C and D; 6/6 responses, P = 0.01, paired Student’s t-test). The same was not seen for cells with increased spontaneous activity in NE conditions (1 of 9 responses).
The increased baseline activity of some MCs in NE conditions may result in a paradoxical decrease in these cells’ ability to discriminate cues from background. We therefore calculated the discriminability index (d′) for stimulus responses in control and NE conditions (comparing across-trial stimulus responses with control Ringer stimulation). In the absence of NE, the d′ statistic remained unchanged between the baseline and test period, even in cells that underwent spontaneous rate decreases (Fig. 2E). In contrast, NE-exposed cells that showed enhanced spontaneous activity showed a significant decrease in d′ (Fig. 2F; P = 0.0133, paired, 2-tailed Student’s t-test, n = 9 responses, n = 4 cells). These data indicate that NE-associated increases in spontaneous activity result in decreased chemosignal discriminability.
Most AOB neurons do not respond to NE with immediate response suppression.
In AOB slices, NE has been reported to promote global suppression of MC activity upon NE wash-in (Araneda and Firestein 2006). Given the heterogeneity of spontaneous and stimulus-driven activity during NE application in the ex vivo preparations, we further investigated AOB neuronal responses to stimuli during NE application (Figs. 3 and 4). As noted above, our experimental design allowed us to evaluate the responsiveness of AOB neurons to the same stimuli across repeated trials. Across the population, we found that the changes in the average ΔR between baseline and test conditions were not significantly different (P = 0.5848, Mann-Whitney-Wilcoxon test) between control and NE-exposed responses (0.0233 ± 0.5749 for control, −0.0806 ± 0.5104 for NE, means ± SD). We investigated the impact of measured recording depth on evoked changes, finding a trend toward decreased ΔR ratio (i.e., increased inhibition by NE) correlation between cell depth and the degree of suppression (r2 = 0.204, P = 0.0524, n = 19 responses). This effect is small but indicates that NE penetration into the external cellular layer of the AOB in the ex vivo preparation is effective and may indicate that deeply situated MCs are more likely to undergo response suppression in the presence of NE. The lack of a strong enhancing or suppressing effect of NE was initially surprising given the previous evidence of MC and IGC modulation by NE (Araneda and Firestein 2006; Smith et al. 2009). When we inspected the NE-associated responses more closely, we observed two forms of apparent NE-associated changes: one that was immediate and nonmonotonic, and another that was gradual and monotonic.
Fig. 3.
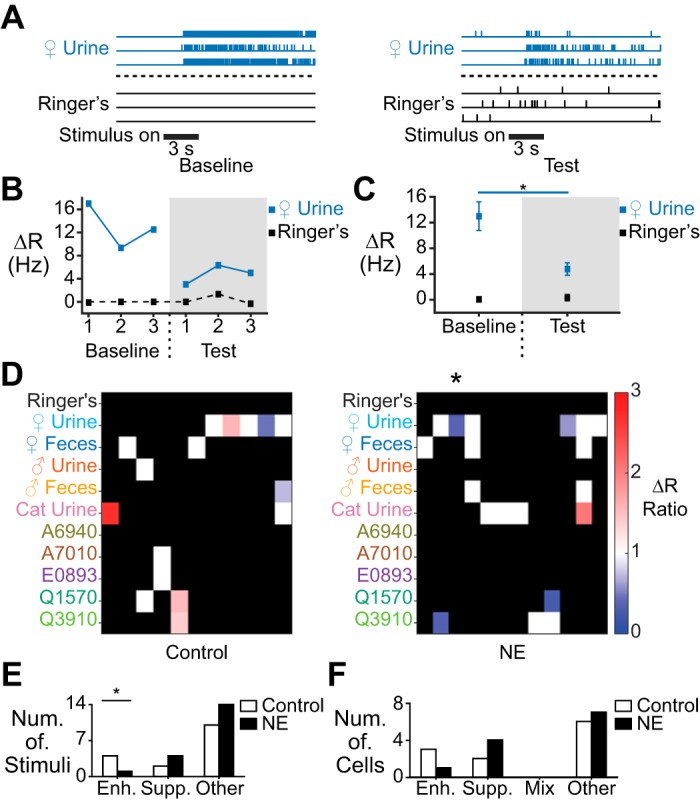
NE elicits immediate, nonmonotonic suppression in a small fraction of AOB neurons. A: raster plot showing stimulus-evoked spiking responses in a cell demonstrating immediate, nonmonotonic response suppression in the presence of NE. B: per-trial ΔR values for the cell shown in A. C: average ΔR for the cell in A and B. *P = 0.027 (unpaired, 2-tailed Student’s t-test). D: heat map of average stimulus responses in control cells (left; n = 11) and NE-exposed cells (right, n = 12). Nonmonotonic enhancement is shown in red and suppression in blue. White pixels indicate no change, and black indicates that the cell did not respond to the stimulus in either the baseline or test period. Asterisk marks the column showing the response of the cell represented in A–C. E: per-stimulus counts of significant nonmonotonic enhancement (Enh.), suppression (Supp.), or no change (n = 16 for control, n = 19 for NE). The number of responses exhibiting nonmonotonic enhancement was significantly decreased during NE exposure (*P = 0.032 shuffle test; P = 0.03 binomial test). “Other” indicates responses that did not show nonmonotonic changes. F: per-cell count of enhancement, suppression, or a mix of both (n = 11 cells for control, n = 12 for NE). “Other” indicates cells that did not demonstrate any nonmonotonic changes.
Fig. 4.
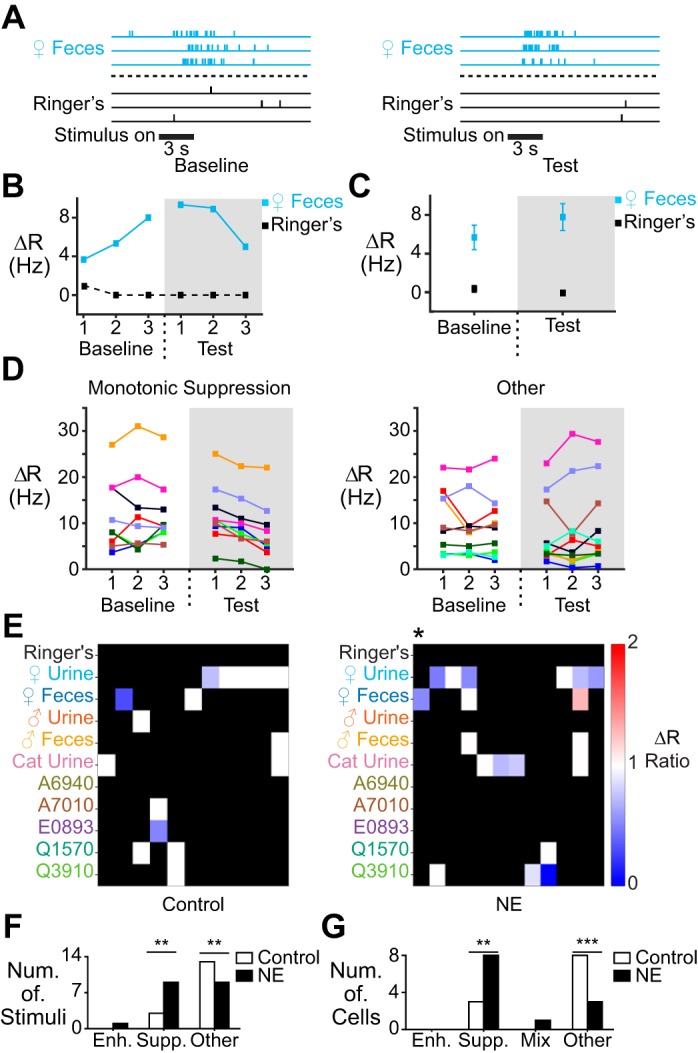
NE-associated monotonic response suppression. A: raster plot showing stimulus-evoked spiking responses in a cell demonstrating monotonic suppression in the presence of NE. B: per-trial ΔR values for the cell shown in A. C: average ΔR for the cell in A and B. D: across-trial ΔR values for responses that either exhibited monotonic suppression during NE delivery (left; n = 9) or did not (right; n = 10). E: heat map of stimulus responses in control (left; n = 11) and NE-exposed cells (right; n = 12) that exhibited monotonic enhancement (red), suppression (blue), or neither (white). Hues indicate the net difference in ΔR at the third (final) stimulus delivery in test period compared with final stimulus delivery in the baseline period. F: per-stimulus counts of monotonic enhancement, suppression, or no monotonic change in control and NE-exposed conditions. The number of responses exhibiting monotonic suppression was significantly increased (**P = 0.004 shuffle test; P = 0.0009 binomial test), whereas the number of unchanged responses was significantly decreased during NE exposure (**P = 0.001 shuffle test; P = 0.0009 binomial test; n = 16 for control, n = 19 for NE). G: per-cell counts of monotonic enhancement or suppression. There were significantly more cells with a suppressed response (**P = 0.005 shuffle test; P = 0.0008 binomial test) and significantly fewer cells with no significant changes (**P = 0.0008 shuffle test; P = 0.0008 binomial test; n = 11 for control, n = 12 for NE).
We defined a nonmonotonic change as a stimulus-driven response that showed a statistically significant difference in the ΔR value in the test period compared with the baseline period without a monotonic change across the three repeated stimulus trials (see materials and methods; Fig. 3, A–D). Nonmonotonic changes in ΔR occurred in both control and NE-exposed groups (Fig. 3D). On a per-stimulus basis, nonmonotonic ΔR suppression was not statistically significant compared with shuffle test expectations (P = 0.2078; Fig. 3E). The same lack of effect was also seen on a per-cell basis (P = 0.1611; Fig. 3F). We did observe a slightly lower number of stimulus responses that underwent nonmonotonic ΔR enhancement in NE conditions (P = 0.0317 shuffle test, P = 0.03 binomial test; Fig. 3E), but this effect was modest. Overall, the data indicate that NE application does not uniformly induce immediate/nonmonotonic stimulus suppression as might have been expected on the basis of previous slice studies (Araneda and Firestein 2006; Smith et al. 2009).
Many AOB neurons respond to NE with gradual, monotonic stimulus suppression.
Monotonic response suppression has been observed in brain regions other than the AOB (Devilbiss and Waterhouse 2004). In this form of suppression, stimulus-driven activity decreases with each delivery of a stimulus (Fig. 4, A–D). Unlike nonmonotonic suppression, monotonic suppression is cumulative and experience dependent, and may be especially relevant to neuronal plasticity that develops over longer and/or more sustained exposure to environmental chemosignals. A caveat to this form of suppression is that the gradual reduction in response magnitudes may not always produce a statistically significant across-trial change in ΔR over the time course of these experiments (Fig. 4C).
Monotonic suppression occurred rarely in control cells (3/16 responses) but was more prevalent in NE-treated cells (9/19 responses; P = 0.0041 shuffle test; P = 0.009 binomial test; Fig. 4, D–F). This increase in monotonic suppression was also significant when evaluated on a per-cell basis (8/12 cells; P = 0.005 shuffle test, P = 0.008 binomial test; Fig. 4G). In contrast to the relatively mild effects of NE exposure on immediate, nonmonotonic suppression, these analyses revealed that many AOB neurons undergo gradual, experience-dependent response suppression in the presence of NE.
To assess the cumulative influence of NE on nonmonotonic and monotonic suppression, we applied a binary classification to each stimulus response in control and NE-exposed conditions (Fig. 5). Use of this binary classification strategy allowed us to include monotonic suppression in our analysis, which, as mentioned previously, was not always mirrored by a strong change in the average ΔR (Fig. 4, A–C). On both a per-stimulus and a per-cell basis, we found a significant increase in the amount of combined suppression during NE exposure (P = 0.00075 shuffle test, P = 0.0002 binomial test per stimulus; P = 0.0083 shuffle test, P = 0.001 binomial test per cell; Fig. 5, B and C). The increased overall suppression was paralleled by a decrease in the number of unchanged responses (P = 0.0355 shuffle test, P = 0.04 binomial test). It was noteworthy that the responses to many stimuli remained unchanged in the presence of NE (Fig. 5B), further evidence that NE does not uniformly suppress AOB neuronal responses to chemosensory stimuli.
Fig. 5.
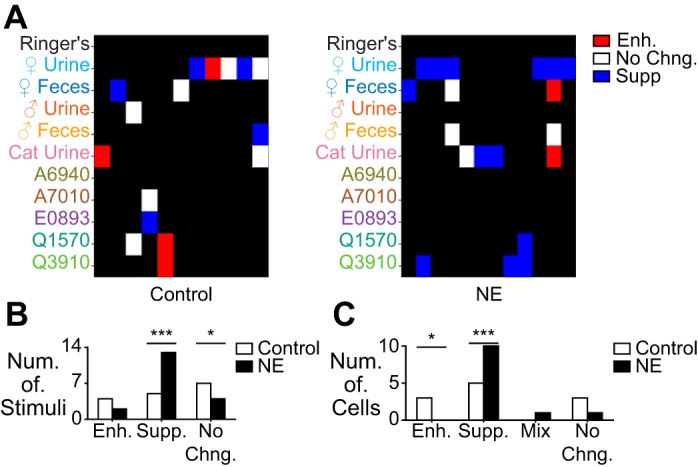
Combined nonmonotonic and monotonic suppression during NE exposure. A: heat map of stimulus responses that showed monotonic or nonmonotonic suppression (blue), enhancement (red), or no change (No Chng.) in control (left; n = 11) or NE-exposed cells (right; n = 12). B: per-stimulus counts of suppression, enhancement, or no change. The overall number of suppressed stimuli was significantly increased (***P = 0.0009 shuffle test; P = 0.0002 binomial test), and the number of unchanged responses was significantly decreased (*P = 0.035 shuffle test; P = 0.04 binomial test; n = 19 for NE, n = 16 for control). C: per-cell counts of enhancement, suppression, a mixture of enhancement and suppression, or no change. The number of cells with enhanced responses was significantly decreased (*P = 0.021 shuffle test; P = 0.02 binomial test), and the number with suppressed responses was significantly increased (***P = 0.008 shuffle test; P = 0.001 binomial tests).
DISCUSSION
Studying the effects of NE on the AOB in ex vivo preparations has several advantages over both acute slice and in vivo approaches. Principally, the ex vivo preparation allows one to study the responses of AOB neurons to naturalistic chemosensory stimulation of the VNO, which is impossible in acute slices. Moreover, with the ex vivo approach one can precisely control the concentration of delivered chemosignals and the timing of stimulus delivery. This is also possible using anesthetized in vivo preparations, but the impacts of systemic anesthetics on GABAergic signaling (Ishizawa 2007) may interfere with the normal function of the reciprocal synapses between AOB MCs and interneurons. A final advantage of this preparation is that it directly exposes the AOB, a structure that is only 800 µm deep, to rapid bath superfusion and pharmacological administration. A noteworthy limitation of this approach, however, is that the experimental dissection severs centrifugal fibers, including noradrenergic fibers, and in doing so may alter basal neuromodulatory tone. Another caveat is that our extracellular single-unit electrophysiological recordings, which isolate MCs on the basis of electrode depth and extracellular spike waveform (Hendrickson et al. 2008; Meeks and Holy 2009; Meeks et al. 2010), do not exclude the possibility that some of the recorded neurons were from cells other than MCs (e.g., external granule cells; Larriva-Sahd 2008). On balance, we have found that for many experiments, the benefits outweigh the limitations and open new routes to studying unanswered questions about AOB circuit function.
In the MOB, NE-associated increases in GABA lead to a decrease in spontaneous MC activity and enhanced signal-to-noise ratios for detected odorants (Linster and Fontanini 2014; Linster et al. 2011). In the AOB, some studies have indicated that NE causes disinhibition of MCs, which may contribute to potentiation of MC activity (Dong et al. 2009). Others, however, have found that NE application increases GABAergic tone, leading to a general suppression of MC activity (Araneda and Firestein 2006; Smith et al. 2009). In ex vivo preparations, we found that spontaneous activity is slightly increased in the AOB. A similar increase in spontaneous MC activity was observed after artificial mating, but it was not clear whether this effect was dependent on noradrenergic signaling (Otsuka et al. 2001). Importantly, we found that the subpopulation of MCs that experience spontaneous increases in firing rate also show a diminished capacity to discriminate stimuli from background. Overall, these data indicate that the effects of NE on spontaneous AOB neuronal activity are subtle and nonuniform, and result in a net decrease in the signal-to-noise ratio for detected chemosignals.
One hypothesis of NE-associated pheromonal memory formation suggests that NE promotes selective suppression of stimuli that are encountered during the period of NE release (Araneda and Firestein 2006; Brennan 2009; Brennan and Binns 2005). Consistent with this hypothesis, we found increased suppression of many stimuli that were delivered during NE application. However, this suppression is heterogeneous and does not occur with all stimuli. Many single cells that responded to several stimuli in our panel showed NE-associated suppression of some stimuli, whereas others were unchanged. This apparent stimulus specificity was unexpected.
The stimulus specificity of NE response suppression may indicate that some MC-GC synapses are primed for NE-associated suppression, but others are not. The mechanisms of such a phenomenon are unclear. MC-GC reciprocal synapses are located along the multiple primary dendrites of AOB MCs, and AOB MCs integrate across chemosignal classes by innervating multiple glomeruli (Kahan and Ben-Shaul 2016; Meeks et al. 2010; Tolokh et al. 2013; Wagner et al. 2006). Heterogeneous NE-mediated suppression may result from differential inhibition of MC dendrites by internal or external granule cells or may even occur at the level of glomerular inputs, as has been reported in the MOB (Eckmeier and Shea 2014). Since adrenergic receptors are localized to the external cellular layer and internal cellular layer of the AOB (Domyancic and Morilak 1997; Rosin et al. 1996; Talley et al. 1996), it seems most likely that IGCs or the more recently described external granule cells (Larriva-Sahd 2008) are the prime targets of noradrenergic signaling.
The lack of suppression of some stimulus responses during NE exposure also raises questions related to the potential biological implications of this resistance to suppression. The persistence of NE-independent stimulus responses may be important for sustaining the capacity to detect chemosignals containing important information that should not be suppressed by an LC-engaging experience. For example, it might be disadvantageous to suppress MC activation by chemosensory cues that are unrelated to mating (e.g., predator-associated cues) that happen to be detected while NE is present in the AOB. A broader inquiry into the relationship between stimulus identity and the capacity for NE-mediated suppression is needed to determine whether this is the case.
In addition to stimulus-specific suppression, we also found that NE promoted distinct modes of inhibition in the AOB. The most prominent form of NE-mediated suppression was a gradual, monotonic decrease in response magnitude over repeated trials of the same stimulus. This effect has been reported in cortical regions (Devilbiss and Waterhouse 2004) but has not been reported in the AOB. Monotonic suppression is an activity-dependent process that blunts the effects of chemosensory stimuli over time and/or repeated exposure. The slow accumulation of stimulus-dependent suppression seems to be a strong match for the slow actions of chemosignals on the AOB. The process of dissolving environmental chemosignals in nasal/vomeronasal mucus and pumping them actively into the VNO takes many seconds, and MC responses to brief chemosensory encounters can last many seconds to minutes (Luo et al. 2003). More studies will be needed to determine whether this gradual, monotonic form of suppression is seen in vivo and, if so, whether it is associated with experience-dependent forms of AOB plasticity and pheromonal learning.
In summary, our data indicate that NE is not a simple suppressive gate for chemosensory information flowing through the AOB. Instead, NE has nuanced, heterogeneous effects that include changes in both spontaneous and stimulus-driven activity. Future experiments are necessary to tease apart the distinct mechanisms, but our findings suggest that specific MCs, and perhaps specific reciprocal synapses, respond differently to noradrenergic modulation.
GRANTS
This work was partially supported by National Institutes of Health Grants T32NS069562 (W. I. Doyle), T32GM007062 (W. I. Doyle), R00DC01170 (J. P. Meeks), and R01DC015784 (J. P. Meeks).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.I.D. and J.P.M. conceived and designed research; W.I.D. and J.P.M. performed experiments; W.I.D. and J.P.M. analyzed data; W.I.D. and J.P.M. interpreted results of experiments; W.I.D. and J.P.M. prepared figures; W.I.D. and J.P.M. drafted manuscript; W.I.D. and J.P.M. edited and revised manuscript; W.I.D. and J.P.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Salma Ferdous for assistance in preparing solutions and all members of the Meeks laboratory for helpful comments and critiques.
REFERENCES
- Araneda RC, Firestein S. Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J Neurosci 26: 3292–3298, 2006. doi: 10.1523/JNEUROSCI.4768-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Huguenard JR, Prince DA. Adrenergic modulation of GABAA receptor-mediated inhibition in rat sensorimotor cortex. J Neurophysiol 79: 937–946, 1998. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur J Neurosci 16: 2371–2382, 2002. doi: 10.1046/j.1460-9568.2002.02413.x. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28: 574–582, 2005. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science 250: 1223–1226, 1990. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Brennan PA. Outstanding issues surrounding vomeronasal mechanisms of pregnancy block and individual recognition in mice. Behav Brain Res 200: 287–294, 2009. doi: 10.1016/j.bbr.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Binns EK. Vomeronasal mechanisms of mate recognition in mice. Chem Senses 30, Suppl 1: i148–i149, 2005. doi: 10.1093/chemse/bjh157. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM, Keverne EB. Neurotransmitter release in the accessory olfactory bulb during and after the formation of an olfactory memory in mice. Neuroscience 69: 1075–1086, 1995. doi: 10.1016/0306-4522(95)00309-7. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol 51: 457–481, 1997. doi: 10.1016/S0301-0082(96)00069-X. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature 184: 105, 1959. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature 450: 899–902, 2007. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Davison IG, Katz LC. Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J Neurosci 27: 2091–2101, 2007. doi: 10.1523/JNEUROSCI.3779-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci 24: 10773–10785, 2004. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of α1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol 386: 358–378, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Dong C, Godwin DW, Brennan PA, Hegde AN. Protein kinase Cα mediates a novel form of plasticity in the accessory olfactory bulb. Neuroscience 163: 811–824, 2009. doi: 10.1016/j.neuroscience.2009.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WI, Dinser JA, Cansler HL, Zhang X, Dinh DD, Browder NS, Riddington IM, Meeks JP. Faecal bile acids are natural ligands of the mouse accessory olfactory system. Nat Commun 7: 11936, 2016. doi: 10.1038/ncomms11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WI, Hammen GF, Meeks JP. Ex vivo preparations of the intact vomeronasal organ and accessory olfactory bulb. J Vis Exp: e51813, 2014. doi: 10.3791/51813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmeier D, Shea SD. Noradrenergic plasticity of olfactory sensory neuron inputs to the main olfactory bulb. J Neurosci 34: 15234–15243, 2014. doi: 10.1523/JNEUROSCI.0551-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol 180: 533–544, 1978. doi: 10.1002/cne.901800309. [DOI] [PubMed] [Google Scholar]
- Fang LY, Quan RD, Kaba H. Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci Lett 438: 133–137, 2008. doi: 10.1016/j.neulet.2007.12.070. [DOI] [PubMed] [Google Scholar]
- Fu X, Yan Y, Xu PS, Geerlof-Vidavsky I, Chong W, Gross ML, Holy TE. A molecular code for identity in the vomeronasal system. Cell 163: 313–323, 2015. doi: 10.1016/j.cell.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J Neurosci 30: 1027–1037, 2010. doi: 10.1523/JNEUROSCI.3585-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. eLife 3: e03025, 2014. doi: 10.7554/eLife.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Osakada T, Matsumoto A, Matsuo N, Haga-Yamanaka S, Nishida T, Mori Y, Mogi K, Touhara K, Kikusui T. Self-exposure to the male pheromone ESP1 enhances male aggressiveness in mice. Curr Biol 26: 1229–1234, 2016. doi: 10.1016/j.cub.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol 86: 2173–2182, 2001. [DOI] [PubMed] [Google Scholar]
- Hendrickson RC, Krauthamer S, Essenberg JM, Holy TE. Inhibition shapes sex selectivity in the mouse accessory olfactory bulb. J Neurosci 19: 12523–12534, 2008. doi: 10.1523/JNEUROSCI.2715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26: 4426–4436, 2006. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa Y. Mechanisms of anesthetic actions and the brain. J Anesth 21: 187–199, 2007. doi: 10.1007/s00540-006-0482-x. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature 297: 227–229, 1982. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Jia C, Chen WR, Shepherd GM. Synaptic organization and neurotransmitters in the rat accessory olfactory bulb. J Neurophysiol 81: 345–355, 1999. [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci 16: 6319–6329, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaba H, Keverne EB. The effect of microinfusions of drugs into the accessory olfactory bulb on the olfactory block to pregnancy. Neuroscience 25: 1007–1011, 1988. doi: 10.1016/0306-4522(88)90053-X. [DOI] [PubMed] [Google Scholar]
- Kahan A, Ben-Shaul Y. Extracting behaviorally relevant traits from natural stimuli: benefits of combinatorial representations at the accessory olfactory bulb. PLOS Comput Biol 12: e1004798, 2016. doi: 10.1371/journal.pcbi.1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AW, Ackels T, Kuo TH, Cichy A, Dey S, Hays C, Kateri M, Logan DW, Marton TF, Spehr M, Stowers L. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell 157: 676–688, 2014. doi: 10.1016/j.cell.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature 437: 898–901, 2005. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. The accessory olfactory bulb in the adult rat: a cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J Comp Neurol 510: 309–350, 2008. doi: 10.1002/cne.21790. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmayer P, S PC, Maul-Pavicic A, Jäger M, Li XH, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306: 1033–1037, 2004. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Leszkowicz E, Khan S, Ng S, Ved N, Swallow DL, Brennan PA. Noradrenaline-induced enhancement of oscillatory local field potentials in the mouse accessory olfactory bulb does not depend on disinhibition of mitral cells. Eur J Neurosci 35: 1433–1445, 2012. doi: 10.1111/j.1460-9568.2012.08070.x. [DOI] [PubMed] [Google Scholar]
- Liberles SD. Mammalian pheromones. Annu Rev Physiol 76: 151–175, 2014. doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Fontanini A. Functional neuromodulation of chemosensation in vertebrates. Curr Opin Neurobiol 29: 82–87, 2014. doi: 10.1016/j.conb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Nai Q, Ennis M. Nonlinear effects of noradrenergic modulation of olfactory bulb function in adult rodents. J Neurophysiol 105: 1432–1443, 2011. doi: 10.1152/jn.00960.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science 299: 1196–1201, 2003. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Kaba H, Moriya K, Yoshida-Matsuoka J, Costanzo RM, Norita M, Ichikawa M. Remodeling of reciprocal synapses associated with persistence of long-term memory. Eur J Neurosci 19: 1668–1672, 2004. doi: 10.1111/j.1460-9568.2004.03271.x. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol 285: 339–349, 1989. doi: 10.1002/cne.902850305. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Arnson HA, Holy TE. Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci 13: 723–730, 2010. doi: 10.1038/nn.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Holy TE. An ex vivo preparation of the intact mouse vomeronasal organ and accessory olfactory bulb. J Neurosci Methods 177: 440–447, 2009. doi: 10.1016/j.jneumeth.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Hayar A, Linster C, Ennis M. Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J Neurophysiol 101: 2472–2484, 2009. doi: 10.1152/jn.91187.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci 28: 6407–6418, 2008. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BV, Curtis D, Sham PC. A note on the calculation of empirical P values from Monte Carlo procedures. Am J Hum Genet 71: 439–441, 2002. doi: 10.1086/341527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Ishii K, Osako Y, Okutani F, Taniguchi M, Oka T, Kaba H. Modulation of dendrodendritic interactions and mitral cell excitability in the mouse accessory olfactory bulb by vaginocervical stimulation. Eur J Neurosci 13: 1833–1838, 2001. doi: 10.1046/j.0953-816x.2001.01557.x. [DOI] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell 141: 692–703, 2010. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W, Shepherd GM, Reese TS, Brightman MW. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol 14: 44–56, 1966. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol 8: 75, 2010. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of α2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol 372: 135–165, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Rosser AE, Keverne EB. The importance of central noradrenergic neurones in the formation of an olfactory memory in the prevention of pregnancy block. Neuroscience 15: 1141–1147, 1985. doi: 10.1016/0306-4522(85)90258-1. [DOI] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci 28: 10711–10719, 2008. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Araneda RC. Cholinergic modulation of neuronal excitability in the accessory olfactory bulb. J Neurophysiol 104: 2963–2974, 2010. doi: 10.1152/jn.00446.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Hu R, DeSouza A, Eberly CL, Krahe K, Chan W, Araneda RC. Differential Muscarinic Modulation in the Olfactory Bulb. J Neurosci 35: 10773–10785, 2015. doi: 10.1523/JNEUROSCI.0099-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Weitz CJ, Araneda RC. Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb. J Neurophysiol 102: 1103–1114, 2009. doi: 10.1152/jn.91093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol 372: 111–134, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Kaba H. Properties of reciprocal synapses in the mouse accessory olfactory bulb. Neuroscience 108: 365–370, 2001. doi: 10.1016/S0306-4522(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Tolokh II, Fu X, Holy TE. Reliable sex and strain discrimination in the mouse vomeronasal organ and accessory olfactory bulb. J Neurosci 33: 13903–13913, 2013. doi: 10.1523/JNEUROSCI.0037-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci 12: 3985–3991, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron 50: 697–709, 2006. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Zimnik NC, Treadway T, Smith RS, Araneda RC. α1A-Adrenergic regulation of inhibition in the olfactory bulb. J Physiol 591: 1631–1643, 2013. doi: 10.1113/jphysiol.2012.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]