Abstract
Purpose
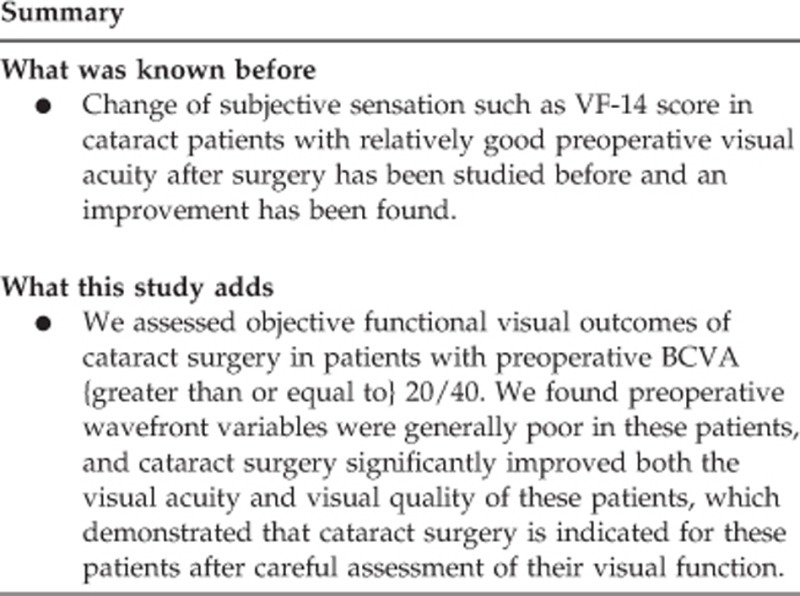
To explore the objective functional visual outcomes of cataract surgery in patients with good preoperative visual acuity.
Methods
We enrolled 130 cataract patients whose best-corrected visual acuity (BCVA) was 20/40 or better preoperatively. Objective visual functions were evaluated with a KR-1W analyzer before and at 1 month after cataract surgery.
Results
The nuclear (N), cortical (C), and N+C groups had very high preoperative ocular and internal total high-order aberrations (HOAs), coma, and abnormal spherical aberrations. At 1 month after cataract surgery, in addition to the remarkable increase of both uncorrected visual acuity and BCVA, both ocular and internal HOAs in the three groups decreased significantly after cataract surgery (all P<0.05). Point spread function and modulation transfer functions were also improved significantly in these patients (all P<0.05).
Conclusions
The objective functional vision of patients with 20/40 or better preoperative BCVA improved significantly after cataract surgery. This finding shows that the arbitrary threshold of BCVA worse than 20/40 in China cannot always be used to determine who will benefit from cataract surgery.
Introduction
Recent advances in phacoemulsification have greatly improved its risk-to-benefit ratio, allowing surgeons to offer cataract surgery to patients with a low risk of complications. However, the optimal timing of cataract surgery is still unknown. Several groups have developed criteria for cataract surgery. In particular, the Preferred Practice Guideline of the American Academy of Ophthalmology1 and the RAND Appropriateness Methodology2 both emphasize the importance of assessing visual function when determining the need for cataract surgery, while other guidelines were based on expert advice.3, 4
In China, a widely accepted criterion for cataract surgery is that the best-corrected visual acuity (BCVA) of the patient is lower than 20/40.5 However, there are always a proportion of cataract patients who have good preoperative visual acuity but complain of poor vision. Therefore, visual acuity seems to provide an inadequate assessment of visual function in these patients.
Wavefront analysis has several advantages in providing greater details on high-order aberrations (HOAs) that might cause significant visual disturbances, such as coma and spherical aberration.6 Previously, cataract surgeons evaluated visual quality mainly through contrast sensitivity and glare disability, which are now thought to be inadequate for these patients,7, 8, 9, 10 assessment of wavefront data may serve as a good alternative to these conventional tests in patients with good preoperative visual acuity.
As most internal aberrations of the eye arise from the crystalline lens, aberrometers that can directly measure the internal aberrations allow surgeons to evaluate changes in the internal optical quality of the eye after replacing the cloudy lens with an intraocular lens (IOL).11 Several new aberrometers (e.g. KR-1W, iTrace, and OQASII) can calculate internal aberrations of the eye directly. Although these aberrometers use different measurement principles, they can provide useful information on causes of functional visual complaints in patients with good visual acuity and benefit of cataract surgery.
With KR-1W, we in the current study evaluated objective functional visual outcomes of cataract surgery in patients with BCVA≥20/40 preoperatively, aiming to determine whether these patients can still benefit from cataract surgery.
Materials and methods
The Institutional Review Board of the Eye and ENT Hospital of Fudan University approved this prospective study. This study was registered at www.clinicaltrials.gov (accession number NCT02182921). All procedures adhered to the tenets of the Declaration of Helsinki Principles and were conducted in accordance with the approved research protocol. Informed consent was obtained from each patient before enrollment.
Patients
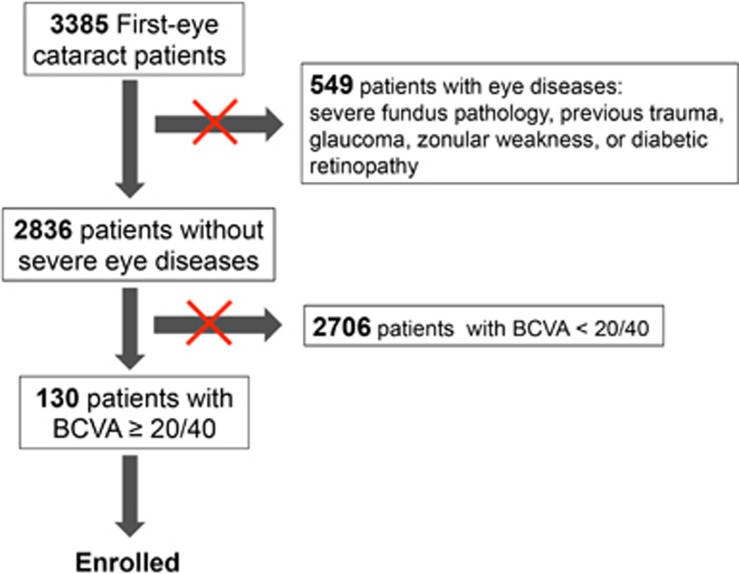
We recruited patients who underwent first-eye cataract surgery and whose preoperative BCVA was 20/40 or better in this study. Between September 2013 and March 2014, we performed first-eye cataract surgery in 3385 patients, of which 130 were eligible for this study and enrolled. Eyes with severe fundus pathology, previous trauma, glaucoma, zonular weakness, or diabetic retinopathy were excluded from this study. Figure 1 shows how our total 'first eye cataract patients' were reduced in number by the various exclusion criteria to the number enrolled in the study.
Figure 1.
The flowchart shows how our total 'first eye cataract patients' were reduced in number by the various exclusion criteria to the number enrolled in the study.
Preoperative examinations
Routine ophthalmic examinations, including assessment of visual acuity, refraction, tonometry, fundoscopy, B-scan ultrasonography, and measurement of axial length (AXL) were performed before surgery. UCVA (uncorrected visual acuity), BCVA, and the spherical equivalent (SE) were recorded. Then, the pupils were dilated using Mydrin P (Santen Pharmaceutical Co., Osaka, Japan). After mydriasis, standard anterior segment photographs were taken (Topcon, Tokyo, Japan), and the type and severity of lens opacification were assessed using the Lens Opacities Classification System, version III (LOCS III) by one experienced ophthalmologist (XJZ). Wavefront aberrometry was performed using a KR-1W analyzer (Topcon, Tokyo, Japan), which simultaneously measures the ocular, corneal, and internal HOAs.7, 12, 13 Wavefront aberration data were recorded at a pupil diameter of 6 mm. The following aberrations were recorded: coma; trefoil; spherical aberration; tetrafoil; secondary astigmatism; and 3rd order, 4th order, and total HOAs of Zernike polynomials, which were calculated as root mean square values. The aberrometer also yielded Strehl ratio values from the point spread function (PSF) and modulation transfer function (MTF) curves as indices of the eye's optical quality. The KR-1W analyzer could not perform wavefront analysis in posterior subcapsular cataract (PSC) patients because of central opacity.
Surgical technique
All the surgeries were carried out by one surgeon (Prof. YL). A 2.6 mm clear corneal incision was created after topical anesthesia with oxybuprocaine hydrochloride eye drops (Santen Pharmaceutical Co., Osaka, Japan), and was followed by continuous curvilinear capsulorhexis with a diameter of about 5.5 mm. Then, hydrodissection, chopping, and phacoemulsification were performed, followed by irrigation and aspiration of the cortex, and insertion of an aspheric IOL (ZCB00, Abbott Medical Optics, Santa Ana, CA, USA) into the capsular bag. After removing the viscoelastics, the corneal incisions were hydrated. No stitches were used in any patient. Cataract surgery was uneventful in all 130 patients. Starting 1day after surgery, the patients administered prednisolone acetate (Allergen Pharmaceutical Ireland, Westport, County Mayo, Ireland) and levofloxacin eye drops (Santen Pharmaceutical Co. Ltd., Osaka, Japan) four times per day for 2 weeks, and pranoprofen (Senju Pharmaceutical Co. Ltd., Osaka, Japan) four times per day for 1 month.
Postoperative evaluations
Slit lamp examination, assessment of visual acuity, refraction, tonometry, and fundoscopy were performed 1 month after surgery. The overall satisfaction after surgery was evaluated and recorded using a 10 point scale, ranging from 1=very bad to 10=excellent by our secretary who was independent of the surgical team. KR-1W wavefront aberrometry and anterior segment photography were repeated after mydriasis. Wavefront aberration data were also recorded at a pupil diameter of 6 mm.
Statistical analysis
All data are expressed as the mean±standard deviation. The χ2 test was used to determine differences in categorical variables. One-way analysis of variance (ANOVA) was used to analyze quantitative data, and Tukey test was used to compare the mean values between individual groups. Paired t-test was used to compare data before and after cataract surgery values of P<0.05 were regarded as statistically significant. All analyses were performed using SPSS software (version 11.0; SPSS Inc., Chicago, IL, USA).
Results
Patient baseline characteristics
Overall, there were 3.8% (130/3385) first-eye cataract patients whose preoperative BCVA≥20/40 during the study period. The average preoperative UCVA and BCVA were 0.43±0.16 logMAR and 0.24±0.07 logMAR, respectively. Six types of cataract were identified: nuclear cataract (N, 36.2%), cortical cataract (C, 27.7%), N+C (23.1%), PSC (6.1%), N+PSC (3.8%), and C+PSC (3.1%).
The characteristics of patients are presented in Table 1. Because of differences in the sample sizes of each group, statistical analyses were only performed in the N, C, and N+C groups. There were no statistically significant differences among the 3 groups in terms of age, BCVA (ANOVA followed by the Tukey test, all P>0.05), gender or eye(s) operated on (χ2 test, all P>0.05). However, preoperative UCVA was better in the C group than in the N group (ANOVA followed by the Tukey test, P=0.049). AXL was significantly longer and SE was significantly greater in the N group than in the other 2 groups (ANOVA followed by the Tukey test, all P<0.05).
Table 1. Baseline characteristics of the patients according to the type of cataract.
| Number(%) | N | C | N+C | PSC | N+PSC | C+PSC |
|---|---|---|---|---|---|---|
| 47 (36.2%) | 36 (27.7%) | 30 (23.1%) | 8 (6.1%) | 5 (3.8%) | 4 (3.1%) | |
| Age (years) | 66.60±11.49 | 66.06±8.13 | 68.53±9.68 | 62.00±11.07 | 63.00±7.94 | 67.00±3.56 |
| Gender (male/female) | 22/25 | 14/22 | 14/16 | 4/4 | 3/2 | 1/3 |
| Operated Eye (right/left) | 21/26 | 22/14 | 14/16 | 5/3 | 2/3 | 1/3 |
| AXL (mm) | 25.06±2.34 | 23.51±1.50a | 23.99±2.34a | 23.37±0.49 | 25.61±2.46 | 23.29±0.64 |
| UCVA (logMAR) | 0.48±0.25 | 0.38±0.13a | 0.40±0.13 | 0.46±0.13 | 0.46±0.30 | 0.40±0.21 |
| BCVA (logMAR) | 0.24±0.08 | 0.24±0.07 | 0.23±0.06 | 0.28±0.03 | 0.27±0.04 | 0.27±0.05 |
| SE(Diopters) | −4.89±4.55 | 0.10±1.89a | −0.61±2.75a | −0.03±3.09 | −4.44±5.07 | 0.32±0.45 |
Abbreviations: AXL, axial length; BCVA, best-corrected visual acuity; C, cortical cataract; IOL, intraocular lens; logMAR, logarithm of the minimal angle of resolution; N, nuclear cataract; PSC, posterior subcapsular cataract; SE, spherical equivalent; UCVA, uncorrected visual acuity.
P<0.05 vs the N group (one-way analysis of variance followed by the Tukey test).
The LOCS III nuclear color (NC) grade was ≤3 in all of the patients in the N group. The LOCS III grade was C3–C5 in the C group (≥ C4 in 80.6% (29/36) of patients). The LOCS III grade was P2–P4 in all patients in the PSC group.
Preoperative wavefront characteristics
Because the KR-1W analyzer could not assess visual quality in the PSC group, we compared the indices of visual quality among the N, C, and N+C groups.
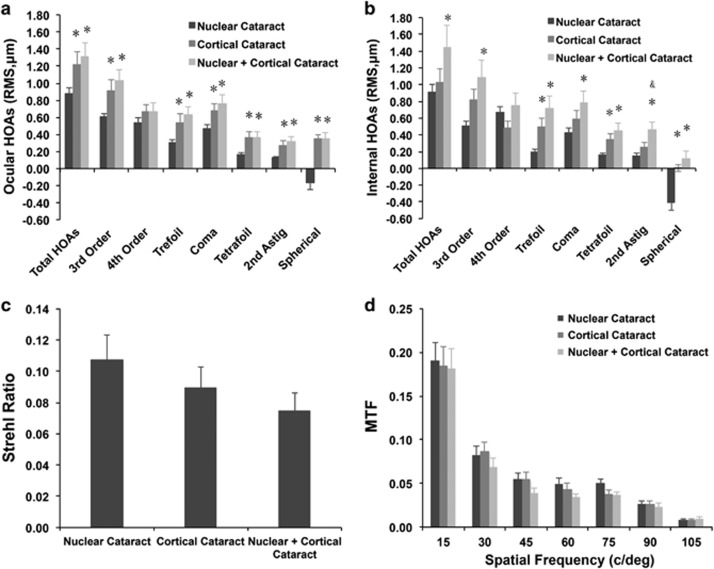
In the N, C, and N+C groups, the ocular total HOA values were 0.88±0.43, 1.22±0.74, and 1.31±0.81 μm; coma were 0.47±0.32, 0.67±0.44, and 0.76±0.52 μm; and spherical aberration were −0.16±0.59, 0.35±0.22, and 0.35±0.40 μm, respectively. Total HOA, 3rd order HOA, coma, trefoil, tetrafoil, secondary astigmatism, and spherical aberration were significantly greater in the C and N+C groups compared with the N group (Figure 2a; ANOVA followed by the Tukey test, all P<0.05).
Figure 2.
Preoperative objective visual quality in patients with a nuclear cataract (N group), cortical cataract (C group), or combined nuclear and cortical cataract (N+C group). (a) Ocular high-order aberrations (HOAs). (b) Internal HOAs. (c) Strehl ratio value. (d) Modulation transfer function curves. *P<0.05 vs the nuclear cataract group (one-way analysis of variance followed by the Tukey test). &P<0.05 vs the cortical cataract group (one-way analysis of variance, followed by the Tukey test). c/deg, cycles/degree; RMS, root mean square.
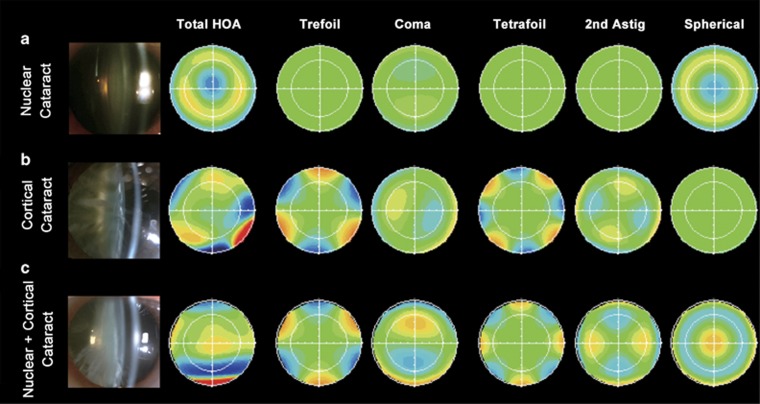
The internal total HOA values were 0.91±0.59, 1.03±0.81, and 1.44±1.36 μm; coma were 0.43±0.38, 0.59±0.47, and 0.78±0.74 μm; and spherical aberration were −0.41±0.63, 0.01±0.21, and 0.11±0.47 μm, respectively. Total HOA, 3rd order HOA, coma, trefoil, tetrafoil, secondary astigmatism, and spherical aberration were significantly greater in the N+C group than in the N group (Figure 2b; ANOVA followed by the Tukey test, all P<0.05). Trefoil, tetrafoil, and spherical aberration were significantly greater in the C group than in the N group (Figure 2b; ANOVA followed by the Tukey test, all P<0.05). Figure 3 shows the typical compositions of the internal HOAs in each group. Of note, the N group was characterized by negative spherical aberration, and the C group was characterized by greater trefoil and tetrafoil, which might be due to morphologic changes, such as water clefts, and wedge- or band-shaped opacities in the lens periphery. The N+C group was characterized by positive spherical aberration and greater trefoil, tetrafoil, and coma, possibly because of combined effects of nuclear and cortical opacity.
Figure 3.
Typical features of the internal higher-order aberrations in nuclear cataract (N group), cortical cataract (C group), or combined nuclear and cortical cataract (N+C group) groups. (a) N group. (b) C group. (c) N+C group. Astig, astigmatism; HOA, high-order aberration.
Benefits of cataract surgery
At 1 month after cataract surgery, the UCVA improved from 0.43±0.16 logMAR to 0.17±0.17 logMAR (paired t-test, P<0.001), and the BCVA improved from 0.24±0.07 logMAR to 0.03±0.09 logMAR (paired t-test, P<0.001). Overall, 86.92% (113/130) of patients had a BCVA <0.1 logMAR and 96.92% (126/130) had a BCVA<0.2 logMAR. The overall satisfaction score was as high as 9.17±0.69.
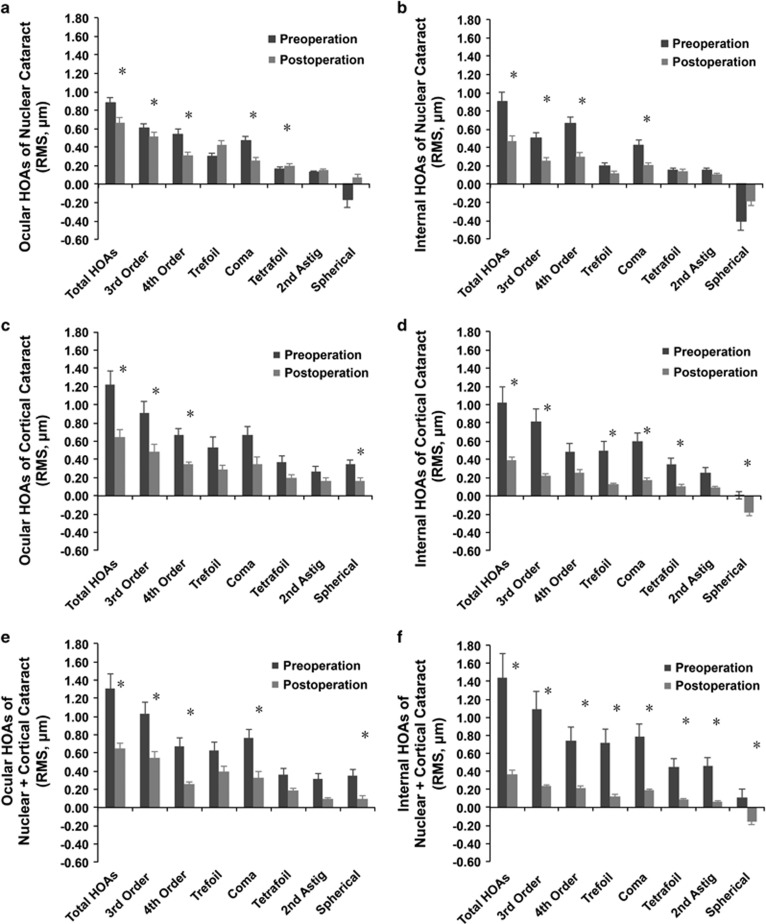
For ocular HOAs, significant decreases in total, 3rd order, and 4th order HOAs were found in each group following surgery. For internal HOAs, significant decreases in total HOA, 3rd order HOA, and coma were also found in these three groups (Figure 4, paired t-test, all P<0.05). In the C group, significant changes were specifically observed in the internal trefoil and tetrafoil (paired t-test, P=0.004 and 0.012, respectively), and in the N+C group, all the internal aberrations assessed were decreased (Figure 4, paired t-test, all P<0.05).
Figure 4.
Comparisons of pre- and postoperative higher-order aberrations (HOAs). (a) Changes in ocular HOAs in the nuclear cataract (N) group. (b) Changes in internal HOAs in the N group. (c) Changes in ocular HOAs in the cortical cataract (C) group. (d) Changes in internal HOAs in the C group. (e) Changes in ocular HOAs in the N+C group. (f) Changes in internal HOAs in the N+C group. *P<0.05 vs the preoperative value (paired t-test). Astig, astigmatism; c/deg, cycles/degree; HOA, higher-order aberrations; RMS, root mean square.
Consequently, PSF and MTF data were increased remarkably in all 3 groups: the preoperative Strehl Ratio was 0.09±0.08, which improved to 0.18±0.12 postoperatively (paired t-test, P<0.001). The preoperative MTF values at spatial frequencies of 15, 30, 45, 60, 75, 90, 105 c/deg were all increased significantly after surgery (paired t-test, all P<0.001).
Discussion
Visual acuity is usually considered as the primary indicator for cataract surgery.5, 14, 15 In China, this demarcation line is usually set at BCVA<20/40. However, many patients having good visual acuity still experience difficulties performing activities of daily living because of cataract-related visual disturbances. Therefore, visual acuity alone might be insufficient for determining the suitability of cataract surgery in these patients. In the current study, we assessed objective functional visual outcomes of cataract surgery in patients with preoperative BCVA≥20/40. We found preoperative wavefront variables were generally poor in these patients, and cataract surgery significantly improved both the visual acuity and visual quality of these patients, which demonstrated that cataract surgery is indicated for these patients after careful assessment of their visual function.
There were some interesting characteristics in this special population. First, most of the patients in the C and PSC groups were not myopic before cataract surgery, whereas the patients in the N group were myopic because of lenticular or axial factors. Second, although the LOCS III grade was≥C4 in 80.6% (29/36) of patients in the C group, the preoperative UCVA was significantly better in this group than in the other groups, which may explain why many patients with severe cortical cataract (identified by clinician) do not report equivalent visual dysfunction. Therefore, careful assessment of visual function and an adequate explanation of the outcomes of surgery are necessary before deciding to perform cataract surgery in cortical patients. Third, different types of cataract had unique compositions of the internal HOAs (Figure 3), which may correspond to the related morphologic changes.
Poor preoperative wavefront results might help to explain the complaints of poor vision and allow correlation to be evaluated in patients with good preoperative BCVA. Previous studies reported that in cataract-free eyes, the total HOA ranged from 0.27 to 0.58 μm,16, 17, 18, 19 coma from 0.14 to 0.21 μm,20, 21, 22 and spherical aberration from 0.02 to 0.22.16, 18, 20, 23, 24, 25, 26 However, the total HOA (0.88, 1.22, and 1.31 μm) and coma (0.47, 0.67, 0.76 μm) were significantly increased in the good visual acuity cataract patients of our study compared with those in the cataract-free eyes of previous studies,16, 17, 18, 19, 20, 21, 22 indicating visual deteriorations in these patients. Spherical aberrations of our patients were either more negative (−0.16 μm in the N group) or more positive (0.35 μm in C and N+C groups) than those of cataract-free eyes reported previously.16, 18, 20, 23, 24, 25, 26 Internal aberrations may better represent the lens-induced changes, which were all significantly increased compared with the cataract-free eyes reported previously.27, 28, 29 Thus, the relatively aggravated wavefront results might imply the source of complaints of poor vision from good visual acuity cataract patients.
Specific components of wavefront analysis may also predict specific visual complaints. For example, in a prior report, glare was associated with the total HOA and spherical aberration, and double vision was mainly associated with coma.30 It is also notable that cortical cataract patients often reported starburst, which may be due to the high rates of peripheral aberrations such as trefoil and tetrafoil according to our data. Therefore, remarkably elevated HOAs might be an important source of visual disturbance in cataract patients with good visual acuity.31, 32
Patients with good preoperative visual acuity but poor visual function can benefit from cataract surgery. Our patients reported high satisfaction scores after cataract surgery. Similar results were reported by Amesbury et al,33 who found that the subjective visual function was significantly improved by cataract surgery in patients with a preoperative BCVA better than 20/20. However, they only reported subjective visual outcomes in patients with BCVA ≥20/20. And our study added objective evidence to the benefit of cataract surgery in patients with BCVA ≥20/40, which is a more common criterion for cataract surgery in China. In these patients, high HOAs may have a more important role than lens opacity in terms of visual dysfunction, and removal of the lens eliminates most lens-associated aberrations. Thus, changes in internal aberration might better illustrate the effect of replacing the crystalline lens with an IOL. Remarkably, the internal total HOA, third order HOA, and coma decreased in the N, C, and N+C groups, consistent with significant improvements of visual function in these patients. Besides, the visual complaints could also be reflected by the MTF and PSF values. Preivous studies34, 35, 36 reported that low values of PSF and MTF were related to the symptoms of halos, starburst, hazy vision, double or multiple image and night vision complaints, and cataract surgery can significantly improve the MTF and PSF values. Our study also found the improvement of visual complaints, accompanied by the increase of MTF and PSF postoperatively. Since the purpose of this study was to explore the objective visual function, we did not describe these in detail.
It is worth noting that although the main emphasis of our study was the objective visual function of cataract patients with good visual acuity, especially the internal aberrations, yet wavefront analysis alone could not suffice to justify the surgical intervention. It might inform patients' decisions, but there are many other factors needed to be taken into account.
In conclusion, the results of our study indicated that patients with preoperative BCVA 20/40 or better can still benefit from cataract surgery. This finding shows that arbitrary BCVA thresholds of worse than 20/40 cannot always be used to determine who will benefit from cataract surgery.
Acknowledgments
We have completed and submitted the ICMJE form for disclosure of potential conflicts of interest, and have none to report. Publication of this article was supported by the National Natural Science Foundation of China (Grants Nos. 81100653, 81270989, and 81470613), the National Health and Family Planning Commission of the People's Republic of China (Grant No. 201302015) and International Science and Technology Cooperation Foundation of Shanghai (Grant No. 14430721100).
Author contributions
Design of the study: XJZ; conduct of the study: HFY; collection and management of the data: WWH, HFY, and JY; analysis and interpretation of the data: WWH, HFY, and JHD; and preparation, review, and approval of manuscript: XJZ and YL.
Footnotes
The authors declare no conflict of interest.
References
- American Academy of OphthalmologyPreferred Practice Pattern. The Academy, San Francisco, 1989. [Google Scholar]
- Lee PP CC, Hilborne LH et al. (eds). Cataract Surgery: a Literature Review and Ratings of Appropriateness and Cruciality. Santa Monica, CA, USA: RAND, 1993. [Google Scholar]
- Lundstrom M, Albrecht S, Hakansson I, Lorefors R, Ohlsson S, Polland W et al. NIKE: a new clinical tool for establishing levels of indications for cataract surgery. Acta Ophthalmol Scand 2006; 84(4): 495–501. [DOI] [PubMed] [Google Scholar]
- Wright CJ, Chambers GK, Robens-Paradise Y. Evaluation of indications for and outcomes of elective surgery. CMAJ 2002; 167(5): 461–466. [PMC free article] [PubMed] [Google Scholar]
- Quintana JM, Arostegui I, Alberdi T, Escobar A, Perea E, Navarro G et al. Decision trees for indication of cataract surgery based on changes in visual acuity. Ophthalmology 2010; 117(8): 1471–1478 8 e1-3. [DOI] [PubMed] [Google Scholar]
- Applegate RA, Marsack JD, Ramos R, Sarver EJ. Interaction between aberrations to improve or reduce visual performance. J Cataract Refract Surg 2003; 29(8): 1487–1495. [DOI] [PubMed] [Google Scholar]
- Lombardo M, Lombardo G. Wave aberration of human eyes and new descriptors of image optical quality and visual performance. J Cataract Refract Surg 2010; 36(2): 313–331. [DOI] [PubMed] [Google Scholar]
- Perez GM, Manzanera S, Artal P. Impact of scattering and spherical aberration in contrast sensitivity. J Vis 2009; 9: 1–10. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Shimizu K, Iijima A, Kobashi H. Factors influencing contrast sensitivity function in myopic eyes. PLoS One 2014; 9(11): e113562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal P, Benito A, Perez GM, Alcon E, De Casas A, Pujul J et al. An objective scatter index based on double-pass retinal images of a point source to classify cataracts. PLoS One 2011; 6(2): e16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall DA, Srinivasan S, Gray LS. Changes in ocular monochromatic higher-order aberrations in the aging eye. Optom Vis Sci 2013; 90(9): 996–1003. [DOI] [PubMed] [Google Scholar]
- Salmon TO, van de Pol C. Normal-eye Zernike coefficients and root-mean-square wavefront errors. J Cataract Refract Surg 2006; 32(12): 2064–2074. [DOI] [PubMed] [Google Scholar]
- Hartwig A, Atchison DA. Analysis of higher-order aberrations in a large clinical population. Invest Ophthalmol Vis Sci 2012; 53(12): 7862–7870. [DOI] [PubMed] [Google Scholar]
- Norregaard JC, Bernth-Petersen P, Alonso J, Dunn E, Black C, Andersen TF et al. Variation in indications for cataract surgery in the United States, Denmark, Canada, and Spain: results from the International Cataract Surgery Outcomes Study. Br J Ophthalmol 1998; 82(10): 1107–1111. [DOI] [PMC free article] [PubMed]
- Michael R, van Rijn LJ, van den Berg TJ, Barraquer RI, Grabner G, Wilhelm H et al. Association of lens opacities, intraocular straylight, contrast sensitivity and visual acuity in European drivers. Acta Ophthalmol 2009; 87(6): 666–671. [DOI] [PubMed] [Google Scholar]
- Little JA, McCullough SJ, Breslin KM, Saunders KJ. Higher order ocular aberrations and their relation to refractive error and ocular biometry in children. Invest Ophthalmol Vis Sci 2014; 55(8): 4791–4800. [DOI] [PubMed] [Google Scholar]
- Yazar S, Hewitt AW, Forward H, McKnight CM, Tan A, Mountain JA et al. Comparison of monochromatic aberrations in young adults with different visual acuity and refractive errors. J Cataract Refract Surg 2014; 40(3): 441–449. [DOI] [PubMed] [Google Scholar]
- Wan XH, Li SM, Xiong Y, Liang YB, Li J, Wang FH et al. Ocular monochromatic aberrations in a rural Chinese adult population. Optom Vis Sci 2014; 91(1): 68–75. [DOI] [PubMed] [Google Scholar]
- Wang L, Koch DD. Ocular higher-order aberrations in individuals screened for refractive surgery. J Cataract Refract Surg 2003; 29(10): 1896–1903. [DOI] [PubMed] [Google Scholar]
- Levy Y, Segal O, Avni I, Zadok D. Ocular higher-order aberrations in eyes with supernormal vision. Am J Ophthalmol 2005; 139(2): 225–228. [DOI] [PubMed] [Google Scholar]
- Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am A Opt Image Sci Vis 2001; 18(8): 1793–1803. [DOI] [PubMed] [Google Scholar]
- Villegas EA, Alcon E, Artal P. Optical quality of the eye in subjects with normal and excellent visual acuity. Invest Ophthalmol Vis Sci 2008; 49(10): 4688–4696. [DOI] [PubMed] [Google Scholar]
- Mathur A, Atchison DA, Tabernero J. Effect of age on components of peripheral ocular aberrations. Optom Vis Sci 2012; 89(7): E967–E976. [DOI] [PubMed] [Google Scholar]
- Rekas M, Krix-Jachym K, Zelichowska B, Ferrer-Blasco T, Montés-Micó R. Optical quality in eyes with aspheric intraocular lenses and in younger and older adult phakic eyes: comparative study. J Cataract Refract Surg 2009; 35(2): 297–302. [DOI] [PubMed] [Google Scholar]
- Fujikado T, Kuroda T, Ninomiya S, Maeda N, Tano Y, Oshika T et al. Age-related changes in ocular and corneal aberrations. Am J Ophthalmol 2004; 138(1): 143–146. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Markwell EL. Aberrations of emmetropic subjects at different ages. Vision Res 2008; 48(21): 2224–2231. [DOI] [PubMed] [Google Scholar]
- Namba H, Hawasaki R, Narumi M, Sugano A, Homma K, Mishi K et al. Ocular higher-order wavefront aberrations in the Japanese adult population: the Yamagata Study (Funagata). Invest Ophthalmol Vis Sci 2014; 56(1): 90–97. [DOI] [PubMed] [Google Scholar]
- deCastro LE, Sandoval HP, Bartholomew LR, Vroman DT, Solomon KD. High-order aberrations and preoperative associated factors. Acta Ophthalmol Scand 2007; 85: 106–110. [DOI] [PubMed] [Google Scholar]
- Philip K, Martinez A, Ho A, Conrad F, Ale J, Mitchell P et al. Total ocular, anterior corneal and lenticular higher order aberrations in hyperopic, myopic and emmetropic eyes. Vision Res 2012; 52: 31–37. [DOI] [PubMed] [Google Scholar]
- Chalita MR, Chavala S, Xu M, Krueger RR. Wavefront analysis in post-LASIK eyes and its correlation with visual symptoms, refraction, and topography. Ophthalmology 2004; 111(3): 447–453. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Fujikado T, Maeda N, Oshika T, Hirohara Y, Mihashi T. Wavefront analysis in eyes with nuclear or cortical cataract. Am J Ophthalmol 2002; 134(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Sachdev N, Ormonde SE, Sherwin T, McGhee CN. Higher-order aberrations of lenticular opacities. J Cataract Refract Surg 2004; 30(8): 1642–1648. [DOI] [PubMed] [Google Scholar]
- Amesbury EC, Grossberg AL, Hong DM, Miller KM. Functional visual outcomes of cataract surgery in patients with 20/20 or better preoperative visual acuity. J Cataract Refract Surg 2009; 35(9): 1505–1508. [DOI] [PubMed] [Google Scholar]
- Tuan KM, Chernyak D, Feldman ST. Predicting patients' night vision complaints with wavefront technology. Am J Ophthalmol 2006; 141(1): 1–6. [DOI] [PubMed] [Google Scholar]
- Cabot F, Saad A, McAlinden C, Haddad NM, Grise-Dulac A, Gatinel D. Objective assessment of crystalline lens opacity level by measuring ocular light scattering with a double-pass system. Am J Ophthalmol 2013; 155(4): 629–635. [DOI] [PubMed] [Google Scholar]
- McAlinden C, Pesudovs K, Moore JE. The development of an instrument to measure quality of vision: the Quality of Vision (QoV) questionnaire. Invest Ophthalmol Vis Sci 2010; 51(11): 5537–5545. [DOI] [PubMed] [Google Scholar]