Abstract
Key Points
Astronauts have recently been discovered to have impaired vision, with a presentation that resembles syndromes of elevated intracranial pressure on Earth.
Gravity has a profound effect on fluid distribution and pressure within the human circulation. In contrast to prevailing theory, we observed that microgravity reduces central venous and intracranial pressure.
This being said, intracranial pressure is not reduced to the levels observed in the 90 deg seated upright posture on Earth. Thus, over 24 h in zero gravity, pressure in the brain is slightly above that observed on Earth, which may explain remodelling of the eye in astronauts.
Abstract
Astronauts have recently been discovered to have impaired vision, with a presentation that resembles syndromes of elevated intracranial pressure (ICP). This syndrome is considered the most mission‐critical medical problem identified in the past decade of manned spaceflight. We recruited five men and three women who had an Ommaya reservoir inserted for the delivery of prophylactic CNS chemotherapy, but were free of their malignant disease for at least 1 year. ICP was assessed by placing a fluid‐filled 25 gauge butterfly needle into the Ommaya reservoir. Subjects were studied in the upright and supine position, during acute zero gravity (parabolic flight) and prolonged simulated microgravity (6 deg head‐down tilt bedrest). icp was lower when seated in the 90 deg upright posture compared to lying supine (seated, 4 ± 1 vs. supine, 15 ± 2 mmHg). Whilst lying in the supine posture, central venous pressure (supine, 7 ± 3 vs. microgravity, 4 ± 2 mmHg) and ICP (supine, 17 ± 2 vs. microgravity, 13 ± 2 mmHg) were reduced in acute zero gravity, although not to the levels observed in the 90 deg seated upright posture on Earth. Prolonged periods of simulated microgravity did not cause progressive elevations in ICP (supine, 15 ± 2 vs. 24 h head‐down tilt, 15 ± 4 mmHg). Complete removal of gravity does not pathologically elevate ICP but does prevent the normal lowering of ICP when upright. These findings suggest the human brain is protected by the daily circadian cycles in regional ICPs, without which pathology may occur.
Keywords: bedrest, idiopathic intracranial hypertension, ocular remodeling, posture, space
Key Points
Astronauts have recently been discovered to have impaired vision, with a presentation that resembles syndromes of elevated intracranial pressure on Earth.
Gravity has a profound effect on fluid distribution and pressure within the human circulation. In contrast to prevailing theory, we observed that microgravity reduces central venous and intracranial pressure.
This being said, intracranial pressure is not reduced to the levels observed in the 90 deg seated upright posture on Earth. Thus, over 24 h in zero gravity, pressure in the brain is slightly above that observed on Earth, which may explain remodelling of the eye in astronauts.
Abbreviations
- BP
blood pressure
- CSF
cerebral spinal fluid
- HDT
head‐down tilt
- ICP
intracranial pressure
- IOP
intraocular pressure
Introduction
When humans sit upright on Earth, the head‐to‐foot hydrostatic gradient (Gz) pulls fluid towards the feet. Conversely when lying in the supine posture, typically whilst asleep at night, fluid redistributes towards the head (Rowell, 1986). Thus, gravity profoundly affects pressure, volume and flow in the human circulation with habitual changes in posture causing diurnal fluctuations in the distribution of fluid and regional pressures.
On Earth, these pressure fluctuations are normal and generally do not cause pathology. However, there are specific human conditions that are exacerbated or in some cases caused by these fluid shifts. For example, idiopathic intracranial hypertension and its symptoms are worse in the supine position, and in some cases may be detectable only at night (Torbey et al. 2004). Intracranial hypotension occurs primarily in the upright position, and both these conditions can cause severe and intractable headache (Ducros & Biousse, 2015).
In the absence of gravity, e.g. the microgravity environment encountered by astronauts on the International Space Station, this cephalad redistribution of body fluids, and in particular the cephalad redistribution of cerebral spinal fluid (CSF), has been hypothesized to cause a newly discovered syndrome of deterioration of visual acuity in astronauts (Mader et al. 2011). In microgravity, redistribution of fluid towards the brain is hypothesized to elevate intracranial pressure (ICP) in a manner disproportionate to intraocular pressure (IOP), thus resulting in a reduced pressure gradient (IOP–ICP) across the lamina cribrosa at the posterior aspect of the eye. Indeed, in an acute model of simulated microgravity, head‐down tilt (HDT; −9 and −10 deg) caused increased ICP (Petersen et al. 2016; Eklund et al. 2016) but not IOP (Eklund et al. 2016). Astronauts with this syndrome develop physical findings suggestive of elevated ICP including papilloedema, flattening of the globe of the eye and dilatation of the optic nerve sheath. This analogy and the theoretical link between cephalad fluid shift and intracranial hypertension has led NASA flight surgeons to refer to the syndrome as the Visual Impairment/Intracranial Pressure syndrome, or ‘VIIP’ and it is considered to be NASA's top health risk for long‐duration spaceflight, including exploration missions to Mars (Mader et al. 2011). In addition to the exposure to microgravity on the International Space Station, it has been hypothesized that other environmental conditions (i.e. elevated ambient CO2 concentration) and rigorous strength‐based exercises regularly performed by astronauts to avoid musculoskeletal loss create a ‘perfect storm’ that causes raised ICP and visual impairments in astronauts (Nelson et al. 2014; Michael & Marshall‐Bowman, 2015).
Despite the attractiveness of this theory, however, no direct ICP measurements in microgravity with or without elevated CO2 or resistance exercise have been made. Moreover, invasive studies of cardiovascular adjustments to microgravity have demonstrated that microgravity physiology is complex and at times counterintuitive; for example, central venous pressure (CVP), originally thought to be increased in astronauts, was decreased (Buckey et al. 1993; Videbaek & Norsk, 1997). Despite our conceptual understanding of these fundamental relationships between gravity and human physiology, no investigation has documented the simultaneous adjustments in arterial, venous and cerebral spinal fluid pressures due to the influence of gravity.
Methods
Participants
Five men (age, 35 ± 13 years; height, 177 ± 13 cm; weight 87 ± 22 kg) and three women (age, 36 ± 4 years; height, 167 ± 11 cm; weight 63 ± 10 kg) who previously had an Ommaya reservoir (catheter placed from the lateral ventricle to a cerebral spinal fluid‐filled reservoir under the scalp) inserted for the delivery of prophylactic CNS chemotherapy as part of their treatment for haematological malignancy were recruited to take part in this study. E‐mails were sent to the medical directors of most major medical centres in the US managing adults with haematological malignancies describing the inclusion/exclusion criteria and requesting referrals. A Facebook page was created, and patient support groups were messaged, although the physicians of all interested volunteers were also consulted. All participants had structurally normal brains documented with magnetic resonance imaging, were free of their malignant disease for at least 1 year, had normal cardiovascular systolic and diastolic function documented by echocardiography, had normal complete blood counts including haematocrit, white blood cell count and platelets, and were on no regular medications that affect cerebral haemodynamics. All participants were informed of the purpose and risks of each procedure and signed an informed consent form, which was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center and NASA Johnson Space Centre and followed guidelines set forth in the Declaration of Helsinki.
Data collection
Intracranial pressure
After a single intravenous dose of cefazolin, a fluid‐filled 25 gauge butterfly needle was inserted in the Ommaya reservoir and connected to a pressure transducer (Transpac IV; Abbott, Chicago, IL, USA; Fig. 1). The wings of the butterfly needle were sutured to the scalp surrounding the Ommaya reservoir and secured with a head dressing so participants could move freely without dislodging the butterfly needle. The presence of cardiac and respiratory oscillations concomitant with the appropriate response to cough, Valsalva manoeuvre and head turning was used to confirm that the pressure waveform reflected ICP. The transducer was fixed at the level of the external acoustic meatus throughout all experimental conditions. As such, ICP was accurate through changes in posture and during microgravity. The pressure transducer was periodically zeroed to the atmospheric reference point.
Figure 1. Diagrammatic presentation of the experimental set‐up.
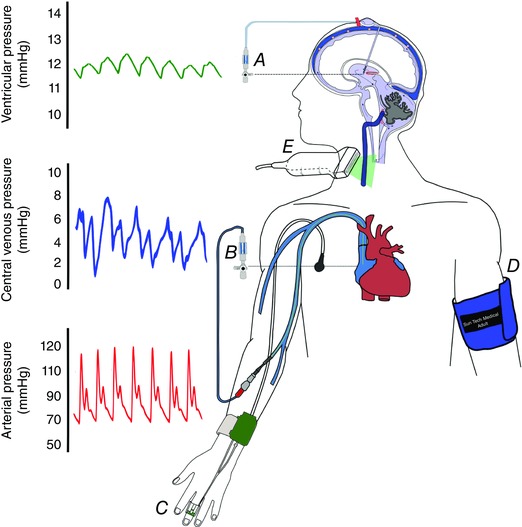
A and B, intracranial pressure (A) and central venous pressure (B) were measured directly via fluid coupled pressure transducers. C–E, beat‐by‐beat arterial blood pressure (C), arterial cuff pressure (D) and jugular venous cross‐sectional area (E) were measured non‐invasively. Note that intracranial, central venous and arterial pressure recordings were referenced to the external auditory meatus and right atrium, respectively (dashed lines). [Color figure can be viewed at wileyonlinelibrary.com]
Central venous pressure
A fluid‐filled 4 French peripherally inserted central catheter was placed using ultrasound guidance into a brachial vein. Thereafter, the catheter was advanced to the level of the right atrium under fluoroscopic guidance and the correct position was confirmed by fluoroscopy and the presence of characteristic CVP waveforms (Fig. 1). The position of the right atrium was marked on the body in the anteroposterior and lateral planes; the pressure transducer (Transpac IV; Abbott) was fixed to the body at the level of the right atrium during parabolic flight (study 2). During changes in posture (study 1) and bedrest (study 3) the pressure transducer was fixed to a pole and levelled to the right atrium with each change in posture. The pressure transducer was periodically zeroed to the atmospheric reference point. Both ICP and CVP pressure transducers were connected to a patient monitor (Tram‐rac, Marquette, Milwaukee, WI, USA) with analog signal digitized through an analog to digital converter (MP‐150, Biopac Systems, Goleta, CA, USA).
Haemodynamics
With changes in posture (study 1) and throughout HDT (study 3), steady‐state arterial blood pressure was measured by electrosphygmomanometry (SunTechMedical Instruments Inc., Morrisville, NC, USA; Fig. 1) with a microphone placed over the brachial artery to detect Korotkoff sounds. During parabolic flight (study 2), bedrest (study 3) and throughout leg press exercise (study 4), beat‐by‐beat arterial blood pressure was also measured by photoplethysmography (Nexfin; BMEYE, Amsterdam, the Netherlands; Fig. 1), with changes in cardiac stroke volume calculated using the Modelflow method (Wesseling et al. 1993; Shibata & Levine, 2011). The beat‐by‐beat blood pressure device was turned off during sleep (study 3). Prior to steady state data collection with changes in posture and during HDT, haemodynamic stability was confirmed by stable cardiac outputs (data not shown) via the acetylene rebreathing technique (model MGA1100, Marquette) (Jarvis et al. 2007).
Jugular venous diameter
As an indirect indicator of cephalad fluid volume (fluid shifts above the heart), the cross‐sectional area of the right internal jugular vein was measured by cardiac‐gated B mode ultrasonography using a 7–12 MHz linear transducer (IE33, Phillips, USA; Fig. 1). The internal jugular vein was imaged 2 cm below the carotid bifurcation with care taken not to compress the vein. Identification of the jugular vein was confirmed by colour Doppler and by asking subjects to perform a small Valsalva manoeuvre. The maximum cross‐sectional area was measured off‐line at the ECG T‐wave in triplicate for each condition (OsiriX, USA).
Experimental protocols
Study 1 assessed changes in ICP with change in posture from the 90 deg sitting upright to supine position. Moreover, whilst in the supine position, subjects placed their head on a pillow to assess the influence of this common procedure in patients subject to long‐term bed rest. Measurements were performed in a quiet, environmentally controlled laboratory with an ambient temperature of ∼25°C. Five minutes of steady state haemodynamics were obtained in the 90 deg sitting and supine positions. Study 2 assessed changes in ICP during removal of all hydrostatic gradients with brief (∼20 s) exposure to microgravity during parabolic flight. Subjects received a single dose of weight‐adjusted intramuscular scopolamine [< 110 lb (1 lb = 0.45 kg), 0.1 mg; 111–160 lb, 0.2 mg; 161–220 lb, 0.3 mg; > 220 lb, 0.4 mg] ∼1 h before flight to prevent nausea and vomiting, which would confound the data. Continuous data were averaged over at least 1 m during 1 g and over six sequential, 20‐s periods of 0 g. Gravity was measured continuously from the onboard accelerometer. Moreover, to document any effect of changes in cabin pressure during parabolic flight, separate pressure transducers were set up to measure cabin pressure directly and pressure within a closed, fluid‐filled length of tubing positioned perpendicular to the direction of flight (i.e. the same direction as the subjects). No changes were observed in either pressure system during parabolic flight.
Study 3 assessed changes in ICP during prolonged exposure to simulated microgravity using a model of −6 deg HDT. During data analysis, careful attention was paid to identify pathological pressure waves as a sign of intracranial instability (Torbey et al. 2004). Furthermore, the pulse amplitude of the ICP waveform was used to assess the compliance of the craniospinal system (Qvarlander et al. 2013 a). Protocol 4 assessed changes in ICP associated with increased ambient CO2 equal to that measured on the International Space Station [ of 0.7% at 4 mmHg (Law et al. 2014)], and with leg press exercise typically undertaken by astronauts to avoid musculoskeletal atrophy in space. Additional CO2 was delivered by a tight fitting face mask. During parabolic flight, participants breathed this gas mixture for 3–5 min during the 1 g turn around (supine condition) and throughout one set of 5–10 parabolas. During bedrest, subjects breathed this CO2 mixture for 5 min in the supine position and after 5 min (acute) and 24 h (prolonged) of −6 deg HDT. During onboard parabolic flight, data were averaged over the final minute of CO2 breathing in 1 g and averaged over the following 5–10 periods of 0 g. In the supine and HDT conditions, data were averaged over the last minute of steady state.
To replicate the typical exercise regimes undertaken by astronauts to avoid musculoskeletal atrophy during spaceflight, leg press exercise was performed whilst breathing additional CO2. Therefore, during parabolic flight (5–10 parabolas), the supine position and after 5 min and 24 h HDT, participants continued to breathe additional CO2 whilst performing moderate to heavy (7–8 on a 0–10 BORG rating of perceived exertion scale; Cooper et al. 1979) leg press exercise with self‐paced breathing. During HDT, participants also completed five repetitions whilst performing Valsalva manoeuvres during the contraction phase and five repetitions whilst forcefully inhaling (Muller manoeuvre) during the contraction phase. During onboard parabolic flight, data were averaged over 1 min in 1 g and during the following 5–10 periods of 0 g with leg press exercise. In the supine and HDT conditions, data were averaged over 1 min prior to and throughout leg press exercise. Data are expressed as the average ICP at baseline and during each exercise condition.
Results
Study 1: changes in posture typically experienced during normal life on Earth
Transitioning from the 90 deg sitting to the supine posture (Fig. 2 A), which removes the Gz hydrostatic gradient, caused an immediate increase in ICP that peaked in ∼13 s and then declined slightly to reach steady state by 3 min (Fig. 2 B). At rest in the 90 deg sitting posture, CVP was 2 ± 3 mmHg and ICP was 4 ± 1 mmHg (Fig. 2 C). Despite the large hydrostatic gradient from the brain to the heart, it is likely that ICP was not lower than this value because the jugular veins are partially collapsed in the upright position (Fig. 2 D), functioning as Starling resistors that interrupt the venous fluid column between the dural venous sinuses and the right atrium, and thus isolating the dural sinus pressure from CVP (Qvarlander et al. 2013 b). ICP was higher in the supine compared to the 90 deg sitting posture in every subject (ΔICP 1.1±2.5 mmHg, Fig. 2 C). The immediate rise in ICP upon becoming supine was probably due to rapid redistribution of CSF and venous blood (ΔCVP 4.4 ± 3.5 mmHg, Fig. 2 C) toward the head. Furthermore, in the supine posture, the jugular veins are widely patent (Fig. 2 D), which permits the CVP to be transmitted directly to the dural venous sinuses, thereby influencing CSF resorption and ICP. This principle, that dural venous sinus pressure dictates ICP with changes in posture (Davson equation; Davson et al. 1973; Qvarlander et al. 2013 b), is supported by our data, which also found that the prediction of ICP based on a simple hydrostatic indifference point for the CSF system slightly underestimated the measured ICP (30 deg, −0.1 ± 1.6; 60 deg, −0.8 ± 2.1 mmHg, Fig. 2 E), implying that another factor, such as collapsibility of the jugular veins, contributes to the absolute change in upright ICP.
Figure 2. Changing from the sitting to supine posture on Earth increases ICP.
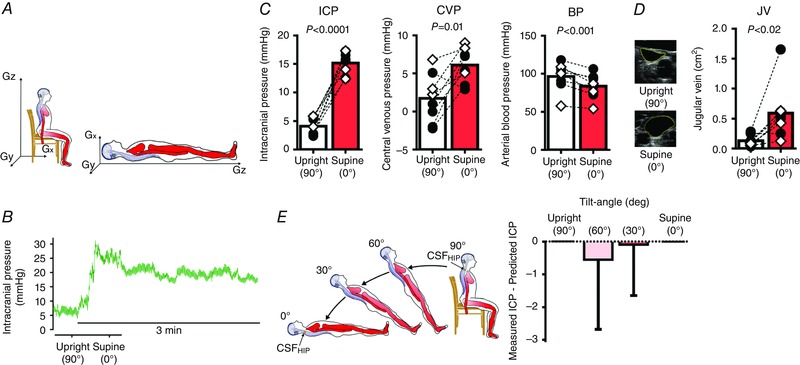
A, experimental set‐up used to quantify pressure changes associated with change in posture on Earth. Gz, head‐to‐foot hydrostatic gradient irrespective of position, which is eliminated in the supine posture. Gx, front‐to‐back hydrostatic gradient, which is present in the supine posture. Gy, side‐to‐side hydrostatic gradient. Needle access to an Ommaya reservoir and placement of a peripherally inserted central catheter permitted intracranial pressure (ICP) and central venous pressure (CVP) measurement by fluid‐coupled pressure transducers, which were referenced to the external auditory meatus and right atrium, such that ICP and CVP were accurate throughout changes in posture. Arterial blood pressure (BP) was obtained by electrosphygmomanometry. B, original recording of changes in ICP in one participant by changing from the 90 deg sitting upright posture to the supine posture. C, mean ICP and CVP are higher, whereas BP is slightly lower in the 90 deg sitting upright posture compared to supine; n = 8, paired t tests. Black circles, males; white diamonds, females. D, original 2D images of jugular veins in the 90 deg sitting upright posture and supine posture from one participant. Jugular vein volume is smaller in the 90 deg sitting upright posture compared to supine; n = 8, paired t test. E, participants (n = 4) were passively placed in the 90, 60, 30 and 0 deg positions for 2 min (left). Hydrostatic indifference point (HIP)CSF, where CSF pressure remains constant despite changes in posture, was calculated for each individual from the 90 deg seated upright and supine ICP. Prediction of ICP in the 30 and 60 deg positions (right) by subtraction of the height of the fluid column superior to the HIPCSF with tilt angle (α). [Color figure can be viewed at wileyonlinelibrary.com]
The mean blood pressure (BP) referenced at the heart, fell (Δ13±7.1 mmHg, Fig. 2 C) with the transition from the 90 deg sitting posture to the supine posture, predominantly because of a decrease in diastolic blood pressure from vasodilatation. However, when the BP was referenced to the external auditory meatus, to account for the heart‐to‐head hydrostatic fluid column to calculate cerebral perfusion pressure (CPP = mean BP – ICP), the BP was actually higher in the supine posture (90 deg sitting, 74 ± 18 vs. supine, 84 ± 16 mmHg, P = 0.01). As a result of these balanced changes in BP and ICP at the level of the brain, CPP remained stable regardless of body posture (90 deg sitting, 70 ± 18 vs. supine, 66 ± 18 mmHg, P = 0.13).
While in the supine position, placing the head on a pillow consistently reduced ICP (supine 14 ± 2 vs. pillow, 10 ± 2 mmHg, P = 0.05).
Study 2: acute microgravity
To investigate the effect of acute microgravity on supine ICP, we examined subjects during parabolic flight (Fig. 3 A), which creates periods of true 0 g and its associated fluid shifts (Fig. 3 B) (Petersen et al. 2011). Throughout the parabolic flight, participants remained in the supine position with their spinal axis (z axis) perpendicular to the direction of flight to minimize any fluid shifts associated with change in the angle of the aircraft. As the aircraft flew level (1 g) for baseline measurements, ICP was stable and comparable to supine measurements made in Study 1 (∼16 mmHg, Fig. 3 C). As the aircraft pulled up to an angle of 45 deg, gravity almost doubled in the Gx (front‐to‐back) direction, which increased the CVP and ICP (Fig. 3 B), presumably due to an increase in intrathoracic pressure (Videbaek & Norsk, 1997). During descent of the aircraft, when gravity was reduced to zero, ICP fell immediately (Fig. 3 B) and was followed a further gradual decline to ∼20% below the 1 g supine value (Δ −3.8 ± 2.9 mmHg, Fig. 3 C). Importantly, although ICP in 0 g decreased compared to the supine posture, it was not as low as ICP in the 90 deg sitting posture on Earth (1 g) (0 g, 13 ± 2.6 mmHg vs. 90 deg sitting, 4.1 ± 1.4 mmHg, P < 0.0001, Fig. 4).
Figure 3. Microgravity reduces ICP in the supine posture.
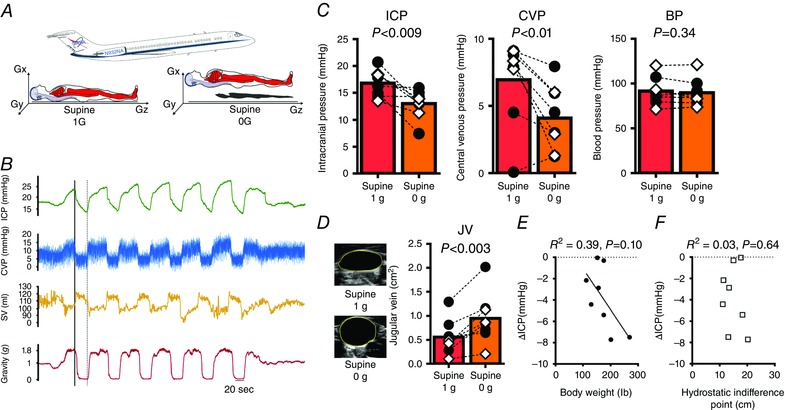
A, successive brief periods of microgravity (∼20 s) were generated during parabolic flight as participants lay in the supine posture, in which the Gz hydrostatic gradient is eliminated but the Gx gradient remains. In 0 g all hydrostatic gradients are absent. B, original recordings in one participant of changes in intracranial pressure (ICP), central venous pressure (CVP), left ventricular stroke volume (SV) and gravity throughout sox parabolas. Increased SV despite a fall in CVP during 0 g confirms cardiac ventricular distension due to a fall in intrathoracic pressure and thus an increase in the transmural cardiac filling pressure. The continuous line and the dashed line indicate the beginning and end of the first 0 g period. ICP increases at the beginning of the parabola as gravity almost doubles in the Gx direction. However, immediately upon entering 0 g, ICP falls rapidly to approximately the supine 1 g value. ICP then continues to fall, and by the end of the 0 g period, ICP is lower than the supine 1 g value. C, mean ICP and CVP decrease, and BP remains stable in 0 g compared to the supine 1 g posture; n = 8, paired t tests. D, original 2D images of jugular veins show further distension in 0 g compared to the supine 1 g posture. E and F, relationships between the fall in ICP in 0 g with (E) body weight and (F) HIPCSF; n = 8, linear regression. Black circles, males; white diamonds, females. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4. Dissociation between 24 h ICP on Earth versus microgravity as a characteristic for optic remodeling during long duration space flight.

A, difference between 24 h intracranial pressure (ICP) on Earth (calculated as 2/3 × 90 deg sitting upright ICP + 1/3 × supine ICP, where supine ICP is assumed to reflect sleep) and during 0 g parabolic flight (assumed to reflect ICP throughout long duration space flight). B, the same data split into males versus females. Although subject numbers are too low for conclusive between‐group statistical analysis, removal of a single suspected outlier (>2SD from the mean) in the male cohort (dashed circle) suggests that females may have a smaller pressure differential between Earth and microgravity; n = 7 males, n = 3 females, unpaired t test. Black circles, males; white diamonds, females. This calculation reflects the 24 h difference in ICP between Earth and microgravity environments, assuming short‐term measurements during parabolic flight reflect long‐term measurements in space. Due to gravity, the brain and eye expect to ‘see’ a low pressure environment throughout two‐thirds of the day, and therefore in microgravity a greater relative posterior optic pressure would be ‘felt’ throughout spaceflight, especially in males, which may explain the increased severity of optic remodelling observed in male versus female astronauts. The single male ‘outlier’ may also explain the atypical male astronauts completely devoid of visual impairment in space.
As noted in previous parabolic flight experiments (Buckey et al. 1993; Videbaek & Norsk, 1997), CVP fell consistently upon entering 0 g (Δ −2.8 ± 2.5 mmHg, Fig. 3 C), probably due to the reduction of intrathoracic pressure in microgravity (Videbaek & Norsk, 1997). Ultrasound demonstrated that the cross‐sectional area of the jugular veins increased during 0 g (Fig. 3 D), which implies that a continuous venous fluid column between the dural venous sinuses and the right atrium exists in microgravity, which influences ICP (Davson et al. 1973). The location of the hydrostatic indifference point for the CSF was not related to the fall in ICP in 0 g (Fig. 3 E); however, there was a modest predictive trend between body weight (a surrogate measure of weight on the chest which is eliminated in 0 g) and the fall in ICP in 0 g (40% of the variance, P = 0.097, Fig. 3 F). Arterial blood pressure was stable between the supine and 0 g conditions (Δ −1.85 ± 5.1 mmHg, Fig. 3 C).
Study 3: 24 h of simulated microgravity with −6 deg HDT
Although the reduction in ICP during acute microgravity (Study 2) was clear and unequivocal, the duration of microgravity with each parabolic arc lasted only ∼20–30 s. Therefore, the effect of longer periods of microgravity on ICP remained unknown. To address this question, we placed subjects in the −6 deg HDT position for 24 h (Fig. 5 A). This widely used Earth‐based model of prolonged microgravity has been shown to cause cephalad fluid shifts similar to those observed in space (Pavy‐Le Traon et al. 2007). Transition from supine to −6 deg HDT caused an immediate, albeit slight increase in ICP (Fig. 5 B) in every subject (Δ 1.8 ± 0.5 mmHg, Fig. 5 C). In contrast, the CVP was unchanged (Δ −0.49 ± 0.5 mmHg, Fig. 5 C).
Figure 5. Twenty‐four hours of simulated microgravity does not elevate ICP.
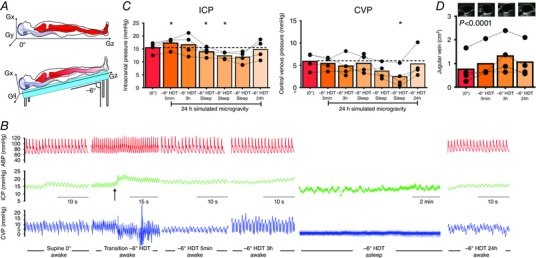
A, diagram of the experimental set‐up used to simulate microgravity. Note that in −6 deg head‐down tilt (HDT), the Gx hydrostatic gradient and a slight foot‐to‐head Gz hydrostatic gradient exist. B, original recordings in one participant of changes in arterial blood pressure (BP), intracranial pressure (ICP) and central venous pressure (CVP) throughout 24 h of simulated microgravity. The beat‐to‐beat blood pressure device was turned off during sleep. Arrow indicates onset of passive tilt to the −6 deg HDT position. Note the compressed time scale in the second graph to demonstrate the transition from supine to HDT. C, in every subject, ICP was slightly higher in the −6 deg HDT position compared to the supine posture, which is entirely explained by the increase in Gz, foot‐to‐head hydrostatic column within the CSF and venous compartments (Fig. 6). During sleep, ICP falls below the supine value and, importantly, ICP returns to the supine baseline value after 24 h of −6 deg HDT; n = 4, one‐way ANOVA, * P < 0.05 vs. 0 deg. C, during sleep in −6 deg HDT, CVP is lower than the supine value, and remains slightly lower than the supine value in three of the four subjects after 24 h of −6 deg HDT; n = 4, one‐way ANOVA, * P < 0.05 vs. 0 deg. D, jugular vein distension persists throughout 24 h of −6 deg HDT, one‐way ANOVA with follow‐up test, 5 min (P = 0.0), 3 h (P = 0.09) and 24 h (P = 0.15) vs. 0 deg. Black circles, males. [Color figure can be viewed at wileyonlinelibrary.com]
Because the head is below the heart in the −6 deg HDT position, the pressure of the cerebral venous and CSF systems, when compared to their respective hydrostatic indifference points, is increased proportional to the length of the hydrostatic column, which explains the increase in ICP (Fig. 6). After 3 h of HDT, the ICP returned to the supine value (Δ 1.1 ± 2.3 mmHg, Fig. 5 C) probably due to the reduction in CVP, which equates to sagittal sinus pressure in the HDT position. At night, during quiet rest and sleep, ICP was slightly reduced compared to the supine posture (Δ −3.1 ± 1.4 mmHg), also corresponding to a slight fall in CVP (Δ −3.45 ± 1.6 mmHg, Fig. 5 C). By the end of 24 h HDT simulated microgravity, when subjects were awake, ICP and CVP had returned to baseline supine values (ICP, 14.8 ± 3.7; CVP, 5.3 ± 3.2 mmHg, Fig. 5 C). This normalization of ICP occurred despite a persistent hydrostatic gradient from the heart to the head over the previous 24 h, as evidenced by persistent jugular venous distension (Fig. 5 D).
Figure 6. Simple hydrostatics explain the increase in intracranial pressure with acute −6 deg head down tilt (HDT).

A, in the −6 deg HDT model, the head is placed below the heart. Therefore, the slight foot‐to‐head (Gz) hydrostatic gradient causes pressure within both the venous and cerebral spinal fluid systems to increase proportional the length of the hydrostatic column superior to their respective hydrostatic indifference points. B, from the supine measurement of central venous pressure and the L‐HIPvein, dural sinus pressure (Pd) was predicted to increase by 2.9 ± 0.2 mmHg. Thus, according to the Davson equation, intracranial pressure (ICP) must rise to exceed dural sinus pressure to maintain cerebral spinal fluid absorption into the sinus. Indeed, the predicted increase in ICP based on the L‐HIPCSF model (1.2 ± 0.6 mmHg) and the measured increase in ICP (1.8 ± 0.5 mmHg) were similar. Nevertheless, the important take home message is that ICP did not increase substantially beyond that expected by simple hydrostatic gradients, and thus cephalad fluid shifts do not cause disproportionate and pathological increases in ICP. [Color figure can be viewed at wileyonlinelibrary.com]
During quiet rest and sleep in HDT, the ICP pulse amplitude was slightly reduced compared to the supine posture, implying that intracranial compliance was unchanged or slightly improved (Fig. 7). One subject was noted to have B‐waves (normally associated with impaired intracranial compliance) sporadically during quiet rest and sleep during HDT; however, the same pattern was seen while this subject was awake in the supine posture, so the B‐waves seen during sleep in the HDT posture were not necessarily due to simulated microgravity.
Figure 7. Sleeping in simulated microgravity does not reduce intracranial compliance.
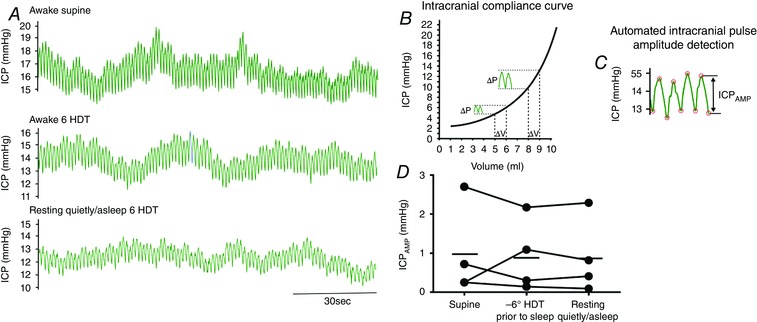
A, original recordings of pulsatile changes in intracranial pressure (ICP) in the awake supine position (top), in the −6 deg HDT position (HDT) just prior to sleep (middle) and during quiet rest/sleeping as document by a Registered Nurse in one human participant. B, pressure–volume intracranial compliance curve. In the steep part of the compliance curve, a 1 ml change in volume (during each heart beat) causes a much larger increase in ICP pulsatility than in the flat portion of the curve; thus, intracranial pulse amplitude (ICPAMP) reflects the compliance state of the intracranial compartment. C, successive diastolic and systolic peaks were detected from continous ICP data by an automated ECG‐gated time‐windowing algorithm. ICPAMP was averaged over 2 min of steady‐state in each condition. D, compared to the supine position, ICPAMP fell slightly in three of the four participants just prior to falling asleep and remained below the supine value while resting quietly/asleep. [Color figure can be viewed at wileyonlinelibrary.com]
Study 4: elevated inspired CO2 concentration and leg press resistance exercise
When compared to the resting condition while breathing ambient air, the addition of 0.7% CO2 (which is the maximum 24‐h peak concentration on the International Space Station; Alexander, 2012) had no effect on ICP either in the supine posture, or at any time during the 24 h of simulated microgravity with HDT bedrest (Fig. 8 A). Furthermore, the addition of 0.7% CO2 during parabolic flight slightly exaggerated the fall in ICP during acute zero gravity (normocapnia, −3.8 ± 2.9; 0.7% CO2, −5.4 ± 2.6 mmHg, Fig. 8 B).
Figure 8. 0.7% carbon dioxide inhalation does not increase ICP during real or simulated microgravity.
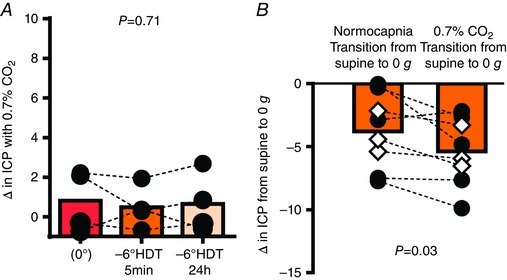
Carbon dioxide (CO2) was administered via a tight fitting facemask for 3–5 min prior to a set of parabolas or steady‐state data collection during bedrest. A, the intracranial pressure (ICP) response to 0.7% CO2 is similar between the supine and acute (5 min) and prolonged (24 h) simulated microgravity conditions. Note that 0.7% CO2 did not clinically increase ICP even after 5 min of HDT despite mean ICP being slightly elevated; n = 4, one‐way ANOVA. B, comparison of the decrease in ICP during microgravity whilst breathing normal air (normoapnia) versus 0.7% CO2; n = 8, paired t test. [Color figure can be viewed at wileyonlinelibrary.com]
CVP and ICP generally increased during the contraction phase of leg press exercise (Fig. 9 A). However, there were important differences observed based on the subject's breathing pattern. For example, when participants performed a Valsalva manoeuvre during leg press – thereby markedly increasing intrathoracic pressure – BP, CVP and ICP rose (Fig. 9 B), and during the relaxation phase, arterial blood pressure, CVP and ICP fell immediately (Fig. 9 B). The synchronized rise and fall in all pressures resulted in an almost constant arterial–venous pressure gradient throughout the brain (Haykowsky et al. 2003) and therefore cerebral perfusion was maintained throughout exercise. When participants performed a Muller manoeuvre, which reduces intrathoracic pressure and CVP, during the leg extension phase, the rise in ICP was attenuated (Fig. 9 C). These observations were consistent in the supine, acute microgravity and prolonged microgravity conditions (Fig. 9 D–G).
Figure 9. Resistance exercise transiently increased ICP if combined with a Valsalva manoeuvre.

Original recordings in one participant of changes in intracranial pressure (ICP), central venous pressure (CVP) and arterial blood pressure (BP) during repetitive leg press exercise while breathing 0.7% carbon dioxide. A, during 0 g, ICP rises during leg extension and falls with leg flexion (relaxation). The synchronized rise and fall in CVP and BP indicates that the participant performed a Valsalva manoeuvre during each leg extension. B, in the HDT position, ICP, CVP and BP rise during leg extension and fall with leg flexion (relaxation) during five leg press exercises with a controlled Valsalva manoeuvre. C, in the HDT position, ICP and BP remain mostly stable during five leg press exercises performed with a controlled Muller manoeuvre. D–G, individual and mean intracranial pressure responses to the combination of 0.7% carbon dioxide and leg press exercise in the (D) supine posture, (E) during acute microgravity, (F) during acute simulated microgravity and (G) during prolonged simulated microgravity. Microgravity, n = 8, paired t tests; supine and HDT conditions, n = 4, one‐way ANOVA, * P ≤ 0.05 vs. baseline. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This series of experiments, which are the first to make direct ICP measurements in humans during 0 g and prolonged simulated microgravity, provide convincing evidence against the hypothesis that ICP is pathologically elevated in 0 g. In fact, removing gravity reduced the ICP compared to the supine posture. Other key findings of this study that are relevant for the physiology of gravity and hydrostatic influences on the circulation of the brain include: (a) in 1 g conditions on Earth, upright ICP is always lower (and substantially so) than supine ICP; (b) gravitational effects on fluid pressures in the head with postural adjustments occur rapidly, with no evidence for a slow progressive rise over time in the prolonged simulated microgravity condition; (c) the reduction in ICP upon exposure to acute microgravity led to a microgravity ICP that was consistently above the upright values; and (d) neither mildly elevated inspired CO2 levels, nor resistance exercise are likely to contribute to or exacerbate an elevation of ICP in space.
Humans typically spend two‐thirds of their time in the upright position (lower ICP) while awake, and one‐third of their time supine (higher ICP) while sleeping at night. Thus, due to the influence of gravity, ICP in humans is low for about two‐thirds of each day. While ground‐based models of microgravity (Petersen et al. 2015; Eklund et al. 2016) suggest that ICP may be elevated in space, in true 0 g, the influence of gravity is removed and ICP falls to an equilibrium point between the upright and supine postures. We speculate here that the absence of diurnal, postural reductions in ICP relative to IOP (Anderson et al. 2016; Petersen et al. 2015; Eklund et al. 2016) in microgravity creates a persistently lower pressure gradient at the posterior aspect of the eye [i.e. a lower translaminar pressure gradient (IOP–ICP) that may result in optic remodelling (Zhao et al. 2015)]. Interestingly, IOP is elevated in 0 g relative to the upright posture on Earth (Anderson et al. 2016), so the effect of microgravity on the pressure gradient at the posterior aspect of the eye (translaminar pressure) may be smaller than expected, yet over prolonged periods of time may be sufficient to cause optic remodelling.
This proposed pathophysiology of visual impairment is in line with the observed time‐dependent, mild to moderate clinical pattern and persistent presence of optic remodelling seen in astronauts on their return to Earth (Mader et al. 2011). The fact that simply placing the head on a pillow lowers ICP, which would be greater with head of bed elevation (Qvarlander et al. 2013 b), explains why individuals clinically or experimentally confined to bed rest do not commonly present with visual abnormalities. The hypothesis of persistent ‘overloading’ of the ICP in 0 g is analogous to the pathological left ventricular remodelling due to regurgitant valvular heart disease, whereby volume loading of the left ventricle occurs with every heartbeat (Gaasch & Meyer, 2008). In contrast, the normal diurnal variation of ICP on Earth may be considered analogous to the intermittent volume loading of the left cardiac ventricle with endurance training, which causes beneficial remodelling. Thus, the adaptive ‘stress‐recovery’ of exercise improves heart health, while unremitting stress causes cardiac dysfunction.
Clinically, female astronauts appear to have less severe visual impairment during long duration space missions than men (Platts et al. 2014). Although the number of females we studied was small, some observations of apparent sex differences may be informative. Compared to males, haemodynamic changes were similar, yet females typically displayed an ICP in 0 g closer to their estimated 24 h ICP on Earth (calculated as one‐third supine; two‐thirds sitting; Fig. 4 B). Such a smaller dissociation between average Earth and microgravity conditions would result in a lower relative pressure gradient behind the eye in females than males, possibly explaining the similar incidence but decreased severity of visual impairment in female astronauts (Platts et al. 2014).
In conclusion, normal changes in posture cause very large changes in ICP in humans. Complete removal of gravitational gradients (zero gravity of space) does not pathologically elevate ICP but does prevent the normal lowering of ICP when standing. Importantly, despite the acute short duration of zero gravity during parabolic flight, we saw no evidence of a progressive rise in ICP due to cephalad fluid shifts with prolonged simulated microgravity in the HDT position. Thus, at present, we have no physiological data to support the hypothesis that ICP should be greater than that observed in zero gravity during parabolic flight. The clinical implication of these findings is that the human brain and eye are protected by the daily circadian cycles in regional ICPs, without which pathology occurs. Creative strategies to maintain relative circadian cycles in ICP should be used in astronauts and clinical patients obligated to bed rest.
Additional information
Competing interests
The authors declare no conflicts of interest.
Author contributions
R.Z., L.A.W., M.A.W., B.D.L. conceived and designed the experiments J.S.L., L.G.P., E.J.H., S.S., W.K.C., R.Z., L.A.W., M.A.W., B.D.L. performed the experiments. All authors contributed to writing the paper.
Funding
This work was supported by the National Space Biomedical Research Institute through NCC 9‐58.
Authors translational perspective.
The human brain and eye are subject to large habitual variations in intracranial pressure (ICP) simply by moving from the supine to the upright posture. Removal of gravity did not pathologically elevate pressure in the brain, but does prevent the normal lowering of ICP when standing. Thus, over 24 h in zero gravity, pressure in the brain is slightly above that observed on Earth, which may explain remodelling of the eye in astronauts. Clinically these data suggest that circadian cycles in ICP should be maintained in astronauts and in patients obligated to bed rest. Moreover, in contrast to contemporary thinking, these data imply that, if chronic, subtle deviations in pressure behind the eye may cause optic remodelling and visual impairment.
Acknowledgements
We especially want to thank the participants for dedicating their time and effort towards these challenging experiments. We would also like to thank Dean Palmer, Mitchell Samels, Braden Everding, Sheryl Livingston, Margot Morris, Cyrus Oufi, Ramanathan Murugappan (Institute for Exercise and Environmental Medicine), Doug Ebert, David Ham, Terry Guess, Kathleen Garcia and Melinda Hailey (Wyle Industries) for their technical support in performing the experiments. We would like to acknowledge the staff at the Clinical Translational Science Center at University of Texas Southwestern Medical Center, the pilots, engineers and coordinators of the Flight Opportunities Program (NASA), the Interventional Radiology Team at St. John Medical Center, Clear Lake, TX, and the physicians, nurses and staff of MM Haq Medical Oncology Center, Houston, TX, for their expertise and assistance in performing the experiments. Finally, we would like to acknowledge all the oncologists and oncology nurse practitioners for patient referrals for this project.
References
- Alexander DJ, Gibson CR, Hamilton DR MD, Lee SMC, Mader TH, Otto C, Oubre CM, Pass AF, Platts SH, Scott JM, Smith SM, Stenger MB, Westby CM & Zanello SB (2012). Risk of spaceflight‐induced intracranial hypertension and vision alterations. Available at: https://humanresearchroadmap.nasa.gov/evidence/reports/viip.pdf
- Anderson AP, Swan JG, Phillips SD, Knaus DA, Kattamis NT, Toutain‐Kidd CM, Zegans ME, Fellows AM & Buckey JC (2016). Acute effects of changes to the gravitational vector on the eye. J Appl Physiol (1985) 120, 939–946. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE & Blomqvist CG (1993). Central venous pressure in space. N Engl J Med 328, 1853–1854. [DOI] [PubMed] [Google Scholar]
- Cooper DF, Grimby G, Jones DA & Edwards RH (1979). Perception of effort in isometric and dynamic muscular contraction. Eur J Appl Physiol Occup Physiol 41, 173–180. [DOI] [PubMed] [Google Scholar]
- Davson H, Domer FR & Hollingsworth JR (1973). The mechanism of drainage of the cerebrospinal fluid. Brain 96, 329–336. [DOI] [PubMed] [Google Scholar]
- Ducros A & Biousse V (2015). Headache arising from idiopathic changes in CSF pressure. Lancet Neurol 14, 655–668. [DOI] [PubMed] [Google Scholar]
- Eklund A, Johannesson G, Johansson E, Holmlund P, Qvarlander S, Ambarki K, Wahlin A, Koskinen LO & Malm J (2016). The pressure difference between eye and brain changes with posture. Ann Neurol 80, 269–276. [DOI] [PubMed] [Google Scholar]
- Gaasch WH & Meyer TE (2008). Left ventricular response to mitral regurgitation: implications for management. Circulation 118, 2298–2303. [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, Eves ND, Warburton DE & Findlay MJ (2003). Resistance exercise, the Valsalva maneuver, and cerebrovascular transmural pressure. Med Sci Sports Exerc 35, 65–68. [DOI] [PubMed] [Google Scholar]
- Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG & Pawelczyk JA (2007). Simultaneous determination of the accuracy and precision of closed‐circuit cardiac output rebreathing techniques. J Appl Physiol (1985) 103, 867–874. [DOI] [PubMed] [Google Scholar]
- Law J, Van Baalen M, Foy M, Mason SS, Mendez C, Wear ML, Meyers VE & Alexander D (2014). Relationship between carbon dioxide levels and reported headaches on the international space station. J Occup Environ Med 56, 477–483. [DOI] [PubMed] [Google Scholar]
- Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R & Polk JD (2011). Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long‐duration space flight. Ophthalmology 118, 2058–2069. [DOI] [PubMed] [Google Scholar]
- Michael AP & Marshall‐Bowman K (2015). Spaceflight‐induced intracranial hypertension. Aerosp Med Hum Perform 86, 557–562. [DOI] [PubMed] [Google Scholar]
- Nelson ES, Mulugeta L & Myers JG (2014). Microgravity‐induced fluid shift and ophthalmic changes. Life (Basel) 4, 621–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavy‐Le Traon A, Heer M, Narici MV, Rittweger J & Vernikos J (2007). From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol 101, 143–194. [DOI] [PubMed] [Google Scholar]
- Petersen LG, Damgaard M, Petersen JC & Norsk P (2011). Mechanisms of increase in cardiac output during acute weightlessness in humans. J Appl Physiol (1985) 111, 407–411. [DOI] [PubMed] [Google Scholar]
- Petersen LG, Petersen JC, Andresen M, Secher NH & Juhler M (2016). Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol Regul Integr Comp Physiol 310, R100–104. [DOI] [PubMed] [Google Scholar]
- Platts SH, Bairey Merz CN, Barr Y, Fu Q, Gulati M, Hughson R, Levine BD, Mehran R, Stachenfeld N & Wenger NK (2014). Effects of sex and gender on adaptation to space: cardiovascular alterations. J Womens Health (Larchmt) 23, 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarlander S, Lundkvist B, Koskinen LO, Malm J & Eklund A (2013. a). Pulsatility in CSF dynamics: pathophysiology of idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 84, 735–741. [DOI] [PubMed] [Google Scholar]
- Qvarlander S, Sundstrom N, Malm J & Eklund A (2013. b). Postural effects on intracranial pressure: modeling and clinical evaluation. J Appl Physiol (1985) 115, 1474–1480. [DOI] [PubMed] [Google Scholar]
- Rowell LB (1986). Human Circulation: Regulation During Physical Stress. Oxford University Press, Oxford. [Google Scholar]
- Shibata S & Levine BD (2011). Biological aortic age derived from the arterial pressure waveform. J Appl Physiol (1985) 110, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbey MT, Geocadin RG, Razumovsky AY, Rigamonti D & Williams MA (2004). Utility of CSF pressure monitoring to identify idiopathic intracranial hypertension without papilledema in patients with chronic daily headache. Cephalalgia 24, 495–502. [DOI] [PubMed] [Google Scholar]
- Videbaek R & Norsk P (1997). Atrial distension in humans during microgravity induced by parabolic flights. J Appl Physiol (1985) 83, 1862–1866. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ & Schreuder JJ (1993). Computation of aortic flow from pressure in humans using a nonlinear, three‐element model. J Appl Physiol (1985) 74, 2566–2573. [DOI] [PubMed] [Google Scholar]
- Zhao D, He Z, Vingrys AJ, Bui BV & Nguyen CT (2015). The effect of intraocular and intracranial pressure on retinal structure and function in rats. Physiol Rep 3, e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]


