Abstract
Key points
The cerebellum is the core structure controlling gaze stability. Chronic cerebellar diseases and acute alcohol intoxication affect cerebellar function, inducing, among others, gaze instability as gaze‐evoked nystagmus.
Gaze‐evoked nystagmus is characterized by increased centripetal eye‐drift. It is used as an important diagnostic sign for patients with cerebellar degeneration and to assess the ‘driving while intoxicated’ condition.
We quantified the effect of alcohol on gaze‐holding using an approach allowing, for the first time, the comparison of deficits induced by alcohol intoxication and cerebellar degeneration.
Our results showed that alcohol intoxication induces a two‐fold increase of centripetal eye‐drift.
We establish analysis techniques for using controlled alcohol intake as a model to support the study of cerebellar deficits.
The observed similarity between the effect of alcohol and the clinical signs observed in cerebellar patients suggests a possible pathomechanism for gaze‐holding deficits.
Abstract
Gaze‐evoked nystagmus (GEN) is an ocular‐motor finding commonly observed in cerebellar disease, characterized by increased centripetal eye‐drift with centrifugal correcting saccades at eccentric gaze. With cerebellar degeneration being a rare and clinically heterogeneous disease, data from patients are limited. We hypothesized that a transient inhibition of cerebellar function by defined amounts of alcohol may provide a suitable model to study gaze‐holding deficits in cerebellar disease. We recorded gaze‐holding at varying horizontal eye positions in 15 healthy participants before and 30 min after alcohol intake required to reach 0.6‰ blood alcohol content (BAC). Changes in ocular‐motor behaviour were quantified measuring eye‐drift velocity as a continuous function of gaze eccentricity over a large range (±40 deg) of horizontal gaze angles and characterized using a two‐parameter tangent model. The effect of alcohol on gaze stability was assessed analysing: (1) overall effects on the gaze‐holding system, (2) specific effects on each eye and (3) differences between gaze angles in the temporal and nasal hemifields. For all subjects, alcohol consumption induced gaze instability, causing a two‐fold increase [2.21 (0.55), median (median absolute deviation); P = 0.002] of eye‐drift velocity at all eccentricities. Results were confirmed analysing each eye and hemifield independently. The alcohol‐induced transient global deficit in gaze‐holding matched the pattern previously described in patients with late‐onset cerebellar degeneration. Controlled intake of alcohol seems a suitable disease model to study cerebellar GEN. With alcohol resulting in global cerebellar hypofunction, we hypothesize that patients matching the gaze‐holding behaviour observed here suffered from diffuse deficits in the gaze‐holding system as well.
Keywords: alcohol, cerebellum, gaze‐evoked nystagmus, gaze‐holding, model for cerebellar disease
Key points
The cerebellum is the core structure controlling gaze stability. Chronic cerebellar diseases and acute alcohol intoxication affect cerebellar function, inducing, among others, gaze instability as gaze‐evoked nystagmus.
Gaze‐evoked nystagmus is characterized by increased centripetal eye‐drift. It is used as an important diagnostic sign for patients with cerebellar degeneration and to assess the ‘driving while intoxicated’ condition.
We quantified the effect of alcohol on gaze‐holding using an approach allowing, for the first time, the comparison of deficits induced by alcohol intoxication and cerebellar degeneration.
Our results showed that alcohol intoxication induces a two‐fold increase of centripetal eye‐drift.
We establish analysis techniques for using controlled alcohol intake as a model to support the study of cerebellar deficits.
The observed similarity between the effect of alcohol and the clinical signs observed in cerebellar patients suggests a possible pathomechanism for gaze‐holding deficits.
Abbreviations
- AA
after alcohol intake
- BA
before alcohol intake
- BAC
blood alcohol content
- EPN
end‐point nystagmus
- GEN
gaze evoked nystagmus
- LE
left eye
- MAD
median absolute deviation
- NH
nasal hemifield
- RE
right eye
- TH
temporal hemifield
- VPNI
velocity‐to‐position neural integrator
Introduction
All neural commands generating eye movements are processed by a brainstem neural network (Godaux & Cheron, 1996; Nakamagoe et al. 2000) commonly called velocity‐to‐position neural integrator (VPNI), converting eye velocity into position commands for ocular motoneurons. The VPNI alone, however, does not provide an appropriate level of tonic innervation to hold gaze in an eccentric position, as the integrator is inherently leaky (Robinson, 1973, 1974). In healthy individuals, the cerebellum compensates for the VPNI leakiness (Leech et al. 1977; Zee et al. 1980; Glasauer, 2003), preventing the eyes from being rapidly pulled back towards the resting position by the elastic forces of the extraocular muscles (Cannon & Robinson, 1987).
Despite cerebellar control, physiological horizontal centripetal eye‐drift that increases with gaze eccentricity occurs in darkness (Bertolini et al. 2013).
Cerebellar diseases may cause an increased centripetal drift velocity which, in turn, elicits centrifugal saccades that aim to keep the eyes at their eccentric position. This sequence of centripetal slow phases and centrifugal quick phases, so called gaze‐evoked nystagmus (GEN), appears especially when midline/paramedian vermal and caudal structures are affected (Leech et al. 1977; Leigh & Zee, 2015).
A physiological centrifugal nystagmus (so‐called end‐point nystagmus, EPN) may also appear in healthy subjects at extreme gaze eccentricities (Abel et al. 1978a, b ; Elzenman et al. 1990; Shallo‐Hoffmann et al. 1990).
Deficient cerebellar control of the VPNI leads to prominent centripetal eye‐drift even at small gaze angles (Tarnutzer et al. 2015), resulting in blurred vision and oscillopsia (Leigh & Zee, 2015). Previously, we described different patterns of eye‐drift in patients with neurodegenerative cerebellar disease of various origins and unknown neuropathological differences, possibly related to the age at disease onset (Tarnutzer et al. 2015). With cerebellar ataxia being a rare disease (estimated prevalence 0.2‰; Klockgether, 2012), data from patients are indeed limited.
Impaired gaze stability has also been demonstrated in healthy individuals under the influence of alcohol (Aschan & Bergstedt, 1975; Lehti, 1976; Rubenzer & Stevenson, 2010). Acute alcohol intoxication [blood alcohol content (BAC) >1‰] significantly increases the incidence of EPN (Citek et al. 2003) and decreases the gaze eccentricity causing nystagmus (Lehti, 1976; Tharp et al. 1981; Goding & Dobie, 1986). Additionally, chronic ethanol consumption alters the function and morphology of several brain structures involved in eye movement control (Mauritz et al. 1979; Fadda & Rossetti, 1998; Setta et al. 1998), and is one of the most common causes of progressive cerebellar degeneration in adults (Klockgether, 2010).
We hypothesized that a transient cerebellar inhibition by defined amounts of alcohol may provide a model to study gaze‐holding deficits in cerebellar disease. A description of changes in gaze‐evoked drift associated with alcohol intake, however, is missing. Previous studies focused on the occurrence of nystagmus, without reporting the amount of eye‐drift (Tharp et al. 1981; Goding & Dobie, 1986; Booker, 2001, 2004, Citek et al. 2003, 2011; Whyte et al. 2010). Thus, measuring eye‐drift velocity induced by consumption of a controlled amount of alcohol, we aimed to: (1) identify the alterations of the normal gaze‐holding behaviour specific to alcohol intake, (2) assess if these temporary effects are comparable to those observed in cerebellar patients and (3) evaluate whether the controlled intake of alcohol in healthy subjects represents a valid disease model for cerebellar degeneration. Recently, we described the non‐linear behaviour of eye‐drift velocity (Abel et al. 1978a; Optican & Zee, 1984) using a tangent function (Bertolini et al. 2013). Such a model is particularly advantageous as it allows us to summarize gaze‐holding behaviour using a two‐parameter function, facilitating the quantitative comparison of different datasets [e.g. before vs. after alcohol as well as previously recorded cerebellar patients (Tarnutzer et al. 2015)].
We also investigated asymmetries in gaze‐holding control between temporal and nasal eccentricities. While asymmetries in the saccadic system (Versino et al. 1996; Ramat et al. 1999) and vestibulo‐ocular reflex (Bertolini & Ramat, 2011) are well known, similar differences in gaze‐holding were only hypothesized (Abel et al. 1978b; Shallo‐Hoffmann et al. 1990). We speculate that alcohol, enhancing eye‐drift, may reveal such asymmetries.
Methods
Subjects and ethical approval
The statistical distribution of eye‐drift velocity in 20 healthy human subjects [mean (SD) 41 (11) years old] described previously (Bertolini et al. 2013; Tarnutzer et al. 2015) suggested that data from at least 14 subjects are needed to reveal a significant increase of 1 deg s–1 in the centripetal drift velocity at extreme gaze, having a power (probability of rejecting the null hypothesis when the alternative hypothesis is true) of 0.80.
Consequently, we recruited 15 healthy subjects [five females, 31.36 (7.3) years old]. The subjects were informed about the nature of the experiment and all experimental procedures were fully explained. Every participant signed a written informed consent form. The Ethics Committee of the Canton of Zurich approved the experimental protocol (KEK‐ZH‐2012‐0150), which was in accordance with the ethical standard laid down in the 2013/1969 Declaration of Helsinki for medical research involving human subjects.
None of the participants had a history of neurological disorders including dizziness/vertigo or gait imbalance or took any drugs that may affect gaze‐holding. Only two subjects wore their usual contact lenses during the experiment, as their myopia could have affected their performance during the test. One subject was excluded due to an incomplete dataset, as recordings after alcohol intake had to be cancelled because of nausea and vomiting.
Experimental setting
During the entire experiment, each subject was seated upright on a turntable mounted on three servo‐controlled motor‐driven axes (Acutronic, Jona, Switzerland). To stabilize the subject's head and limit head movements, individually moulded thermoplastic masks (Sinmed BV, Reeuwijk, The Netherlands) were used. Safety belts were applied to minimize trunk‐movement‐related artifacts.
The visual stimulus was generated using a remotely controlled LED, attached to a hemispherical full‐field screen at 1.5 m distance. The LED was mounted at eye level straight‐ahead. The screen was connected to a platform that could be rotated along an earth‐vertical axis (position resolution = 0.01 deg).
Horizontal eye movements were recorded using a head‐mounted video‐oculography (VOG) device (Eyeseecam, Munich, Germany), a video system using two infrared cameras mounted on swimming goggles. The position of both eyes was sampled at 220 Hz, with a spatial resolution of 0.01 deg root mean square (Schneider et al. 2005; Dera et al. 2006).
A calibration procedure was performed at the beginning of the experiment requiring the subject to look at a sequence of fixation points (21 points forming a grid of gaze angles from −25 to +25 deg with 10 deg steps along the horizontal axis, and from −10 to +10 deg with 10 deg steps along the vertical axis) projected on the hemispherical screen using a laser galvanometer. The relationship between the output values of the VOG system and eye angular positions on the hemispherical screen was obtained by fitting a second‐order polynomial function (Bertolini et al. 2013).
Experimental procedure
Every subject underwent two identical sessions: before alcohol intake (baseline recording) and 30 min after the ingestion of the amount of alcohol (in grams) estimated to reach a BAC of 0.6‰. The grams of alcohol were calculated on a subject‐by‐subject basis using the Widmark formula (Widmark, 1981) (parameters required: subject's height, weight, sex). The estimated quantity was converted to millilitres of red wine 13% alc. vol.
The achieved BAC was then estimated from the BrAC (breath alcohol content) using a breath alcohol tester (Dräger Alcotest 6510, Lübeck, Germany), with conventional single breath technique to avoid any bias related to different breathing techniques (Jones, 1982). To confirm that BAC values remained stable during the whole experiment, BrAC was measured immediately before and after each block of our experiment (i.e. approximately every 10 min).
The baseline recording allows discounting any confounding factor known to affect GEN and its prevalence (e.g. age, between‐subject variability, alertness, physical status of the subjects) (Rubenzer & Stevenson, 2010; Whyte et al. 2010; Bertolini et al. 2013). As each experimental session lasted around 1 h and the two sessions were separated by a maximum of 1 h, the risk that tiredness may change significantly during the test (i.e. before and after alcohol intake) was small.
The paradigm was identical to the one previously described and validated for studying gaze‐holding in healthy subjects (Bertolini et al. 2013) and patients with cerebellar neurodegeneration (Tarnutzer et al. 2015). It can be summarized as follows: in a completely dark environment, the subject was asked to fixate a briefly flashing red LED (50 ms every 2 s) moving at 0.5 deg s–1 in the range of horizontal gaze eccentricity from 40 deg right to 40 deg left (with respect to the primary gaze position for each eye), without moving the head.
Both eyes were concurrently recorded, but one eye was covered with an optic filter, allowing eye tracking but preventing vision. This approach was chosen to avoid possible double vision due to GEN.
This paradigm was recorded twice, with the LED initially moving either leftward or rightward (the direction of the first movement was randomized across subjects). During each trial the flashing LED reached an eccentricity of 40 towards the side of the viewing eye and of 20 towards that of the covered eye as the target was usually not visible for larger gaze angles on the side of the covered eye due to both occlusion from the VOG goggles structure and the subject's nose. The entire process was repeated changing the covered eye (the order of the covered eye was randomized across subjects).
Data pre‐processing
Eye movement data were analysed using interactive functions written in MATLAB (MatLab 8.2; The MathWorks, Inc., Natick, MA, USA). Instantaneous eye velocity was obtained computing the derivative of horizontal eye movements.
Only the slow phases of the eye movements were considered when analysing eye‐drift velocity at different gaze eccentricities, removing the fast phases (saccades) and eye‐blink‐related artifacts using an automatic custom velocity‐based algorithm (for details of the procedure see Bertolini et al. 2013). Missing data (e.g. due to brief interruption of pupil tracking by the VOG software) were not interpolated. Data were downsampled from 220 to 100 Hz. No other data preprocessing was done.
Data grouping
We performed three different analyses of the recorded eye‐velocity data, each time addressing a different question for which a specific procedure for pooling the data was required.
First, we evaluated the alcohol effect on the overall ability to hold gaze on a target. For each subject we pooled the data from both eyes recorded during all trials (trials differ by the starting direction of the target displacement and by the covered eye; see ‘Experimental procedure’ section above for details). To adopt a gaze‐based reference system, we took the positions of the eyes when looking at the target straight ahead as zero position and, accordingly, we defined the gaze eccentricity as the angular position of the LED with respect to zero (gaze angles to the subject's right were defined as positive). We estimated the velocity bias when looking straight ahead, by computing the median of instantaneous eye‐drift velocities recorded within the range of ±2.5 deg of gaze eccentricity and subtracted it from all data points. This allowed us to compare the dependency of eye‐drift from gaze eccentricity independently from minor discrepancies of the straight‐ahead position across trials and subjects. This analysis compared two conditions: before and after the intake of alcohol (BA and AA, respectively).
Our second analysis considered the behaviour of both eyes separately to test for possible disconjugate effects of alcohol. The procedure was identical to the one described above to pool the data, with the exception that the data acquired from each eye were kept separate, building up two subgroups (named LE for left eye and RE for right eye, respectively) for both conditions studied (i.e. BA and AA).
The third analysis aimed at evaluating asymmetries in gaze‐holding mechanisms assessing the differences between eye‐drift after fixation in temporal and nasal hemifields. Such analysis required an additional step to separate the data from the two eyes with respect to the eye null position.
Specifically, while in Abel et al. (1978b) gaze‐holding asymmetries were observed defining an ‘abducting and adducting eye’ using the direction of the previous saccade, we describe our results in terms of the position of the eyes in the orbit, hence considering either the eye in the temporal hemifield or the eye in the nasal hemifield as TH and NH, respectively. Therefore the TH data were obtained pooling data from all fixation points, irrespective of right or left eye, in the temporal hemifield. TH data then comprise gaze angles lower than eye null position for LE and greater for RE, and therefore producing eye‐drift in temporal–nasal direction (TN). Similarly, the NH data were obtained pooling all fixation points in the nasal hemifield, i.e. gaze angles greater than eye null position for LE and lesser for RE, causing eye‐drift in naso‐temporal direction (NT).
To align left and right eye data for the second analysis and to distinguish temporal and nasal gaze angles in the third analysis, we used the null position of each eye (i.e. the gaze angle showing no drift) as the ‘switch point’. However, we observed that the zero position defined by the target straight ahead as described above was often not appropriate to describe the actual null of either eye. In darkness each eye drifts toward a resting point corresponding to a subject‐specific resting vergence (Jaschinski‐Kruza, 1991; Rosenfield, 1997; Jaschinski et al. 2007). Such vergence may not correspond to the one required to look at the target used in this experiment, leading to disconjugate drifts when looking straight ahead. We therefore estimated null position Nulleye on the raw data of each single eye, fitting the instantaneous velocity of each eye, V eye, with the following linear function of eye eccentricity, E eye, in range from –15 to 15 deg (position–velocity linear relationship for small gaze angles; Bertolini et al. 2013):
| (1) |
The null position Nulleye was computed as the value of E eye with velocity V eye = 0, i.e. Nulleye = q eye/m eye. Fit coefficients, q eye and m eye, were estimated using quantile regression (Koenker & Bassett, 1978). The Nulleye was considered unreliable when the slope m eye was close to zero (threshold: m eye > 0.002s–1) and Nulleye value was outside the range –10 deg< Nulleye ˅Nulleye > 10 deg. In such cases Nulleye was set to zero for both eyes.
Once Nulleye was estimated, its value was used to align data points of the two eyes according to their actual null position (i.e. resting point vergence). Such correction allows us to compare left versus right eye and to distinguish nasal gaze angles from temporal ones, avoiding incorrect alignment of data points from each eye in the position–velocity (PV) plot (discussed in the ‘Differential analysis for temporal and nasal hemifields’ subsection) and overestimating the slope of the PV relationship in temporal hemifield data erroneously using data points from the nasal hemifield.
Data analysis
Our data analysis is similar to that described previously (Bertolini et al. 2013; Tarnutzer et al. 2015) to study the gaze‐holding mechanism in healthy subjects and patients with cerebellar disorders. The analysis is based on a PV plot (i.e. a plot of eye‐drift velocity as a function of gaze eccentricity), commonly adopted to analyse the VPNI time constant by means of a linear fit modelling, but introduces some important differences (Bertolini et al. 2013).
To draw the PV plot, we sorted the eye‐drift velocity of every subject in ascending order of gaze eccentricity. Sorted data were assigned into 17 non‐overlapping, 5 deg wide bins, covering the whole range of gaze angles tested (±40 deg). For each bin the median values of position and velocity were calculated, reducing data noise caused by outliers.
The different procedures described in the ‘Data grouping’ subsection were separately applied to the data acquired in the two conditions tested, BA and AA. This generated several subsets of data to be compared within the three analyses (as defined in the ‘Data grouping’ subsection):
-
1.
Overall gaze BA vs. AA,
-
2.
Left eye BA vs. right eye BA, left eye AA vs. right eye AA, left eye BA vs. AA, right eye BA vs. AA.
-
3.
Temporal hemifield BA vs. nasal hemifield BA, temporal hemifield AA vs. nasal hemifield AA, temporal hemifield BA vs. AA and nasal hemifield BA vs. AA.
For each comparison our analysis was carried out in two steps: a ‘direct comparison’ of data and a model‐based analysis.
Direct comparison
In the ‘direct comparison’, for each subject i we computed the median ratio (ri) of the median velocities of corresponding bins. This was repeated for each pair of subsets compared, which were in turn named S1 and S2. Formally the computation is expressed by:
| (2) |
where, for the ith subject, Vji ,S1 represents the median velocity of the jth bin in subset S1, while Vji ,S2 represents the median velocity of the same bin in subset S2.
The distribution of median ratios across subjects was tested using the Wilcoxon signed‐rank test to verify whether the subsets compared (S1, S2) were statistically different.
Model‐based approach
In addition to the ‘direct comparison’, we performed a further analysis using a model‐based approach comparing each pair of subsets. As suggested in early studies (Abel et al. 1978a; Optican & Zee, 1984) and recently confirmed (Bertolini et al. 2013), we assumed a non‐linear relationship between eye position and drift velocity, in contrast to the common assumption of linear growth between drift velocity and gaze eccentricity that does not allow appreciation of the differences observed across a sample of patients with cerebellar diseases (Tarnutzer et al. 2015). Specifically, in each subset analysed, for the ith subject, the instantaneous drift velocity (V i) was independently fitted, using the following function of gaze eccentricity (E i):
| (3) |
The mathematical model in eqn (3) is a modified version of those presented by Bertolini et al. (2013) and Tarnutzer et al. (2015). It consists of a tangent function where independent changes of the two parameters k 1 and k 2 lead to changes of two distinct features describing the behaviour of the drift velocity V as a function of the gaze angle E. Specifically, the ‘shaping coefficient’ k 1 modifies the shape of the tangent function to capture rapid deterioration of gaze‐holding performance beyond a certain eccentricity of gaze, i.e. how marked the non‐linear behaviour is; the ‘scaling coefficient’ k 2 instead scales the whole function independently from the gaze angle, keeping the tangent shape unchanged [see fig. 1 in Tarnutzer et al. (2015) for a detailed description].
Figure 1. Horizontal eye position before and after alcohol consumption.
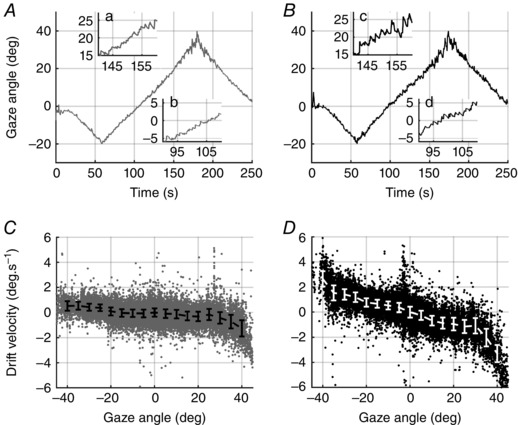
Horizontal eye position recorded in a single trial from a typical subject before (A, C) and after alcohol consumption (B, D). Positive angles correspond to right gaze eccentricities as seen by the subject. In A and B, right eye position is plotted as a function of time. Insets (a–d): centrifugal nystagmus is already present at the same gaze eccentricity, but slow phase velocity of nystagmus is strongly increased by alcohol consumption. In C and D, horizontal eye‐drift velocity is plotted against gaze position. Data points: instantaneous velocities of slow phases; saccades were removed during preprocessing of data. Solid bars: median (MAD) of instantaneous eye‐drift velocity. Greater gaze instability is caused by alcohol intake. Such an effect is visible as a homogenous increase of eye‐drift for all gaze angles (D) compared to the baseline condition (C).
Moreover, compared to the previous versions of the tangent function presented (Bertolini et al. 2013; Tarnutzer et al. 2015), the modelling in eqn (3) reduces the number of estimated parameters from three to two, as we now remove the offset velocity directly on raw data instead of using a third coefficient k 3. This simplification, although mainly methodological, allowed us to focus directly on the two relevant parameters.
The ratios (, ) of each fit coefficient in two paired subsets (S1, S2) were then computed for every subject as follows:
| (4) |
The statistical distributions of ratios (, ) across the subjects were tested by means of a Wilcoxon signed‐rank test, and were compared to a population with median equal to one.
Gaze‐holding dataset comparison
To verify that our dataset of 14 subjects (before alcohol intake) was comparable to previously reported gaze‐dependent eye‐drift, we compared it with a gaze‐holding dataset of 20 healthy human subjects described by Bertolini et al. (2013) excluding two subjects who also participated in our experiment. For each subject, we independently fitted the median velocity computed over gaze eccentricity bins using eqn (3), pooling all data from left and right eye within each subject, and compared the resulting parameters.
Statistical analysis
Median and median absolute deviation (MAD) were used as statistical descriptors of the data, as they are weakly affected by outliers (Leys et al. 2013).
For all paired comparisons, we performed a Kolmogorov–Smirnov test. Since data were non‐normally distributed, a bilateral Wilcoxon signed rank test was then used after testing the symmetry of the data by means of the Wilcoxon test for symmetry. In the same way, we tested the difference of two independent samples using the Wilcoxon rank sum test.
For multiple comparisons, a Bonferroni correction was used to ensure a conservative measure of significance. We considered a P‐value of < 0.05 (after correction in multiple comparisons) as statistically significant.
Least square regression and quantile regression (Koenker & Bassett, 1978) were used as methods for data fitting when the normality of the data were confirmed or not, respectively.
To measure the strength of the relationship between the tangent coefficients, because the linearity of analysed variables was not confirmed, Kendall's tau, a non‐parametric correlation index, was used.
Results
Our results show that alcohol intoxication induces a faster centripetal drift of the eye, increasing with increasing eccentricity, with respect to sober subjects (see insets in Fig. 1 A, B).
Such behaviour is efficiently summarized in the PV plot, which shows all the data points corresponding to the recorded slow phase velocity as a function of eye eccentricity (Fig. 1 C, D). Specifically, the observed effect of 0.6‰ BAC can be quantified as doubling the drift velocity at all gaze angles, possibly causing GEN at lower gaze eccentricities.
Alcohol effect on gaze‐holding
At baseline, BAC was zero in all subjects. The median level of BAC across our subjects 30 min after alcohol intake was in accordance with Widmark's formula prediction (Widmark, 1981) [0.58 (0.06)‰ BAC; 31 (4) min; median (MAD)]. This value remained quite stable during the whole recording period [sample distribution of median of BAC for each subject, 0.61 (0.02)‰ BAC; sample distribution of BAC variability, i.e. MAD, for each subject: 0.03 (0.02)‰ BAC].
A comparison of the eye movements from the BA and AA condition is shown in Fig. 1 A, B for a typical subject. Alcohol consumption clearly reduced the gaze angle where nystagmus becomes clearly recognizable. This is due to a higher eye‐drift velocity at the same gaze eccentricity, as illustrated on the PV plots (Fig. 1 C, D).
This pattern was confirmed in the whole dataset by computing the median ratio of the AA versus the BA condition for every subject (see ‘Direct comparison’ in the Methods). The Wilcoxon test for paired data revealed that the median ratio distribution [2.21 (0.55)] was significantly higher than one (P = 0.002), confirming that a BAC of 0.6‰ affects the gaze‐holding mechanism by increasing centripetal eye‐drift velocity more than 2‐fold.
Fitting the tangent function in eqn (3) independently for each subject and computing the ratios of estimated coefficients (eqn 4) allowed us to investigate the mechanisms behind these increases in drift velocity. No statistical difference was found for the shaping coefficient k 1 [median ratio = 1.09 (0.38), P = 0.22, Wilcoxon signed‐rank test for paired data]. On the other hand, the ratio of the scaling coefficient k 2 [1.96 (0.82)] was statistically different from one (P = 0.001), suggesting that changes in drift velocity induced by alcohol were due to a proportional increase of drift velocity at all studied gaze angles.
The pure scaling effect induced by alcohol is clearly visible in Fig. 2, which compares the mean of individual velocity curves before and after alcohol consumption, pooling all subjects. The shape of the curve from the AA condition (black solid curve) appears almost unchanged when compared to the BA condition, showing a steady increase of eye‐drift velocity as a function of gaze eccentricity. A simple algebraic multiplication of the point‐by‐point velocity from the BA curve (dark grey dashed curve) by a scaling factor of 2 (light grey dashed curve) reproduces the experimental data and thus indicates that a BAC of 0.6‰ induces no change in the shape of the PV relationship of gaze‐evoked eye‐drift (black solid curve).
Figure 2. BAC on eye‐drift velocity as a function of eye gaze angle.
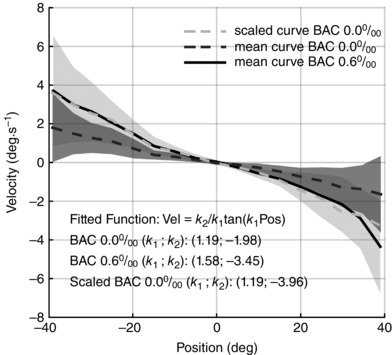
Effect of 0.6‰ BAC on eye‐drift velocity as a function of eye gaze angle. Each line represents the mean drift velocity of all subjects in the different conditions, while the shaded area represents the mean (SD). The light grey dashed line is a scaled version of the data recorded before alcohol intake (dark grey dashed line), perfectly overlapping with the data recorded after alcohol intake (black solid line), confirming the pure scaling effect of 0.6‰ BAC. Such an effect is further confirmed by the scaling parameter of the tangent model (k 2), which was estimated on the plotted curves (the estimated parameters are reported in the figure).
Differential analysis of the two eyes
Comparing drift velocities from both eyes, an eye‐specific offset in the resting (or null) position was observed in some subjects. Such offset biased the pairing of gaze eccentricity of the two eyes when comparing drift velocities. According to our criterion for a reliable estimate of the null point (for a detailed description of criteria to estimate the null see ‘Data grouping’ subsection in Methods), we estimated the null position Nulleye for each eye. A reliable estimate was possible for 8 of the 14 subjects from the BA condition [Offset RE: –4.68 (2.28) deg; Offset LE: 4.69 (2.28) deg) and for 11 out of 14 subjects from the AA condition [Offset RE: –4.00 (1.90) deg; Offset LE: 5.91 (2.34) deg], respectively (see eqn 1). To allow an unbiased comparison of the drift velocity between the two eyes, the reliably estimated offsets were removed. No correction was performed for the remaining subjects (see ‘Data grouping’ subsection). The results of the bias removal are shown in Fig. 3 B and D for a typical subject. Specifically, the figure demonstrates how the data points from LE and RE (in BA and AA conditions, respectively, Fig. 3 B and D) showed a better overlap after bias subtraction than in the original PV plot (Fig. 3 A, C).
Figure 3. Position–velocity plots of a typical subject.
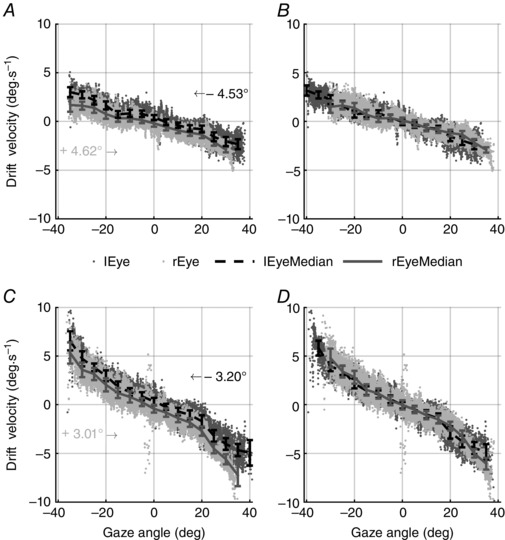
PV plots of a typical subject with data points of the two eyes aligned (B, D) or not (A, C) according to their actual null position (eqn 1). Data from both eyes (light grey and dark grey, respectively, for right and left eye) are plotted separately for before (A, B) and after (C, D) alcohol intake conditions. Data points: instantaneous velocities of slow phases; saccades were removed during data preprocessing. Solid bars: median (MAD) of instantaneous drift velocity. In A and C, independently of alcohol consumption, an eye‐specific offset can be easily observed as the data points for each eye are not overlapping. Such an offset was estimated by means of eqn (1) and used to shift data as shown by arrows in A and C. Only when the eyes are correctly aligned (B, D) can their PV plots be compared.
After offset correction, the distributions of median ratios (see eqn 2) of LE and RE were not statistically different from 1 in any condition [BA: 0.97 (0.19), P = 0.65; AA: 0.99 (0.09), P = 0.82; Wilcoxon signed‐rank test]. This implies that the VPNI acts identically for both eyes with respect to their specific null position, and that this symmetry is not affected by the consumption of alcohol.
The results were further confirmed by comparing the estimated tangent coefficients (eqn 4) in each data subset (see Table 1). The median of k 1 ratios between RE and LE [BA: 1.01 (0.34), P = 0.54; AA: 1.01 (0.18), P = 0.94] and that of k 2 ratios [BA: 0.96 (0.29), P = 0.83; AA: 1.03 (0.07), P = 0.21] were indeed not different from 1 both before and after alcohol consumption.
Table 1.
Tangent model coefficient distributions
| Pooled data | Shaping coefficient k 1 (s–1) [median (MAD)] | Scaling coefficient (s–1) [median (MAD)] |
|---|---|---|
| Both eyes healthy subjects (Bertolini et al. 2013) | 1.3848 (0.4917) | 1.4815 (0.8885) |
| Both eyes BA intake | 1.2777 (0.5538) | 1.8517 (0.8280) |
| Both eyes AA intake | 1.6595 (0.1297) | 2.9838 (1.2666) |
| Left eye BA intake | 1.5060 (0.2112) | 1.3740 (0.9731) |
| Right eye BA intake | 1.2697 (0.7215) | 1.6904 (0.8653) |
| Left eye AA intake | 1.4219 (0.2988) | 2.7650 (0.9454) |
| Right eye AA intake | 1.6053 (0.4709) | 2.9564 (1.2444) |
| Nasal eye BA intake | 1.5191 (0.4448) | 1.0037 (0.7354) |
| Temporal eye BA intake | 1.3301 (0.3851) | 2.1944 (0.6290) |
| Nasal eye AA intake | 1.5145 (0.7224) | 3.5158 (2.0575) |
| Temporal eye AA intake | 1.5364 (0.1924) | 3.2121 (1.3454) |
With respect to the effects of a BAC of 0.6‰, our analysis revealed that the same homogeneous scaling effect of eye‐drift velocity found for the pooled data (shown in Fig. 2) was observed at the level of each single eye.
Specifically, the direct comparison of the AA and BA conditions (computing the distribution of median ratios according to eqn (2)) revealed significant differences in the data of both eyes [RE: 2.08 (0.42), P = 0.002; LE: 1.69 (0.30), P = 0.005]. Similarly to the pooled analysis, no significant difference in the shaping coefficient k 1 was found for RE or LE alone [medians of ratio between AA and BA conditions were 1.03 (0.44), P = 0.41, and 1.07 (0.14), P = 0.31, respectively for RE and LE], while ratios of k 2 estimated in AA to k 2 estimated in the BA condition were statistically higher than 1 for both eyes [RE: 2.20 (1.06), P = 0.04; LE: 1.95 (0.57), P = 0.007].
Differential analysis for temporal and nasal hemifields
By comparing gaze angles in temporal and nasal hemifields considering separately the data acquired in the two tested conditions, we observed that the different ocular dynamics of the ocular plant shown in saccades data did not affect gaze‐holding features. In the BA condition no significant difference was found between NH and TH (P = 0.064) either using eqn (2) to compare medians within each bin (where S1 and S2 represent NH BA and TH BA, respectively, and their median of distribution of medians ratios was 0.68 (0.23)] or comparing the tangent coefficients estimated from NH BA and TH BA. No differences were indeed found either in the shaping coefficient k 1 or the scaling coefficient k 2, since both median ratios were not statistically different from 1 [k 1 NH BA/ k 1 TH BA: 0.99 (0.08), P = 0.52; k 2 NH BA/k 2 TH BA: 0.61 (0.35); P = 0.084].
Similarly, in the AA condition a direct comparison of NH and TH did not reveal significant differences [median ratio distribution: 1.0 (0.44), P = 0.52]. The ratios of tangent coefficients k 1 and k 2 in both directions were not different from 1 [k 1 NH AA/k 1 TH AA: 0.96 (0.43), P = 0.79; k 2 NH AA/k 2 TH AA: 0.94 (0.38); P = 0.68], as shown in Table 1.
In line with the results obtained with the other grouping strategies, analysis of the effects of alcohol consumption, through direct comparison of data pooled by drift direction, showed a statistically significant difference between BA and AA conditions [medians of the ratio between AA and BA conditions for TH: 1.68 (0.42), P = 0.01; and NH: 2.76 (1.23), P = 0.004]. The comparison of the parameters of the fitted function (eqn 4) revealed that the change in the gaze‐holding behaviour was due to a pure scaling of eye velocity as the Wilcoxon signed‐rank test showed that only the median ratio of k 2, either for TH [k 1 TH AA/k 1 TH BA: 1.04 (0.19), P = 0.30; k 2 TH AA/k 2 TH BA: 1.58 (0.31), P = 0.027] and NH [k 1 NH AA/k 1 NH BA: 0.99 (0.17), P = 0.97; k 2 NH AA/k 2 NH BA: 2.46 (2.20), P = 0.019] was significantly different from 1.
Gaze‐holding dataset comparison
The first two rows of Table 1 show the distribution of tangent coefficients estimated using our dataset and the gaze‐holding dataset described by Bertolini et al. (2013). Despite small differences, neither the shaping coefficient k 1 nor the scaling coefficient k 2 showed any statistically significant difference with respect to the values of healthy subjects in Bertolini et al. (2013) (Wilcoxon rank sum test: P = 0.79 and P = 0.24, respectively). The absence of relevant differences also emerges from Fig. 4, where the averages of individual medians of velocity bins are shown for both datasets.
Figure 4. Position–velocity plot of two different datasets of healthy subjects.
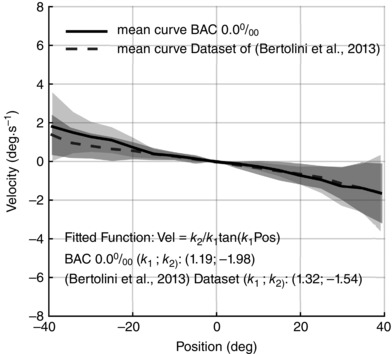
Each line represents the mean drift velocity of all subjects, while the shaded area represents the mean (SD). Positive angles correspond to right gaze eccentricities as seen by the subject. Data recorded on our subjects before alcohol intake (black solid line) are almost indistinguishable from the dataset of 20 healthy subjects described by Bertolini et al. (2013) (dark grey dashed line), confirming that our dataset includes subjects with physiological gaze‐dependent eye‐drift. The plotted curves were also fitted with the tangent function (the estimated parameters are reported in the figure). Both the shaping (k 1) and scaling (k 2) parameters are comparable in the two datasets.
Correlation analysis
The correlation analysis between tangent coefficients k 1 and k 2 confirmed that the tangent model allow us to distinguish two patterns of gaze‐holding behaviours. Independently of the condition being analysed, k 1 and k 2 did not show a significant correlation (BA: τ < |0.30|, P > 0.05; AA: τ < |0.30|, P > 0.05; using Kendall non‐parametric correlation coefficient τ), proving that eqn (3) provides two uncorrelated features to summarize gaze‐holding behaviour.
Discussion
Chronic alcohol consumption causes progressive changes in cerebellar morphology and functionality (Fadda & Rossetti, 1998; Klockgether, 2010). Thus, alcoholics can show symptoms similar to those typical of patients with hereditary cerebellar disease. Impaired gaze stability, an ocular‐motor sign shared by various cerebellar diseases, is encountered also during acute alcohol intoxication, as a consequence of the loss of efficiency of the VPNI due to transient cerebellar impairment.
Using a validated methodology (Bertolini et al. 2013; Tarnutzer et al. 2015), we quantified the changes in the gaze‐holding behaviour induced by alcohol. By measuring eye‐drift velocity as a continuous function over ±40 deg of gaze eccentricity and fitting a two‐parameter tangent function to the data, we showed a consistent effect of 0.6‰ BAC in all subjects. The effect was similar at all gaze eccentricities, causing a 2‐fold increase of the centripetal eye‐drift velocity.
Our finding was confirmed using three different approaches. First, the distribution of median ratios obtained as the ratio of each subject's raw data recorded after alcohol (AA) to that before alcohol (BA) (i.e. without model assumptions) showed a gaze‐independent increase of median drift velocity by a factor close to 2 [2.21 (0.55)]. Second, using the tangent function, we demonstrated that alcohol has a pure scaling effect on eye‐drift velocity, as only the scaling coefficient k 2 was significantly increased after alcohol intake. As the ratio of k 2 in AA to BA conditions [1.96 (0.82)] is also close to 2, we conclude that the observed increase in medians could be explained by the scaling factor. Third, the velocity curve ‘average subject BA’ multiplied by a factor of 2 almost perfectly overlaps with the curve ‘average subject AA’ (Fig. 1).
Non‐pathological GEN at gaze angles smaller than expected for EPN was previously reported in healthy subjects after alcohol consumption (Lehti, 1976; Goding & Dobie, 1986; Booker, 2001, 2004; Citek et al. 2003). Previous studies focused on the nystagmic response only considering that the observation of nystagmus is used to assess gaze‐holding deficits in patients and to assess the ‘driving while intoxicated’ condition through visual inspection (Tharp et al. 1981; McKnight et al. 2002; Citek et al. 2003; Rubenzer & Stevenson, 2010). Yet, the results of these studies are inconsistent and have prevented a shared consensus on the use of GEN to assess alcohol intoxication. The core of this dispute (Citek, 2010; Whyte et al. 2010) lies in the consistency of the alcohol‐induced GEN between individuals and on the discriminability of such an effect from normal variations due to other factors.
To our knowledge, the experiment presented in this paper is the first to assess the effect of alcohol on the amount of gaze‐dependent eye‐drift, i.e. the deficit causing nystagmus, and therefore to directly investigate the mechanism of alcohol‐induced gaze instability. Due to this approach our results shed new light on the contrasting findings reported in the literature. First, we determined that the effect of alcohol on gaze‐holding is consistent across subjects. Second, we showed that eye‐drift velocity after alcohol intake depends on that before alcohol consumption.
The distinction between these two statements is important when evaluating the relationship between GEN and BAC. In our experiment, the impact of alcohol intake was extrapolated from intra‐individual comparisons of gaze‐holding performance immediately before and shortly after drinking. Despite eye‐drift velocity, BA varied considerably among subjects (Booker, 2004; Rubenzer & Stevenson, 2010), leading to variable drift velocities AA, and a BAC of 0.6‰ always caused BA velocity to roughly double. Therefore, our results suggest that even if an alcohol effect is consistently doubling eye‐drift velocity, the manifestation of small amounts of nystagmus, which is governed by drift velocity but is also influenced by other factors, will be highly unpredictable at low BAC (and even in sober subjects) due to the large variability of BA drift velocities between subjects.
The findings presented in this study also allow a better understanding of the mechanism linking cerebellar impairment and gaze‐holding deficits. In patients with cerebellar disease (Tarnutzer et al. 2015), the tangent function model (Bertolini et al. 2013) revealed three distinct subgroups of patients, namely: a ‘pure scaling’ subgroup, showing a consistent increase of eye‐drift velocity with respect to normal values at all gaze angles; a ‘shape‐change’ subgroup, with abnormal drift velocity only for large gaze angles; and a subgroup showing a mixture of the two behaviours. Although the authors observed that patients with symptom onset at a later stage in life presented a ‘pure scaling’ behaviour, the heterogeneity of patient populations in Tarnutzer et al. (2015) prevented linking gaze‐holding behaviours and medical findings.
The current experiment reveals that 0.6‰ BAC causes a ‘pure scaling’ effect. We hypothesize that such a gaze‐independent (i.e. global) decrease in gaze‐holding abilities reflects diffuse cerebellar loss of function. This decrease, although of lesser magnitude, resembles the change observed in the pure scaling patient subgroup, reinforcing the hypothesis (Tarnutzer et al. 2015) that such patients may suffer from more diffuse cerebellar loss‐of‐function as compared to patients with a shape‐changing pattern.
Such similarity suggests that a controlled amount of alcohol intake provides a promising human model to study the effect of global cerebellar hypofunction to better understand the pathomechanisms of progressive cerebellar degeneration. As the healthy cerebellum prolongs the VPNI time constant, alcohol intake may reduce this time constant and, consequently, lead to an increase of eye‐drift velocity for all gaze angles, i.e. to a ‘pure scaling effect’ due to global reduction of cerebellar control.
Regarding the mechanism inducing such an effect, different explanations are possible. First, it can be linked to the inhibitory effect of alcohol on the cerebellum, reducing cerebellar blood flow (Volkow et al. 1988) or to diffuse alteration of Purkinje cell function (Sinclair & Lo, 1981; Basile et al. 1983; George & Chu, 1984; Carta et al. 2006). Second, the cerebellar cortex is one of the most sensitive brain regions to alcohol (Klemm et al. 1976), and alcohol consumption seems to alter the firing pattern of cycling and spontaneous activity of Purkinje cells, introducing irregularities in their discharge (Sinclair & Lo, 1981; George & Chu, 1984; Franklin & Gruol, 1987; Seo & Suh, 2001; Servais et al. 2005). As the firing activity of Purkinje cells encodes specific physiological functions (Kaczmarek & Levitan, 1987), alcohol consumption may alter cerebellar functions affecting motor coordination, equilibrium (Servais et al. 2005; Carta et al. 2006) and gaze‐holding mechanisms.
It is worth noting that the alcohol‐induced GEN may not be due only to a cerebellar deficit, as a possible interaction with the brainstem neural integrator may be not excluded. In fact, although alcohol greatly affects cerebellar functionality (Klemm et al. 1976; Dar, 2015; Luo, 2015; Valenzuela & Jotty, 2015), evidence from other studies such as the delayed transmission of acoustic brainstem potentials suggests an alcohol‐related alteration even in the brainstem (Chu et al. 1978; Squires et al. 1978a, b ; Porjesz & Begleiter, 1981).
In contrast to previous reports (Abel et al. 1978b; Shallo‐Hoffmann et al. 1990), the analyses performed to separate data from both eyes and hemifields showed no differences. The PV plots of LE and RE, however, did not completely overlap when plotted separately (Fig. 3 A, C). We believe that such a difference represents an artifact induced by the physiological drift of the eyes toward the resting point of vergence. Indeed, in the absence of an adequate visual stimulus (in most gaze‐holding studies the target flashes), the eyes drift towards their resting point, defined not only by vertical and horizontal position, but also by vergence.
As on average the fixation point of vergence at rest lies at about 1 m distance (Jaschinski‐Kruza, 1991; Rosenfield, 1997), although widely variable among subjects, and such distance frequently differs from the one between the target and the subject (e.g. 1.5 m in our set‐up), the eyes frequently perform vergence movements induced by tonic vergence (Rosenfield, 1997; Jaschinski et al. 2007).
In the PV plot this causes eye‐specific, positional offsets between the eye null and the null position in the target frame of reference (i.e. the resting point of vergence and our PV plot zero, respectively). Such eye‐specific offsets result in a discrepancy between the null positions of the two eyes matching the one observed in our data shown on the PV plot (Fig. 5). These differences need to be taken into account to distinguish gaze angles in temporal from nasal hemifields, as the null position of the eye needs to be extrapolated from the data. Using the fixation straight ahead as null point to separate eye movement directions may have led to the previously observed asymmetries (Abel et al. 1978b; Shallo‐Hoffmann et al. 1990). We avoided this confounder by shifting PV curves of each eye on the basis of the null position separately estimated for each eye.
Figure 5. Gaze angle versus drift velocity.
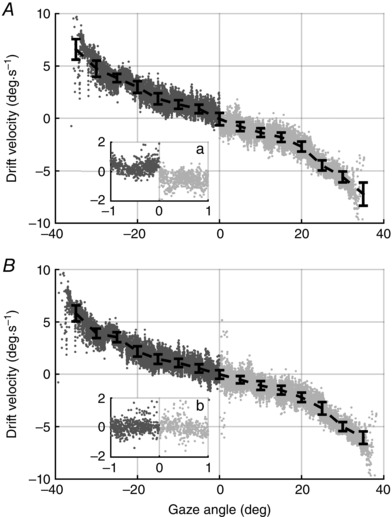
Gaze angle drift velocity relationship in the temporal hemifield (TH) estimated on data shown in Fig. 3 C and D, respectively, with (C) and without (B) eye‐specific positional offset. Dark grey and light grey data points: instantaneous velocities of slow phases, respectively, from the left and the right eye of Fig. 3. Black solid bars: median (MAD) of instantaneous drift velocity. In both A and B, TH data points were obtained pooling the left and right eye, considering gaze eccentricities being lesser and greater than the null position (i.e. zero of the PV plot), respectively. Using data shown in Fig. 3 C, a discontinuity is visible between data points from the left (dark grey dots) and right eye (light grey dots) in inset a. Such ambiguity is due to an incorrect alignment of the eyes in Fig. 3 C. Conversely, B, using data from correctly aligned eyes (Fig. 3 D), does not show any discontinuity between the data from the left and right eye (inset b).
Of note, comparison of the parameters of the tangent function describing gaze‐holding between TH and NH showed no significant differences between directions in both the BA and the AA condition. This consistency is important, because the high variability in the k 2 coefficient of TH and NH BA might have hidden an actual difference between directions. Alcohol intake, causing a scaling effect on both gaze angles in nasal and temporal hemifields, would, however, amplify such a difference, making it visible in the AA dataset. The absence of any significant difference for k 2 in the AA condition (Table 1) therefore supports the conclusion that such differences are absent also in the BA condition.
Additional Information
Competing interests
The authors declare no competing financial interests.
Author contributions
G.B. and A.A.T conceived, designed the research and with F.R. performed the experiments. F.R. implemented the analytical tools, interpreted the data, provided intellectual content, performed the statistical analysis, drafted and revised the final manuscript; G.B. and A.A.T provided intellectual content, revised and drafted the final manuscript. S.R. and D.S. participated in interpretation of data, wrote and critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and declare that all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The research was supported by the Swiss Foundation for Alcohol Research (Grant Reference Number: 253), the Swiss National Science Foundation and the Dr. Dabbous Foundation.
Acknowledgements
The authors thank Marco Penner and Christopher Bockisch for technical support.
References
- Abel LA, Dell'Osso LF & Daroff RB (1978a). Analog model for gaze‐evoked nystagmus. IEEE Trans Biomed Eng 25, 71–75. [DOI] [PubMed] [Google Scholar]
- Abel LA, Parker L, Daroff RB & Dell'Osso LF (1978b). End‐point nystagmus. Investig Ophthalmol Vis Sci 17, 539–544. [PubMed] [Google Scholar]
- Aschan G & Bergstedt M (1975). Positional alcoholic nystagmus (PAN) in man following repeated alcohol doses. Acta Otolaryngol Suppl 330, 15–29. [DOI] [PubMed] [Google Scholar]
- Basile A, Hoffer B & Dunwiddie T (1983). Differential sensitivity of cerebellar Purkinje neurons to ethanol in selectivity outbred lines of mice: maintenance in vitro independent of synaptic transmission. Brain Res 264, 69–78. [DOI] [PubMed] [Google Scholar]
- Bertolini G & Ramat S (2011). Velocity storage in the human vertical rotational vestibulo‐ocular reflex. Exp Brain Res 209, 51–63. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Tarnutzer AA, Olasagasti I, Khojasteh E, Weber KP, Bockisch CJ, Straumann D & Marti S (2013). Gaze holding in healthy subjects. PLoS One 8, e61389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker JL (2001). End‐position nystagmus as an indicator of ethanol intoxication. Sci Justice 41, 113–116. [DOI] [PubMed] [Google Scholar]
- Booker JL (2004). The Horizontal Gaze Nystagmus test: fraudulent science in the American courts. Sci Justice 44, 133–139. [DOI] [PubMed] [Google Scholar]
- Cannon SC & Robinson DA (1987). Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J Neurophysiol 57, 1383–1409. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M & Valenzuela CF (2006). Alcohol potently modulates climbing fiber→Purkinje neuron synapses: Role of metabotropic glutamate receptors. J Neurosci 26, 1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu NS, Squires KC & Starr A (1978). Auditory brain stem potentials in chronic alcohol intoxication and alcohol withdrawal. Arch Neurol 35, 596–602. [DOI] [PubMed] [Google Scholar]
- Citek K (2010). Gen is not HGN. Investig Ophthalmol Vis Sci 51, 6900–6901. [DOI] [PubMed] [Google Scholar]
- Citek K, Ball B & Rutledge DA (2003). Nystagmus testing in intoxicated individuals. Optometry 74, 695–710. [PubMed] [Google Scholar]
- Citek K, Elmont AD, Jons CL, Krezelok CJ, Neron JD, Plummer TA & Tannenbaum T (2011). Sleep deprivation does not mimic alcohol intoxication on field sobriety testing. J Forensic Sci 56, 1170–1179. [DOI] [PubMed] [Google Scholar]
- Dar MS (2015). Ethanol‐induced cerebellar ataxia: cellular and molecular mechanisms. Cerebellum 14, 447–465. [DOI] [PubMed] [Google Scholar]
- Dera T, Boning G, Barete S & Schneider E (2006). Low‐latency video tracking of horizontal, vertical and torsional eye movements as a basis for 3DOF realtime motion control of a head‐mounted camera. Conf Proc IEEE Int Conf Syst Man Cybern 6, 5191–5196. [Google Scholar]
- Elzenman M, Cheng P, Sharpe JA & Frecker RC (1990). End‐point nystagmus and ocular drift: an experimental and theoretical study. Vision Res 30, 863–877. [DOI] [PubMed] [Google Scholar]
- Fadda F & Rossetti ZL (1998). Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56, 385–431. [DOI] [PubMed] [Google Scholar]
- Franklin CL & Gruol DL (1987). Acute ethanol alters the firing pattern and glutamate response of cerebellar Purkinje neurons in culture. Brain Res 416, 205–218. [DOI] [PubMed] [Google Scholar]
- George F & Chu N‐S (1984). Effects of ethanol on Purkinje cells recorded from cerebellar slices. Alcohol 1, 353–358. [DOI] [PubMed] [Google Scholar]
- Glasauer S (2003). Cerebellar contribution to saccades and gaze holding: a modeling approach. Ann N Y Acad Sci 1004, 206–219. [DOI] [PubMed] [Google Scholar]
- Godaux E & Cheron G (1996). The hypothesis of the uniqueness of the oculomotor neural integrator: direct experimental evidence in the cat. J Physiol 492, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding GS & Dobie RA (1986). Gaze nystagmus and blood alcohol. Laryngoscope 96, 713–717. [DOI] [PubMed] [Google Scholar]
- Jaschinski‐Kruza W (1991). Eyestrain in VDU users: viewing distance and the resting position of ocular muscles. Hum Factors 33, 69–83. [DOI] [PubMed] [Google Scholar]
- Jaschinski W, Jainta S, Hoormann J & Walper N (2007). Objective vs subjective measurements of dark vergence. Ophthalmic Physiol Opt 27, 85–92. [DOI] [PubMed] [Google Scholar]
- Jones AW (1982). How breathing technique can influence the results of breath‐alcohol analysis. Med Sci Law 22, 275. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK & Levitan IB (1987). Neuromodulation: The Biochemical Control of Neuronal Excitability. Oxford University Press, Oxford. [Google Scholar]
- Klemm WR, Mallari CG, Dreyfus LR, Fiske JC, Forney E & Mikeska JA (1976). Ethanol‐induced regional and dose‐response differences in multiple‐unit activity in rabbits. Psychopharmacology (Berl) 49, 235–244. [DOI] [PubMed] [Google Scholar]
- Klockgether T (2010). Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol 9, 94–104. [DOI] [PubMed] [Google Scholar]
- Klockgether T (2012). Sporadic adult‐onset ataxia of unknown etiology In Handbook of Clinical Neurology, ed. Sankara HS. & Alexandra D, pp. 253–262. Elsevier, Amsterdam. [DOI] [PubMed] [Google Scholar]
- Koenker RW & Bassett G (1978). Regression quantiles. Econometrica 46, 33–50. [Google Scholar]
- Leech J, Gresty M, Hess K & Rudge P (1977). Gaze failure, drifting eye movements, and centripetal nystagmus in cerebellar disease. Br J Ophthalmol 61, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti HMJ (1976). The effect of blood alcohol concentration on the onset of gaze nystagmus. Blutalkohol 13, 411–414. [Google Scholar]
- Leigh RJ & Zee DS (2015). The Neurology of Eye Movements, 5th edn. Oxford University Press, Oxford. [Google Scholar]
- Leys C, Ley C, Klein O, Bernard P & Licata L (2013). Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol 49, 764–766. [Google Scholar]
- Luo J (2015). Effects of ethanol on the cerebellum: advances and prospects. Cerebellum 14, 383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritz KH, Dichgans J & Hufschmidt A (1979). Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain 102, 461–482. [DOI] [PubMed] [Google Scholar]
- McKnight AJ, Langston EA, McKnight AS & Lange JE (2002). Sobriety tests for low blood alcohol concentrations. Accid Anal Prev 34, 305–311. [DOI] [PubMed] [Google Scholar]
- Nakamagoe K, Iwamoto Y & Yoshida K (2000). Evidence for brainstem structures participating in oculomotor integration. Science 288, 857–859. [DOI] [PubMed] [Google Scholar]
- Optican LM & Zee DS (1984). A hypothetical explanation of congenital nystagmus. Biol Cybern 50, 119–134. [DOI] [PubMed] [Google Scholar]
- Porjesz B & Begleiter H (1981). Human evoked brain potentials and alcohol. Alcohol Clin Exp Res 5, 304–317. [DOI] [PubMed] [Google Scholar]
- Ramat S, Das VE, Somers JT & Leigh RJ (1999). Tests of two hypotheses to account for different‐sized saccades during disjunctive gaze shifts. Exp Brain Res 129, 500–510. [DOI] [PubMed] [Google Scholar]
- Robinson DA (1973). Models of the saccadic eye movement control system. Kybernetik 14, 71–83. [DOI] [PubMed] [Google Scholar]
- Robinson DA (1974). The effect of cerebellectomy on the cat's vestibulo‐ocular integrator. Brain Res 71, 195–207. [DOI] [PubMed] [Google Scholar]
- Rosenfield M (1997). Tonic vergence and vergence adaptation. Optom Vis Sci 74, 303–328. [DOI] [PubMed] [Google Scholar]
- Rubenzer SJ & Stevenson SB (2010). Horizontal gaze nystagmus: a review of vision science and application issues. J Forensic Sci 55, 394–409. [DOI] [PubMed] [Google Scholar]
- Schneider E, Bartl K, Bardins S, Dera T, Boening G & Brandt T (2005). Eye movement driven head‐mounted camera: it looks where the eyes look. Conf Proc IEEE Int Conf Syst Man Cybern 3, 2437–2442. [Google Scholar]
- Seo WS & Suh CK (2001). Acute effect of ethanol on firing patterns of Purkinje cells in the rat cerebellar slice preparation. Yonsei Med J 42, 384–389. [DOI] [PubMed] [Google Scholar]
- Servais L, Bearzatto B, Delvaux V, Noël E, Leach R, Brasseur M, Schiffmann SN & Guy C (2005). Effect of chronic ethanol ingestion on Purkinje and Golgi cell firing in vivo and on motor coordination in mice. Brain Res 1055, 171–179. [DOI] [PubMed] [Google Scholar]
- Setta F, Jacquy J, Hildebrand J & Manto MU (1998). Ataxia induced by small amounts of alcohol. J Neurol Neurosurg Psychiatry 65, 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallo‐Hoffmann J, Schwarze H, Simonsz HJ & Muhlendyck H (1990). A reexamination of end‐point and rebound nystagmus in normals. Investig Ophthalmol Vis Sci 31, 388–392. [PubMed] [Google Scholar]
- Sinclair JG & Lo GF (1981). The effects of ethanol on cerebellar purkinje cell discharge pattern and inhibition evoked by local surface stimulation. Brain Res 204, 465–471. [DOI] [PubMed] [Google Scholar]
- Squires KC, Chu NS & Starr A (1978a). Auditory brain stem potentials with alcohol. Electroencephalogr Clin Neurophysiol 45, 577–584. [DOI] [PubMed] [Google Scholar]
- Squires KC, Chu NS & Starr A (1978b). Acute effects of alcohol on auditory brainstem potentials in humans. Science 201, 174–176. [DOI] [PubMed] [Google Scholar]
- Tarnutzer AA, Weber KP, Schuknecht B, Straumann D, Marti S & Bertolini G (2015). Gaze holding deficits discriminate early from late onset cerebellar degeneration. J Neurol 262, 2015. [DOI] [PubMed] [Google Scholar]
- Tharp V, Burns M, Moskowitz H & NHTSA (1981). Development and Field Test of Psychophysical Tests for DWI Arrest. U.S. Department of Transport, National Highway Traffic Safety Administration, Final Report, Publication No. DOT‐HS‐805‐864.
- Valenzuela CF & Jotty K (2015). Mini‐review: Effects of ethanol on GABAA receptor‐mediated neurotransmission in the cerebellar cortex – recent advances. Cerebellum 14, 438–446. [DOI] [PubMed] [Google Scholar]
- Versino M, Hurko O & Zee DS (1996). Disorders of binocular control of eye movements in patients with cerebellar dysfunction. Brain 119, 1933–1950. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE & Dewey S (1988). Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res 24, 201–209. [DOI] [PubMed] [Google Scholar]
- Whyte CA, Petrock AM & Rosenberg M (2010). Occurrence of physiologic gaze‐evoked nystagmus at small angles of gaze. Investig Ophthalmol Vis Sci 51, 2476–2478. [DOI] [PubMed] [Google Scholar]
- Widmark EMP (1981). Principles and Applications of Medicolegal Alcohol Determination. Biomedical Publications, Davis, CA. [Google Scholar]
- Zee DS, Leigh RJ, Mathieu‐Millaire F, Leigh JR & Mathieu‐Millaire F (1980). Cerebellar control of ocular gaze stability. Ann Neurol 7, 37–40. [DOI] [PubMed] [Google Scholar]


