Abstract
Microglia are the only immune cells that permanently reside in the central nervous system (CNS) alongside neurons and other types of glial cells. The past decade has witnessed a revolution in our understanding of their roles during normal physiological conditions. Cutting‐edge techniques revealed that these resident immune cells are critical for proper brain development, actively maintain health in the mature brain, and rapidly adapt their function to physiological or pathophysiological needs. In this review, we highlight recent studies on microglial origin (from the embryonic yolk sac) and the factors regulating their differentiation and homeostasis upon brain invasion. Elegant experiments tracking microglia in the CNS allowed studies of their unique roles compared with other types of resident macrophages. Here we review the emerging roles of microglia in brain development, plasticity and cognition, and discuss the implications of the depletion or dysfunction of microglia for our understanding of disease pathogenesis. Immune activation, inflammation and various other conditions resulting in undesirable microglial activity at different stages of life could severely impair learning, memory and other essential cognitive functions. The diversity of microglial phenotypes across the lifespan, between compartments of the CNS, and sexes, as well as their crosstalk with the body and external environment, is also emphasised. Understanding what defines particular microglial phenotypes is of major importance for future development of innovative therapies controlling their effector functions, with consequences for cognition across chronic stress, ageing, neuropsychiatric and neurological diseases.
Abbreviations
- BBB
blood–brain barrier
- BDNF
brain‐derived neurotrophic factor
- BM
bone marrow
- CNS
central nervous system
- E
embryonic day
- gw
gestational weeks
- IBA1
ionised calcium‐binding adapter molecule 1
- IL
interleukin
- LTP
long‐term potentiation
- SGZ
subgranular zone
- SVZ
subventricular zone
- TGFβ
transforming growth factor β
- TNFα
tumour necrosis factor α
- TSPO
translocator protein
- YS
yolk sac
Introduction
In the past decade, studies on microglia have expanded from investigating their function as resident macrophages of the brain and mediators of injury, neuroinflammation
and neurodegeneration (reviewed in (Prinz et al. 2011; Cartier et al. 2014; Prinz & Priller, 2014) to understanding their origins and non‐immunological roles in the central nervous system (CNS). Advances in genetic tools that allowed specific fate mapping of microglia (Ginhoux et al. 2010; Goldmann et al. 2013; Parkhurst et al. 2013; Yona et al. 2013; Wieghofer et al. 2015), in vivo imaging techniques, which revealed microglial activities in the brain milieu (Davalos et al. 2005; Nimmerjahn et al. 2005; Wake et al. 2009; Tremblay et al. 2010; Li et al. 2012), and high throughput gene expression analyses that differentiated microglia from their myeloid relatives and other brain cells (Butovsky et al. 2012; Gautier et al. 2012; Chiu et al. 2013; Hickman et al. 2013; Butovsky et al. 2014), have aided the rapid progress towards unravelling the multiple facets of microglia in shaping brain development and maintenance of homeostasis throughout the lifetime of an organism. Recent data have led to the call for a new approach to the classification of microglia in the family of mononuclear phagocytes (Guilliams et al. 2014), as well as a reconsideration of the description of microglial phenotypes based on their macrophage attributes M1 and M2 (Martinez & Gordon, 2014; Heppner et al. 2015), suggesting a major paradigm shift in the field. In this review we focus on recent findings shedding light on the physiological roles of microglia during development, adulthood and ageing, and discuss their impact on our understanding of diseases.
Origin of brain microglia
The colonisation of the CNS by microglial cells is evolutionarily conserved across vertebrate species and takes place even before formation of the neuroectoderm‐derived glial cell types, i.e. astrocytes and oligodendrocytes (Verney et al. 2010; Schlegelmilch et al. 2011; Swinnen et al. 2013). Unlike neuroectodermal cells, microglia are of myeloid origin. Several studies employing genetic fate mapping tools systematically delineated the source of microglia and confirmed that they arise mainly from yolk sac (YS) primitive macrophages (Ginhoux et al. 2010; Hoeffel et al. 2012; Schulz et al. 2012; Kierdorf et al. 2013; Gomez Perdiguero et al. 2015; Hoeffel et al. 2015), reside in the brain throughout life, and maintain their numbers through a process of self‐renewal (Hashimoto et al. 2013).
Normal blood circulation is essential for seeding of the CNS by YS macrophages. De novo formation and remodelling of blood vessels in the mouse embryo occur between embryonic days (E) 8.0 and E10.0 (Walls et al. 2008). Around E7.0, precursors of haematopoietic and endothelial cells expressing vascular endothelial growth factor receptors migrate from the primitive streak to the proximal YS to form the blood islands (Jones, 2011), where the multi‐lineage c‐kit+ erythromyeloid YS precursor cells of microglia reside (Kierdorf et al. 2013). Yolk sac precursors mature from A1 (CD45+ c‐kitlo CX3CR1− F4/80−) to A2 (CD45+ c‐kit− CX3CR1+ F4/80hi) amoeboid macrophages in the blood islands and cephalic mesenchyme before taking on a phenotype of mature macrophages in the neuroepithelium at E10.5 (Roumier et al. 2008; Mizutani et al. 2012). Fate mapping of haematopoietic precursors that express the runt‐related transcription factor Runx1 by genetic targeting between E6.5 and E10.5 revealed that YS microglia are specified between E7.0 and E7.5 (Ginhoux et al. 2010). The absence of brain microglial progenitors despite normal YS haematopoiesis in E9.5–10.5 Ncx‐1knockout mouse embryos with defective blood circulation supports the notion that brain recruitment of YS progenitors depends on a functional circulatory system (Ginhoux et al. 2010).
Microglia enter the brain rudiment via the leptomeninges and lateral ventricles by E9.5 and distribute throughout the cortical wall from both directions at different speeds with varying rates of proliferation and maturation, based on region and developmental stage (Ginhoux et al. 2010; Arnoux et al. 2013; Swinnen et al. 2013). Growing coverage of the CNS by amoeboid macrophages that eventually evolve into ramified microglia is possible due to rapid proliferation of 40–80% of their population between E10.5 and P0 (Kierdorf et al. 2013). Human amoeboid microglia penetrate the developing cerebral cortex at 4.5 gestational weeks (gw) via the pial surface, ventricles and choroid plexus (Monier et al. 2007; Verney et al. 2010). Radial and tangential migration of microglia was observed towards the putative white matter, subplate and cortical plate layers, while pial cells populate the prospective cortical layer I. A second wave of microglial invasion via the vasculature at 12–13 gw is limited to the white matter (Monier et al. 2007; Verney et al. 2010). In the initial two postnatal weeks of mouse development, microglial cell numbers increase, followed by a gradual decline of 50% from week three to six, after which microglial density stabilises (Nikodemova et al. 2015). The increase in microglial apoptosis is concomitant with a decrease in proliferation, contributing to the overall reduction in microglial numbers (Nikodemova et al. 2015).
Despite the exponential increase in studies focused on microglial origin over the past 5 years, fundamental questions remain regarding their cell cycle dynamics. How long do microglia live? Are long‐lived microglial progenitors found within the brain, and do microglia get replaced by bone marrow (BM)‐derived cells? Several reports have excluded the contribution of definitive haematopoiesis to brain microglia by using specific targeting of haematopoietic stem cells generated in the fetal liver or the aorta–gonad–mesonephros in different myeloid‐specific genetic reporter models for Csf‐1r (colony‐stimulating factor‐1 receptor), Flt3 (Fms‐like tyrosine kinase 3), Myb (myeloblastosis), Runx1, and Tie2 (angiopoietin receptor) (Ginhoux et al. 2010; Schulz et al. 2012; Gomez Perdiguero et al. 2015; Hoeffel et al. 2015). While monocytes may be recruited to the neonatal and adult brain where they differentiate into microglia‐like cells, this is more likely to occur under inflammatory conditions (Simard et al. 2006; Monier et al. 2007; Verney et al. 2010; Ginhoux & Prinz, 2015) and can still be phenotypically distinguished from resident microglia cells (O'Koren et al. 2016). These findings were corroborated using a KitMercreMer fate mapping strategy, showing that tissue resident macrophages are derived from haematopoietic stem cells, with the exception of microglia, which represent the only resident macrophages entirely derived from YS precursors in mouse (Sheng et al. 2015).
Interestingly, a Hox8b lineage of haematopoietic mononuclear cells exists within the brain of newborn mice and persists in the adult, contributing a significant percentage of the total microglial population in regions where they are found (Chen et al. 2010). A plausible point of entry for these cells is via the brain ventricular choroid plexus (Shechter et al. 2013), since Hox8b expressing cells were observed to be distributed in a gradient from the pial surface and ventricular lining into the parenchyma during the first two postnatal weeks (Chen et al. 2010). Mice lacking Hox8b expression in microglia reportedly display obsessive‐compulsive‐like behaviour of over‐grooming (Chen et al. 2010). This homeostatic contribution of BM‐derived macrophages to the CNS remains an enigma to be clarified, especially since many fate mapping studies have shown that microglia arise from the YS exclusively. In zebrafish a pulse labelling strategy with temporal–spatial resolution challenged this single source view by showing that the adult wave of brain microglial generation does not have a YS origin, but arises from the ventral walls of the dorsal aorta instead (Xu et al. 2015).
Factors required for microglial development and homeostasis
Recent studies have focused not only on determining the origin of microglia and elucidating how these unique cells renew, but also on identifying factors which may affect their maturation, activation, proliferation and apoptosis under normal physiological conditions. Together they expand the possibilities available to control their effector functions in CNS diseases.
Cell signalling pathways required for microglial proliferation and maturation
The early development of YS microglia precursors is dependent on key transcription factors Pu.1, a member of the Ets family (Rosenbauer & Tenen, 2007) and interferon regulatory factor Irf8, which both function as heterodimers in the determination of brain macrophage phenotype (Beers et al. 2006; Minten et al. 2012; Kierdorf et al. 2013). Furthermore matrix metalloproteinases 8 and 9 are upregulated by A2 and embryonic macrophages during proliferation and invasion of the neuroectoderm (Kierdorf et al. 2013). Another transcription factor that is known to regulate the differentiation of myeloid cells is Runx1, which is expressed in a subpopulation of amoeboid microglia restricted to the ventricles during early postnatal forebrain development (Zusso et al. 2012). Runx1 has been described to mediate microglial proliferation. However by P10, the colocalisation of proliferation marker Ki67 and Runx1 was no longer observed, with microglia shifting toward a ramified morphology, suggesting its role as a maturation factor (Zusso et al. 2012). Nevertheless it is still elusive how Runx1 regulates these events in a spatiotemporal dependent manner. The microRNA miR‐124, which binds the mRNA of transcription factor C/EBPalpha and which in turn downregulates Pu.1, was identified to be specific to microglia among myeloid cell populations (Ponomarev et al. 2011). miR‐124 is functionally conserved in zebrafish and mouse in controlling the motility and phagocytic activities of microglia which transition from amoeboid to ramified forms (Ponomarev et al. 2011; Svahn et al. 2015). In the E13.5 mouse spinal cord, purinergic ionotropic receptor P2X7 additionally mediates the proliferation of embryonic microglia and consequently regulates microglial density (Rigato et al. 2012). While many macrophage and microglial‐specific transcription factors have been uncovered, the interactions between factors and signalling cascades have not been elucidated.
Another essential pathway that defines microglial cell number involves Csf‐1R (CD115) (Fig. 1). Embryos are depleted of microglia in Csf‐1R knockout mice, which also have impaired brain architecture (Erblich et al. 2011). Neuron‐derived interleukin (IL)‐34, the second ligand for Csf‐1R, is more critical for regulating microglial cell density than Csf‐1 in the adult brain; however, its requirement during perinatal development is still controversial (Greter et al. 2012; Wang et al. 2012) (Fig. 1). Mature microglia similarly require Csf‐1R signalling, as mice treated with Csf‐1R inhibitors experience an almost complete loss of microglia in adulthood (Elmore et al. 2014). Similarly, mice deficient in the Csf‐1R adaptor protein DAP12 (DNAX activation protein of 12 kDa) reportedly have reduced microglial cell numbers in adults (Otero et al. 2009), but no overt impact on their density during development (Kierdorf et al. 2013). While young DAP12‐deficient mice do not show neurological symptoms up to 4 weeks of age, synaptic function and plasticity are impaired, suggesting its importance in maintaining microglial physiology and microglia–neuron interaction (Roumier et al. 2004) as discussed below. Interestingly the decline in microglial numbers after the third postnatal week was not mitigated by upregulating brain Csf‐1 levels (Nikodemova et al. 2015), indicating the existence of alternative microglial responses to unknown developmental signals that drive the apoptosis of a population of microglial cells during CNS maturation.
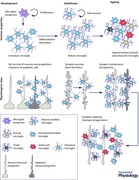
Figure 1. Signalling pathways regulating microglial proliferation, maturation and homeostasis in healthy and diseased conditions .
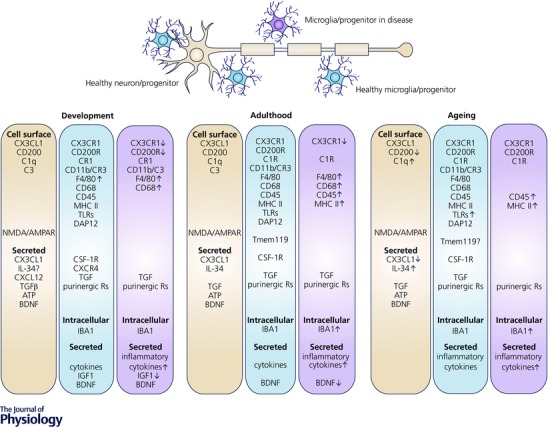
The proper development, differentiation, proliferation and function of both microglia and neurons require a continuous and lifelong crosstalk between the two cell types via complementary ligands and receptors and secreted trophic factors (e.g. cytokines). As microglia (blue) and neurons (brown) mature, they downregulate certain signalling pathways (e.g. Cxcl12‐Cxcr4) and upregulate others (e.g. IL‐34‐Csf‐1R). Dysregulation of either ligands or receptors occurs in a number of disease processes (dysfunctional microglia, purple). Maturation of yolk sac derived microglia within the CNS is dependent on proper signalling via purinergic receptors and cell surface protein Csf‐1R. Similarly, mature microglia require functional Csf‐1R signalling for their maintenance. Microglial ‘signature’ factors recently identified include TGFβ (transforming growth factor β, which appears critical for mediating microglial survival and phenotypic differentiation in the healthy mature brain) and Tmem119 (transmembrane protein 119, a cell surface protein of unknown function). The expression of microglial markers (including IBA1, CD45, MHC class II, and others) is often changed during the course of ageing and disease, coincident with changes in microglial function. R, receptors.
Cell signalling pathways required for microglial homeostasis
Mature microglia in the postnatal brain utilise a vast number of surface molecules in order to quickly respond to their extracellular environment, and are capable of responding to cytokines, chemokines, purines, hormones and neurotransmitters, among others. Similar to other tissue resident macrophages, microglia express common markers such as the fractalkine receptor CX3CR1, Csf‐1R, the integrin CD11b, surface glycoproteins F4/80 and CD68, ionised calcium‐binding adapter molecule 1 (IBA1), and pan‐haematopoietic CD45, but at lower levels than in perivascular macrophages and blood monocytes at steady state (Greter & Merad, 2013) (Fig. 1). High throughput gene expression studies have identified several genes distinguishing microglia from other cell types in the CNS or in the periphery (Serrats et al. 2010; Butovsky et al. 2012, 2014; Gautier et al. 2012; Chiu et al. 2013). These gene expression studies have also highlighted the phenotypic diversity of mature microglia, notably with respect to other tissue macrophages. However, whether these ‘signature’ factors directly contribute to specifying their motility, morphology and functions remains to be demonstrated (Gautier et al. 2012; Chiu et al. 2013; Hickman et al. 2013; Butovsky et al. 2014). A transmembrane protein with unknown function, Tmem119, was recently described to distinguish resident IBA1‐expressing microglia from non‐parenchymal CNS macrophages of the choroid plexus and meninges, as well as CD163+ perivascular cells (Bennett et al. 2016) (Fig. 1). Mannose receptor (CD206) expression was also shown to be limited to these non‐parenchymal CNS macrophages populations in normal, inflamed, injured and diseased CNS (Galea et al. 2005; Gomez‐Nicola et al. 2014). These populations of non‐parenchymal CNS myeloid cells have not been thoroughly characterised thus far and were postulated to derive from definitive haematopoiesis in contrast to YS‐derived microglia (Aguzzi et al. 2013). In addition, the simplified use of ‘M1’ classical activation and ‘M2’ alternative activation as descriptors of microglial phenotypes has fallen out of favour, especially from in vivo studies in disease models (Martinez & Gordon, 2014). Instead, much more attention is being paid to characterising the changes in whole genome expression in response to specific environmental challenges, as opposed to dualistic categorisation into ‘pro‐inflammatory’ versus ‘anti‐inflammatory’ phenotypes.
Among the factors identified so far, TGFβ (transforming growth factor β) (Fig. 1) could be critical for mediating microglial survival and phenotypic differentiation, considering that microglial density is drastically reduced in the CNS of TGFβ receptor‐deficient mice (Butovsky et al. 2014). TGFβ signalling induces microglia to adopt a ramified morphology concomitant with reduced levels of CD86, MHC class II and CD11c, and upregulation of CX3CR1 and IBA1 in vitro (Abutbul et al. 2012). Through its interaction with microglial CX3CR1 receptor, neuron‐derived fractalkine (CX3CL1) is considered an OFF signal keeping microglia in a non‐activated state (Biber et al. 2007) (Fig. 1). It is necessary for the timely recruitment of microglial cells into the early postnatal hippocampus and cerebral cortex (Hoshiko et al. 2012; Ueno et al. 2013; Paolicelli et al. 2014). The loss of CX3CR1 leads to a transient reduction of microglial numbers during the early postnatal period, and concomitant deficit in microglia‐mediated synaptic pruning, weakened synaptic transmission, and decreased functional brain connectivity (Paolicelli et al. 2011; Zhan et al. 2014), with consequences for cognitive behaviours discussed below.
Modulation of microglial process remodelling by purinergic signalling
Microglia are the most dynamic cells of the mature CNS, constantly remodelling their processes (Davalos et al. 2005; Nimmerjahn et al. 2005; Wake et al. 2009; Tremblay et al. 2010; Li et al. 2012), even following death of the organism (Dibaj et al. 2010). Several studies have focused on elucidating the mechanisms regulating microglial cell migration and process motility this past decade (Tremblay et al. 2011), revealing that purinergic signalling through microglial P2RY12 (Fig. 1) drives microglial response to laser injury in vivo (Davalos et al. 2005; Haynes et al. 2006), process remodelling in retinal explants (Fontainhas et al. 2011), as well as filopodial extension in mouse models of status epilepticus (Eyo et al. 2014) and neuropathic pain (Gu et al. 2016). In addition, dendritic neuronal NMDA receptor activation was shown to trigger release of ATP inducing the outgrowth of microglial processes in acute mouse hippocampal slices, suggesting a novel form of neuron–microglia communication that is mediated by purines (Dissing‐Olesen et al. 2014) (Fig. 1). More recently, it was found that extracellular calcium reduction induced microglial processes to converge at distinct sites, targeting neuronal dendrites independently from neuronal action potential firing in mouse brain slices and in vivo. This process is mediated by purinergic signalling through microglial P2RY12 receptors, suggesting that microglial interactions with neurons are guided by cerebral calcium reduction in the healthy brain (Eyo et al. 2015). The source of purinergic signalling, whether astrocytic or neuronal, however, remains unclear due to conflicting results between studies, which could arise from differences in the environmental contexts under investigation.
Heterogeneity of microglia in CNS milieus
Gene expression analyses have identified subtle differences in microglial expression profiles between brain regions under normal physiological conditions (de Haas et al. 2008; Doorn et al. 2015). The attraction of microglia into the ventricular/subventricular zone (SVZ) of the developing cortex is influenced by CXCL12 secreted from basal progenitors lining the ventricular zone via microglial CXCR4 (Arno et al. 2014) (Fig. 1). Genetic ablation of basal progenitors reduces microglial recruitment, while reduction of microglial numbers affects the number of neuronal progenitors in the ventricular and SVZ (Arno et al. 2014). In the white matter, it was unveiled that microglia specifically upregulate the ubiquitin‐specific protease 18, which under healthy conditions mitigates tissue destruction due to the presence of tonic interferon signal in this milieu (Goldmann et al. 2015). Microglia in the adult SVZ and rostral migratory stream also comprise a morphologically and antigenically distinct phenotype, distinguishable by lower expression of purinergic receptor P2RY12, and its lack of ATP‐driven chemotaxis (Ribeiro Xavier et al. 2015). Taken together, specific dynamic interactions between developing neural progenitor cells, neurons and microglia in each microenvironment may be required to determine the proper recruitment of microglia, as well as the maturation and maintenance of local neuronal circuits.
Differences between sexes
In addition to regional heterogeneity, sex‐related differences in microglial cells were lately uncovered. Microglial density was shown to differ between males and females across stages of the lifespan and brain regions that include the preoptic area, the hippocampus, parietal cortex and amygdala, under steady state conditions (Mouton et al. 2002; Schwarz et al. 2012; Lenz et al. 2013), and in response to chronic stress (Bollinger et al. 2015). These effects could be modulated by sex hormones, considering that isolated microglial cells express both oestrogen and progesterone receptors (Sierra et al. 2008). Microglia act in concert with the nervous and endocrine systems during sex determination in development. Inhibition of microglial activation with minocycline (which also affects astrocytes and other inflammatory mediators in a non‐specific manner) during the critical stage for sexual differentiation prevented the shift towards masculinity, defined by an increase in dendritic spine related proteins in the preoptic area (Lenz et al. 2013). Microglial involvement with pain processing, through P2X4R‐induced release of BDNF (brain‐derived neurotrophic factor) (Fig. 1), was further observed in male but not female mice, indicating a mechanistic difference between sexes (Sorge et al. 2015). This effect was mediated by testosterone, as treatment of female animals with testosterone resulted in microglia‐facilitated neuropathic pain instead of T cells‐driven processing in females (Sorge et al. 2015). Overall, the genetic or environmental factors that modulate the sex‐linked determination of microglial morphology, density and function in their respective domains are largely unclear. However, recognition of the differential regulation of microglial ontogeny prompts a critical assessment of existing data based on a particular sex.
Crosstalk with the periphery
Even though microglia reside in the apparently immune‐privileged CNS, an increasing number of studies point to an inter‐relationship between these cells and the periphery in the absence of pathology. Microglia are constantly modulated by blood to brain cytokine diffusion and transport during infection, immune‐related molecules secreted from endothelial cells forming the blood–brain barrier (BBB), and peripheral immune signals from the autonomic nervous system as reviewed in (Dilger & Johnson, 2008). In particular, the gut microbiome, which tightly regulates mood and cognition, was implicated in the normal development and maintenance of microglial cell homeostasis. Rats exposed to helminths prior to birth have different microglial tiling and reduced microglial response to early‐life immune challenges (Williamson et al. 2015). Mice bred in germ‐free conditions have increased microglial density and immaturity, with the effects regulated by short‐chain fatty acids derived from bacterial fermentation by‐products of microbiota (Erny et al. 2015). Rodents raised without gut microbiota also display increased BBB permeability and decreased expression of tight junction proteins on the endothelial cells forming their BBB (Braniste et al. 2014). It is even possible that the introduction of short‐chain fatty acids into otherwise germ‐free mice could allow signalling to peripheral splenic macrophages, which then traffic into the CNS, promoting the maturation of microglia (Mosher & Wyss‐Coray, 2015). These data demonstrate that the steady state of the CNS immune system does not only depend on the responses of microglia to their local environment, but also on a complex system of interactions between the peripheral and central nervous systems.
Physiological functions of microglia in brain development and beyond
Microglia are crucial regulators of brain development and homeostasis via neuronal–microglial interactions, synaptic modelling, scavenging of cellular debris and secretion of trophic factors. How does a single cell type multitask? Adult microglia appear uniform morphologically during steady state conditions, but they are functionally heterogeneous in their physiological responses, which may be attributed to their local environment and dependent on the ongoing neuronal activity (Li et al. 2012; Schafer et al. 2012; Arnoux et al. 2013; Clark et al. 2015).
Microglial regulation of neuronal death and survival
As phagocytes, activated microglia proliferate and accumulate in areas presenting high densities of apoptotic neurons to facilitate neuronal turnover during developmental cell death (Marin‐Teva et al. 2004; Peri & Nusslein‐Volhard, 2008; Swinnen et al. 2013). These events are very well coordinated. For instance, increased rates of cell death in neurogenic regions such as the SVZ along the lateral ventricles lead to the release of macrophage migration inhibitory factor (MIF) triggering microglial proliferation (Arno et al. 2014). When massive neuronal death is induced by neonatal alcohol exposure, microglia in the P7 cortex respond with an acute increase in TNFα (tumour necrosis factor α), IL1β, and CD68 levels and decrease of P2RY12, consistent with a pro‐inflammatory state (Ahlers et al. 2015) (Fig. 1). This probably encourages the clearance of dead neuronal cells, and subsequent deactivation occurs 48 h post ethanol injection – just after the peak of phagocytosis (Ahlers et al. 2015).
Apart from the clearance of dead cells, microglial phagocytosis controls the number of neural precursors in proliferative regions of the developing telencephalon in perinatal rats and macaques (Cunningham et al. 2013). Pharmacological interventions or maternal immune activation disrupting microglial cell numbers inversely change the pool size of neural progenitors and affect proper CNS development (Cunningham et al. 2013). However, further investigations are still required to understand the actual impact of modulating the number of neural precursor cells during early development on adult behaviour. Besides being scavengers, microglia directly support neuronal survival, neurogenesis and oligodendrogenesis in vitro (Butovsky et al. 2006; Walton et al. 2006) and in vivo during pre‐ and postnatal development (Ueno et al. 2013; Arno et al. 2014; Shigemoto‐Mogami et al. 2014). Microglia lining the subcortical white matter tracts in the early postnatal cerebral cortex support the survival of layer V neurons through secretion of the trophic factor insulin‐like growth factor 1 (Ueno et al. 2013) (Fig. 1). Activated microglia in the early postnatal SVZ support neurogenesis and oligodendrogenesis via the release of pro‐inflammatory cytokines such as IL‐1β, IL‐6, TNFα, and IFNγ (interferon γ) (Shigemoto‐Mogami et al. 2014) (Fig. 1). They are also necessary for maintaining homeostasis of the pool of basal progenitors in the forebrain throughout neurogenesis from E14 to E17 (Arno et al. 2014).
In the healthy mature brain, neurogenesis continues throughout adulthood in well‐defined areas where the existence of neural stem cells persists. The two consensus areas in rodents and human are the SVZ and hippocampal subgranular zone (SGZ) (Sierra et al. 2014). In the SGZ, microglial phagocytosis was shown to actively eliminate the excess newborn neurons that die by apoptosis (Sierra et al. 2010; reviewed in Sierra et al. 2014). In the SVZ, depletion of the microglial phenotype expressing low levels of P2RY12 hampered the survival and migration of newly generated neuroblasts to the olfactory bulb (Ribeiro Xavier et al. 2015), suggesting the importance of this unique microglial subpopulation in maintaining the adult olfactory circuitry.
Roles of microglia in neuronal wiring
Once neuronal circuits are established, microglia mainly contribute to the refinement of synaptic connections in the healthy brain (Wake et al. 2009; Tremblay et al. 2010; Bialas & Stevens, 2013). Microglial involvement with the formation of axonal tracts or engulfment of dendritic spines and/or axon terminal fragments during CNS development is conserved from Drosophila (Watts et al. 2004) to rodents (Paolicelli et al. 2011; Schafer et al. 2012; Squarzoni et al. 2014), and presumably humans, based on their topological relationship with axonal tracts described postmortem (Cho et al. 2013).
In the prenatal mouse brain, microglia regulate the wiring of forebrain circuits, controlling the outgrowth of dopaminergic axons into the forebrain and the laminar positioning of neocortical interneurons subsets (Squarzoni et al. 2014). In the postnatal brain, microglia‐mediated synaptic pruning is similarly required for the activity‐dependent wiring of neural circuits (Paolicelli et al. 2011; Schafer et al. 2012). Microglial phagocytosis of synaptic elements is documented during postnatal development, adolescence, adulthood and normal ageing, within the thalamus, cerebral cortex and hippocampus (Tremblay et al. 2010, 2012; Paolicelli et al. 2011; Schafer et al. 2012; Milior et al. 2016), suggesting a crucial role in synaptic plasticity and behavioural adaptation to the environment (Tremblay et al. 2010), notably in response to chronic stress (Milior et al. 2016). In the developing retino‐geniculate system, microglial sculpting of neural circuits was demonstrated to involve TGFβ, which modulates the expression of complement protein C1q, which in turn mediates the downstream complement protein C3 tagging of synapses (Stevens et al. 2007) for elimination via CR3/C3 signalling (Schafer et al. 2012; Bialas & Stevens, 2013) (Fig. 1). Mice lacking TGFβ receptor II in retinal neurons had reduced synaptic localization of complement proteins, as well as impaired microglial engulfment of retinal ganglion cell inputs (Bialas & Stevens, 2013).
During adolescence and adulthood, depleting microglia using transgenic expression of the diphtheria toxin (Parkhurst et al. 2013) or treatment with a Csf‐1R inhibitor (Elmore et al. 2014) also increased synaptic density in the hippocampus (Rice et al. 2015). It was also shown using a transgenic strategy (CX3CR1CreER crossed with BDNFflox) that preventing microglial release of BDNF decreases the formation of cortical dendritic spines in vivo during motor learning (Parkhurst et al. 2013) (Fig. 1). Similar findings of a decreased spinogenesis, paralleling the impairment of synaptic elimination, were obtained upon microglial depletion achieved by crossing the CX3CR1CreER model with diphtheria toxin receptor (DTR)‐inducible R26iDTR mice (Parkhurst et al. 2013). These observations support the notion that microglia are important effectors of structural plasticity in the healthy mature brain, mediating both the formation and elimination of synaptic elements.
Microglial regulation of neuronal activity and synaptic plasticity
Healthy microglia additionally produce a broad spectrum of signalling molecules, from cytokines to neurotransmitters and extracellular matrix proteins which are capable of regulating neuronal activity, as well as synaptic activity and functional plasticity (Bessis et al. 2007; Bechade et al. 2013; Ji et al. 2013). In the larval zebrafish, dynamic interactions between microglial processes and neuronal cells bodies regulate neuronal activity, reducing both spontaneous and visually evoked activities in vivo (Li et al. 2012). In rodents, several ex vivo studies in hippocampal slices revealed various alterations of synaptic maturation, activity and functional plasticity using knockout mice where microglia‐specific signalling pathways are compromised (Wu et al. 2015). For instance, fractalkine signalling deficiency in CX3CR1 knockout mice impaired the functional maturation of thalamo‐cortical synapses during the first postnatal week (Hoshiko et al. 2012). Loss‐of‐function mutation or deletion of microglial DAP12, CD200R or CX3CR1 similarly resulted in reduced or enhanced hippocampal long‐term potentiation (LTP), the most commonly studied paradigm of synaptic activity and plasticity, during adulthood (Roumier et al. 2004, 2008; Maggi et al. 2009; Costello et al. 2011; Rogers et al. 2011) (Fig. 1). CD200R interacts with the glycoprotein CD200 expressed by neurons, oligodendrocytes and astrocytes (Costello et al. 2011). Several studies also revealed modulatory effects of fractalkine on the electrophysiological properties of excitatory synapses, involving NMDA and AMPA receptors, as well as d‐serine, in acute hippocampal slices from juvenile and adult mice (reviewed in Paolicelli et al. 2014). Nevertheless microglial involvement and the downstream pathways related to these effects on neurotransmission remain to be investigated.
Consequences of microglial dysfunction on cognition
Given the plurality of microglial functions, conditions resulting in defective microglia during brain development can lead to impaired clearance of cellular debris and compromised neural connectivity. Dysfunctional or perturbed microglial homeostasis could have direct consequences on the onset of severe neurodegenerative or neuropsychiatric disorders at an early age or later in adulthood. For example, young boys deficient for the ALD protein encoded by ABCD1 gene suffer from a debilitating brain demyelinating disease known as X‐linked adrenoleukodystrophy, which could be halted by partial reconstitution of myeloid and lymphoid populations via gene therapy (Cartier et al. 2009) Previously, glial cells in MECP2 mutants lacking the methyl‐CpG‐binding protein were directly implicated in Rett syndrome, an X‐linked autism spectrum disorder, due to neuronal dysfunction (Maezawa & Jin, 2010; Lioy et al. 2011; Derecki et al. 2012). Derecki et al. (2012) proposed that BM transplantation of wild‐type ‘microglia’ could ameliorate the disease symptoms. However, a collaborative investigation using several mouse models of Rett could not replicate the amelioration of motor deficits and breathing abnormalities, as well as extended lifespan, leading to the termination of clinical trials for BM transplants in children with Rett syndrome (Wang et al. 2015).
During adulthood, impaired microglial remodelling of neuronal circuits could severely impair learning and memory functions. Fractalkine signalling deficiency in CX3CR1 knockout mice, microglial‐BDNF deletion and microglial depletion similarly resulted in cognitive impairment, when assessing motor behaviour, and fear conditioning where the amygdala plays a critical role (Rogers et al. 2011; Parkhurst et al. 2013) (Fig. 1). The CX3CR1 knockout mice also showed altered performance in the Morris water maze testing hippocampus‐dependent learning and memory functions (Maggi et al. 2011; Rogers et al. 2011). Nevertheless, no significant deficits of novel object recognition, a paradigm which depends on the prefrontal cortex, were detected in these models, suggesting the recruitment of different mechanisms (Parkhurst et al. 2013; Zhan et al. 2014). In addition, 21 days of microglial depletion with the Csf‐1R inhibitor did not induce cognitive deficit under various behavioural schemes (Elmore et al. 2014), indicating that longer treatment periods might be required to modify the behaviour. Indeed, microglial depletion using clodronate or oral administration of a Csf‐1R inhibitor only transiently impaired spatial memory without modifying sociability (Torres et al. 2016).
Dysregulation by the environment
Exposure to environmental risk factors (such as air pollution, omega‐3 deficiency, chronic psychological stress, infection, etc.) has profound effects on microglia and the inflammatory milieu in the brain, as well as cognition (Bilbo, 2013; Castanon et al. 2015). These effects are particularly pronounced when exposure occurs during critical windows of development (Knuesel et al. 2014). The underlying mechanisms need to be further investigated, especially in the context of neuropsychiatric disorders, but fractalkine signalling is emerging as an important mediator of microglial response to the environment (Maggi et al. 2011; Milior et al. 2016). In particular, the CX3CR1 knockout mice display social interaction deficits associated with autism spectrum disorders in humans, both early in life and adulthood (Zhan et al. 2014) (Fig. 1). CX3CR1 knockout mice exposed to an enriched environment fail to enhance hippocampal LTP and to improve learning performance in the Morris water maze (Maggi et al. 2011). CX3CR1 knockout mice are also resistant to anxiety‐like behaviour (Wohleb et al. 2014) and microglial alterations (Hellwig et al. 2015; Milior et al. 2016) induced by chronic stress. In particular, microglial phagocytosis of synaptic elements was implicated in the adaptation of the brain and behaviour to chronic stress (Milior et al. 2016). In addition, blockade of stress‐induced microglial activation by minocycline or IL‐1 receptor antagonist overexpression prevents microglial apoptosis and decline upon chronic stress and the emergence of cognitive impairment (Hinwood et al. 2012; Kreisel et al. 2014).
Microglial activation, senescence and priming in normal ageing
Two compelling studies based on heterochronic parabionts (i.e. young and aged mice with linked circulatory systems) claimed that young blood, or its derived factor, was able to reverse cognitive impairment, improve synaptic plasticity, enhance brain vasculature, and increase neurogenesis in aged mouse (Katsimpardi et al. 2014; Villeda et al. 2014). Is ageing one of the greatest risk factors for neurodegenerative diseases? What is the physiological relevance of microglia in essential coping mechanisms during late adulthood and normal ageing? We explore these questions by examining the various microglial phenotypes observed in the aged CNS and their relevance to healthy ageing.
Microglial activation
In the absence of pathology, aged microglia have higher expression of pro‐inflammatory genes and antigen presenting markers such as complement components, TLR (Toll‐like receptor) signalling, inflammasomes, scavenger and MHC class II antigens; increased production of pro‐inflammatory cytokines and reactive oxygen species (ROS); while anti‐inflammatory cytokines including IL‐10 and TGFβ1, and microglial activation inhibitory factors such as CD200 and fractalkine receptors are down‐regulated (Mosher & Wyss‐Coray, 2014) (Fig. 1). These parameters indicate that as humans and rodents age microglia take on a phenotype consistent with a pro‐inflammatory response, even in the absence of overt pathological stimuli (Luo et al. 2010; Choi & Won, 2011; Wong, 2013; Sierra et al. 2014; Ojo et al. 2015; Barrientos et al. 2015 a). These findings were confirmed with positron emission tomography in aged individuals (>50 years old) showing increased detection of the radiotracer (R)‐[11C]PK11195, a marker of translocator protein (TSPO) mainly expressed on the outer mitochondrial membrane of activated microglia (Schuitemaker et al. 2012; Wehrspaun et al. 2015). Microarray gene expression analyses also revealed that surface receptors mediating neuronal–microglial crosstalk and anti‐inflammatory genes are reduced in aged individuals (Schuitemaker et al. 2012; Wehrspaun et al. 2015). Although another group showed that the microglial activation might be related to TSPO polymorphisms rather than ageing (Suridjan et al. 2014), TSPO is still considered an important regulator of microglial activation and phagocytosis through macroglia–microglia interaction (Karlstetter et al. 2014; Wang et al. 2014).
Microglial senescence
Despite their increase in pro‐inflammatory signalling, aged microglia are often regarded as ‘dystrophic’ or ‘senescent’ as they lose the ability to respond adequately to external stimuli and injury (Streit, 2006; Wasserman et al. 2008). Dystrophic microglia were previously identified in aged human brain in situ (Streit et al. 2004, 2009) and in vitro (Caldeira et al. 2014). One study proposed that following stress‐induced depressive‐like condition, dystrophic microglia (including apoptosis, reduction in their numbers within the hippocampus and reduced expression of activation markers) might contribute to reduced hippocampal neurogenesis and increased depressive‐like behaviour in rodents (Kreisel et al. 2014). Compared to 3‐month old mice, aged microglia in 2‐year old mice show different levels of morphological changes, including ∼25% increase in density, ∼50% enlargement in area of soma, shorter and thicker processes with ∼20% reduction in process motility, ∼60% increase in soma motility, and accumulation of cellular debris (Tremblay et al. 2012; Hefendehl et al. 2014). Microglial activation, process motility, and migratory velocity were also diminished in aged retina and cerebral cortex in response to injury (Damani et al. 2011; Hefendehl et al. 2014).
Microglial priming
Microglial priming is widely observed in aged brains from humans, non‐human primates and rodents (Streit & Sparks, 1997; Sheffield & Berman, 1998; Godbout et al. 2005; Frank et al. 2006; VanGuilder et al. 2011). Microglial priming with ageing may additionally result from the accumulation of misfolded and aggregated proteins, such as amyloid‐β and mutant huntingtin, in Alzheimer's and Huntington's diseases, respectively (reviewed in Cartier et al. 2014). This age‐related priming induces an exaggerated inflammatory response to a secondary stimulus (Cunningham, 2013; Perry & Holmes, 2014; Matt & Johnson, 2015). According to early human studies, elderly individuals were more susceptible to peripheral infections, and these infections increased mortality rates for individuals over 65 years of age (Pinner et al. 1996). Extensive studies performed on rodents have produced data that support the hypothesis of higher susceptibility to neurodegeneration due to hyperactivation of microglia during pathological ageing. Peripherally delivered lipopolysaccharide (LPS) stimulation exaggerated gene and protein expressions of pro‐inflammatory cytokines, triggered oxidative stress, and further reduced social behaviour and locomotor activity in aged rodents (Godbout et al. 2005; Sierra et al. 2007; Matt & Johnson, 2015; Barrientos et al. 2015 b). More importantly, prolonged activation can make microglia resistant to regulation, impairing their responses to IL‐10, TGFβ1 and IL‐4, and become irresponsive to neurons signalling through CX3CL1‐CX3CR1 and CD200‐CD200R. These changes could disrupt the fine coordination between the immune and nervous systems (Barrientos et al. 2010, 2015 a; Cunningham, 2013; Norden & Godbout, 2013; Wong, 2013; Biber et al. 2014; Perry & Holmes, 2014; Ojo et al. 2015).
Dark microglia and consequences on cognition
In addition, we recently described a new ‘dark’ microglial phenotype in mouse hippocampus, cerebral cortex, amygdala and hypothalamus that is prevalent during chronic stress, fractalkine signalling deficiency (CX3CR1 knockout model), normal ageing, and Alzheimer's disease pathology (Bisht et al. 2016). Dark microglia exhibit several signs of oxidative stress, including a condensed, electron‐dense cytoplasm and nucleoplasm (giving them a ‘dark’ appearance similar to mitochondria), mitochondrial disruption, and endoplasmic reticulum dilatation (i.e. the best characterised sign of oxidative stress at the ultrastructural level). These cells appear to be extremely active, frequently reaching for synaptic clefts, while extensively encircling axon terminals, dendritic spines and entire synapses with their highly ramified and thin processes. They also express CD11b (composing CR3 involved in synaptic pruning) in their processes encircling synaptic elements (Bisht et al. 2016). Our observations suggest that dark microglia could be implicated in the loss of synapses, which is the best pathological correlate of cognitive decline across ageing and several diseases, including the highly prevalent Alzheimer's disease. In particular, dark microglia could represent a subset of hyperactive microglia that become stressed as a result of their hyperactivity under adaptive pressure, leading to dysregulated interactions with synapses. Considering that chronic stress accelerates cellular ageing, potentiates oxidative stress and neuroinflammation, and predisposes to various diseases across the lifespan, therapeutic interventions aimed at promoting microglial resilience to stress could be crucial for promoting healthy ageing.
Overall, these findings indicate that normal ageing can lead to various microglial abnormalities ultimately affecting their physiological roles in brain plasticity and cognition. While it is still unclear whether microglia are the primary players in neurodegenerative diseases, changes in aged microglia should be considered to provide insights into the development of diagnosis and therapeutic approaches for such diseases (Siskova & Tremblay, 2013).
Summary and outlook
Over the past decade microglia have emerged as important contributors to normal brain physiology. As a consequence, the field recently experienced an explosive growth. Within this context, our review aims at providing a critical update on their unique origin, the factors regulating their maturation and homeostasis, their physiological roles in brain development, function and plasticity, and the implications of their loss or dysfunction to cognitive decline during normal ageing and in diseases. Since microglia actively maintain health during normal physiological conditions, it is of prime importance to elucidate the cellular, molecular and epigenetic mechanisms that determine their phenotype and underlie their motility, phagocytic behaviour, synaptic interactions and release of various mediators modulating cognition. The development of effective treatments requires better understanding of those mechanisms governing microglial effector functions across the lifespan.
Additional information
Competing interests
The authors declare no competing financial interests.
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) RGPIN‐2014‐05308 to M.E.T and German Research Foundation (DFG) TA1029/1‐1 to T.L.T. K.B. is recipient of a scholarship from the Faculté de médecine of Université Laval. We are grateful to Hassan El Hajj for his help with the bibliography and to Amanda Sierra for her critical comments on the manuscript.
Biography
Marie‐Ève Tremblay has been an assistant professor at Université Laval since 2013. Her research focuses on elucidating the roles of microglia in the loss of synapses which best correlate with the impairment of learning and memory across chronic stress, ageing and various diseases. Her long‐term goal is to help develop new therapies using myeloid cells as vectors for effecting targeted changes in neuronal circuits, to promote stress resilience and healthy ageing, and to improve the cognitive functions in individuals suffering from post‐traumatic stress disorders, and various neuropsychiatric and neurodegenerative diseases.

Contributor Information
Tuan Leng Tay, Email: tuan.leng.tay@uniklinik-freiburg.de.
Marie‐Ève Tremblay, Email: tremblay.marie-eve@crchudequebec.ulaval.ca.
References
- Abutbul S, Shapiro J, Szaingurten‐Solodkin I, Levy N, Carmy Y, Baron R, Jung S & Monsonego A (2012). TGF‐beta signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia 60, 1160–1171. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA & Bennett ML (2013). Microglia: scapegoat, saboteur, or something else? Science 339, 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ & Dailey ME (2015). Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX‐dependent neurodegeneration. Glia 63, 1694–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arno B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B, Martino G & Muzio L (2014). Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun 5, 5611. [DOI] [PubMed] [Google Scholar]
- Arnoux I, Hoshiko M, Mandavy L, Avignone E, Yamamoto N & Audinat E (2013). Adaptive phenotype of microglial cells during the normal postnatal development of the somatosensory “Barrel” cortex. Glia 61, 1582–1594. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR & Maier SF (2010). Memory impairments in healthy aging: Role of aging‐induced microglial sensitization. Aging Dis 1, 212–231. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR & Maier SF (2015. a). Neuroinflammation in the normal aging hippocampus. Neuroscience 19, 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA & Maier SF (2015. b). Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol Aging 36, 1483–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechade C, Cantaut‐Belarif Y & Bessis A (2013). Microglial control of neuronal activity. Front Cell Neurosci 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR & Appel SH (2006). Wild‐type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 103, 16021–16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG & Barres BA (2016). New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA 113, E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D & Roumier A (2007). Microglial control of neuronal death and synaptic properties. Glia 55, 233–238. [DOI] [PubMed] [Google Scholar]
- Bialas AR & Stevens B (2013). TGF‐beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Biber K, Neumann H, Inoue K & Boddeke HW (2007). Neuronal ‘On’ and 'Off' signals control microglia. Trends Neurosci 30, 596–602. [DOI] [PubMed] [Google Scholar]
- Biber K, Owens T & Boddeke E (2014). What is microglia neurotoxicity (Not)? Glia 62, 841–854. [DOI] [PubMed] [Google Scholar]
- Bilbo SD (2013). Frank A. Beach award: programming of neuroendocrine function by early‐life experience: a critical role for the immune system. Horm Behav 63, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht K, Sharma K, Lecours C, Sanchez MG, El Hajj H, Milior G, Olmos‐Alonso A, Gomez‐Nicola D, Luheshi G, Vallières L, Branchi I, Maggi L, Livimatola C, Butovsky O & Tremblay ME (2016). Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64, 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Bergeon Burns CM & Wellman CL (2015). Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, Al‐Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B & Pettersson S (2014). The gut microbiota influences blood‐brain barrier permeability in mice. Sci Transl Med 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP & Weiner HL (2014). Identification of a unique TGF‐beta‐dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME & Weiner HL (2012). Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 122, 3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G & Schwartz M (2006). Microglia activated by IL‐4 or IFN‐gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31, 149–160. [DOI] [PubMed] [Google Scholar]
- Caldeira C, Oliveira AF, Cunha C, Vaz AR, Falcao AS, Fernandes A & Brites D (2014). Microglia change from a reactive to an age‐like phenotype with the time in culture. Front Cell Neurosci 8, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein‐Bey‐Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal‐Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l'Homme B, Bougneres P, Von Kalle C, Fischer A, Cavazzana‐Calvo M & Aubourg P (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X‐linked adrenoleukodystrophy. Science 326, 818–823. [DOI] [PubMed] [Google Scholar]
- Cartier N, Lewis CA, Zhang R & Rossi FM (2014). The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol 128, 363–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Luheshi G & Laye S (2015). Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front Neurosci 9, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G & Capecchi MR (2010). Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, Myers RM & Maniatis T (2013). A neurodegeneration‐specific gene‐expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4, 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Cheong JS, Kim JH, Abe H, Murakami G & Cho BH (2013). Site‐specific distribution of CD68‐positive microglial cells in the brains of human midterm fetuses: a topographical relationship with growing axons. Biomed Res Int 2013, 762303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH & Won MH (2011). Microglia in the normally aged hippocampus. Lab Anim Res 27, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Gruber‐Schoffnegger D & Drdla‐Schutting R (2015). Selective activation of microglia facilitates synaptic strength. J Neurosci 35, 4552–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Lyons A, Denieffe S, Browne TC, Cox FF & Lynch MA (2011). Long term potentiation is impaired in membrane glycoprotein CD200‐deficient mice: a role for Toll‐like receptor activation. J Biol Chem 286, 34722–34732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C (2013). Microglia and neurodegeneration: the role of systemic inflammation. Glia 61, 71–90. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martinez‐Cerdeno V & Noctor SC (2013). Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33, 4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN & Wong WT (2011). Age‐related alterations in the dynamic behavior of microglia. Aging Cell 10, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML & Gan WB (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- de Haas AH, Boddeke HW & Biber K (2008). Region‐specific expression of immunoregulatory proteins on microglia in the healthy CNS. Glia 56, 888–894. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG & Kipnis J (2012). Wild‐type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibaj P, Steffens H, Nadrigny F, Neusch C, Kirchhoff F & Schomburg ED (2010). Long‐lasting post‐mortem activity of spinal microglia in situ in mice. J Neurosci Res 88, 2431–2440. [DOI] [PubMed] [Google Scholar]
- Dilger RN & Johnson RW (2008). Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 84, 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing‐Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB & MacVicar BA (2014). Activation of neuronal NMDA receptors triggers transient ATP‐mediated microglial process outgrowth. J Neurosci 34, 10511–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn KJ, Breve JJ, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ & van Dam AM (2015). Brain region‐specific gene expression profiles in freshly isolated rat microglia. Front Cell Neurosci 9, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL & Green KN (2014). Colony‐stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K & Pollard JW (2011). Absence of colony stimulation factor‐1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PloS One 6, e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren‐Shaul H & Mahlakoiv T (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Gu N, De S, Dong H, Richardson JR & Wu LJ (2015). Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci 35, 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A & Wu LJ (2014). Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci 34, 10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W & Wong WT (2011). Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PloS One 6, e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR & Maier SF (2006). mRNA up‐regulation of MHC II and pivotal pro‐inflammatory genes in normal brain aging. Neurobiol Aging 27, 717–722. [DOI] [PubMed] [Google Scholar]
- Galea I, Palin K, Newman TA, Van Rooijen N, Perry VH & Boche D (2005). Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia 49, 375–384. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M & Randolph GJ (2012). Gene‐expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM & Merad M (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F & Prinz M (2015). Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol 7, a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW & Johnson RW (2005). Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 19, 1329–1331. [DOI] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S & Prinz M (2013). A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 16, 1618–1626. [DOI] [PubMed] [Google Scholar]
- Goldmann T, Zeller N, Raasch J, Kierdorf K, Frenzel K, Ketscher L, Basters A, Staszewski O, Brendecke SM, Spiess A, Tay TL, Kreutz C, Timmer J, Mancini GM, Blank T, Fritz G, Biber K, Lang R, Malo D, Merkler D, Heikenwalder M, Knobeloch KP & Prinz M (2015). USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J 34, 1612–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F & Rodewald HR (2015). Tissue‐resident macrophages originate from yolk‐sac‐derived erythro‐myeloid progenitors. Nature 518, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Nicola D, Schetters ST & Perry VH (2014). Differential role of CCR2 in the dynamics of microglia and perivascular macrophages during prion disease. Glia 62, 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M & Becher B (2012). Stroma‐derived interleukin‐34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M & Merad M (2013). Regulation of microglia development and homeostasis. Glia 61, 121–127. [DOI] [PubMed] [Google Scholar]
- Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H & Wu LJ (2016). Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun 55, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R & Yona S (2014). Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia‐Sastre A, Stanley ER, Ginhoux F, Frenette PS & Merad M (2013). Tissue‐resident macrophages self‐maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB & Julius D (2006). The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9, 1512–1519. [DOI] [PubMed] [Google Scholar]
- Hefendehl JK, Neher JJ, Suhs RB, Kohsaka S, Skodras A & Jucker M (2014). Homeostatic and injury‐induced microglia behavior in the aging brain. Aging Cell 13, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig S, Masuch A, Nestel S, Katzmarski N, Meyer‐Luehmann M & Biber K (2015). Forebrain microglia from wild‐type but not adult 5xFAD mice prevent amyloid‐beta plaque formation in organotypic hippocampal slice cultures. Sci Rep 5, 14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM & Becher B (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16, 358–372. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK & El Khoury J (2013). The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA & Walker FR (2012). Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 22, 1442–1454. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M & Ginhoux F (2015). C‐Myb+ erythro‐myeloid progenitor‐derived fetal monocytes give rise to adult tissue‐resident macrophages. Immunity 42, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M & Ginhoux F (2012). Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac‐derived macrophages. J Exp Med 209, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N & Audinat E (2012). Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci 32, 15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Miyauchi J & Tsirka SE (2013). Microglia: an active player in the regulation of synaptic activity. Neural Plast 2013, 627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA (2011). The initiation of blood flow and flow induced events in early vascular development. Semin Cell Dev Biol 22, 1028–1035. [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber BH, Rupprecht R & Langmann T (2014). Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J Neuroinflammation 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ & Rubin LL (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F & Prinz M (2013). Microglia emerge from erythromyeloid precursors via Pu.1‐ and Irf8‐dependent pathways. Nat Neurosci 16, 273–280. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S & Prinssen EP (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10, 643–660. [DOI] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben‐Menachem‐Zidon O, Baratta MV, Maier SF & Yirmiya R (2014). Dynamic microglial alterations underlie stress‐induced depressive‐like behavior and suppressed neurogenesis. Mol Psychiatry 19, 699–709. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R & McCarthy MM (2013). Microglia are essential to masculinization of brain and behavior. J Neurosci 33, 2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL & Du JL (2012). Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell 23, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N & Mandel G (2011). A role for glia in the progression of Rett's syndrome. Nature 475, 497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Ding JQ & Chen SD (2010). Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I & Jin LW (2010). Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci 30, 5346–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Scianni M, Branchi I, D'Andrea I, Lauro C & Limatola C (2011). CX3CR1 deficiency alters hippocampal‐dependent plasticity phenomena blunting the effects of enriched environment. Front Cell Neurosci 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Trettel F, Scianni M, Bertollini C, Eusebi F, Fredholm BB & Limatola C (2009). LTP impairment by fractalkine/CX3CL1 in mouse hippocampus is mediated through the activity of adenosine receptor type 3 (A3R). J Neuroimmunol 215, 36–42. [DOI] [PubMed] [Google Scholar]
- Marin‐Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N & Mallat M (2004). Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547. [DOI] [PubMed] [Google Scholar]
- Martinez FO & Gordon S (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt SM & Johnson RW (2015). Neuro‐immune dysfunction during brain aging: new insights in microglial cell regulation. Curr Opin Pharmacol 26, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, Deflorio C, Lauro C, Alboni S, Limatola C, Branchi I, Tremblay ME & Maggi L (2016). Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav Immun 55, 114–125. [DOI] [PubMed] [Google Scholar]
- Minten C, Terry R, Deffrasnes C, King NJ & Campbell IL (2012). IFN regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS. PloS One 7, e49851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM & Cardona AE (2012). The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol 188, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier A, Adle‐Biassette H, Delezoide AL, Evrard P, Gressens P & Verney C (2007). Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol 66, 372–382. [DOI] [PubMed] [Google Scholar]
- Mosher KI & Wyss‐Coray T (2014). Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol 88, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI & Wyss‐Coray T (2015). Go with your gut: microbiota meet microglia. Nat Neurosci 18, 930–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME & Ingram DK (2002). Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res 956, 30–35. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Kimyon RS, De I, Small AL, Collier LS & Watters JJ (2015). Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol 278, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F & Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Norden DM & Godbout JP (2013). Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Koren EG, Mathew R & Saban DR (2016). Fate mapping reveals that microglia and recruited monocyte‐derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep 6, 20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo JO, Rezaie P, Gabbott PL & Stewart MG (2015). Impact of age‐related neuroglial cell responses on hippocampal deterioration. Front Aging Neurosci 7, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS & Colonna M (2009). Macrophage colony‐stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta‐catenin. Nat Immunol 10, 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K & Tremblay ME (2014). Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D & Gross CT (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ , Hempstead BL, Littman DR & Gan WB (2013). Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F & Nusslein‐Volhard C (2008). Live imaging of neuronal degradation by microglia reveals a role for v0‐ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927. [DOI] [PubMed] [Google Scholar]
- Perry VH & Holmes C (2014). Microglial priming in neurodegenerative disease. Nat Rev Neurol 10, 217–224. [DOI] [PubMed] [Google Scholar]
- Pinner RW, Teutsch SM, Simonsen L, Klug LA, Graber JM, Clarke MJ & Berkelman RL (1996). Trends in infectious diseases mortality in the United States. JAMA 275, 189–193. [PubMed] [Google Scholar]
- Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM & Weiner HL (2011). MicroRNA‐124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP‐alpha‐PU.1 pathway. Nat Med 17, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M & Priller J (2014). Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15, 300–312. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS & Ransohoff RM (2011). Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14, 1227–1235. [DOI] [PubMed] [Google Scholar]
- Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de Menezes JR & Nedergaard M (2015). A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci 35, 11848–11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RA, Spangenberg EE, Yamate‐Morgan H, Lee RJ, Arora RP, Hernandez MX, Tenner AJ, West BL & Green KN (2015). Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J Neurosci 35, 9977–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato C, Swinnen N, Buckinx R, Couillin I, Mangin JM, Rigo JM, Legendre P & Le Corronc H (2012). Microglia proliferation is controlled by P2X7 receptors in a Pannexin‐1‐independent manner during early embryonic spinal cord invasion. J Neurosci 32, 11559–11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC & Gemma C (2011). CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci 31, 16241–16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbauer F & Tenen DG (2007). Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol 7, 105–117. [DOI] [PubMed] [Google Scholar]
- Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A & Bessis A (2004). Impaired synaptic function in the microglial KARAP/DAP12‐deficient mouse. J Neurosci 24, 11421–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Pascual O, Bechade C, Wakselman S, Poncer JC, Real E, Triller A & Bessis A (2008). Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PloS One 3, e2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA & Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch T, Henke K & Peri F (2011). Microglia in the developing brain: from immunity to behaviour. Curr Opin Neurobiol 21, 5–10. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, Scheltens P & van Berckel BN (2012). Microglial activation in healthy aging. Neurobiol Aging 33, 1067–1072. [DOI] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo‐Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ & Geissmann F (2012). A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW & Bilbo SD (2012). Sex differences in microglial colonization of the developing rat brain. J Neurochem 120, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, Garcia‐Bueno B, van Rooijen N, Reyes TM & Sawchenko PE (2010). Dual roles for perivascular macrophages in immune‐to‐brain signaling. Neuron 65, 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S & Schwartz M (2013). Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield LG & Berman NE (1998). Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging 19, 47–55. [DOI] [PubMed] [Google Scholar]
- Sheng J, Ruedl C & Karjalainen K (2015). Most tissue‐resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 43, 382–393. [DOI] [PubMed] [Google Scholar]
- Shigemoto‐Mogami Y, Hoshikawa K, Goldman JE, Sekino Y & Sato K (2014). Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34, 2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz‐Aparicio I, Encinas JM, Comeau S & Tremblay ME (2014). Surveillance, phagocytosis, and inflammation: how never‐resting microglia influence adult hippocampal neurogenesis. Neural Plast 2014, 610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet‐Wadiche LS, Tsirka SE & Maletic‐Savatic M (2010). Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried‐Blackmore A, Milner TA, McEwen BS & Bulloch K (2008). Steroid hormone receptor expression and function in microglia. Glia 56, 659–674. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried‐Blackmore AC, McEwen BS & Bulloch K (2007). Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP & Rivest S (2006). Bone marrow‐derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502. [DOI] [PubMed] [Google Scholar]
- Siskova Z & Tremblay ME (2013). Microglia and synapse: interactions in health and neurodegeneration. Neural Plast 2013, 425845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J & Salter MW (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont‐Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F & Garel S (2014). Microglia modulate wiring of the embryonic forebrain. Cell Rep 8, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW & Barres BA (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Streit WJ (2006). Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci 29, 506–510. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS & Bechmann I (2009). Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol 118, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ & Sparks DL (2004). Dystrophic microglia in the aging human brain. Glia 45, 208–212. [DOI] [PubMed] [Google Scholar]
- Streit WJ & Sparks DL (1997). Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med (Berl) 75, 130–138. [DOI] [PubMed] [Google Scholar]
- Suridjan I, Rusjan PM, Voineskos AN, Selvanathan T, Setiawan E, Strafella AP, Wilson AA, Meyer JH, Houle S & Mizrahi R (2014). Neuroinflammation in healthy aging: a PET study using a novel Translocator Protein 18 kDa (TSPO) radioligand, [(18)F]‐FEPPA. Neuroimage 84, 868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svahn AJ, Giacomotto J, Graeber MB, Rinkwitz S & Becker TS (2015). miR‐124 Contributes to the functional maturity of microglia. Dev Neurobiol 76, 507–518. [DOI] [PubMed] [Google Scholar]
- Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, Brone B, Legendre P & Rigo JM (2013). Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 61, 150–163. [DOI] [PubMed] [Google Scholar]
- Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, West BL, Robinson JK & Tsirka SE (2016). Dynamic microglial modulation of spatial learning and social behavior. Brain Behav Immun 55, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL & Majewska AK (2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A & Nimmerjahn A (2011). The role of microglia in the healthy brain. J Neurosci 31, 16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Zettel ML, Ison JR, Allen PD & Majewska AK (2012). Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia 60, 541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M & Yamashita T (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16, 543–551. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE & Freeman WM (2011). Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation 8, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Monier A, Fallet‐Bianco C & Gressens P (2010). Early microglial colonization of the human forebrain and possible involvement in periventricular white‐matter injury of preterm infants. J Anat 217, 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith L, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley E, Zou B, Simmons D, Xie X, Longo F & Wyss‐Coray T (2014). Young blood reverses age‐related impairments in cognitive function and synaptic plasticity in mice. Nat Med 20, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S & Nabekura J (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29, 3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls JR, Coultas L, Rossant J & Henkelman RM (2008). Three‐dimensional analysis of vascular development in the mouse embryo. PloS One 3, e2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP 2nd, Scheffler B & Steindler DA (2006). Microglia instruct subventricular zone neurogenesis. Glia 54, 815–825. [DOI] [PubMed] [Google Scholar]
- Wang J, Wegener JE, Huang TW, Sripathy S, De Jesus‐Cortes H, Xu P, Tran S, Knobbe W, Leko V, Britt J, Starwalt R, McDaniel L, Ward CS, Parra D, Newcomb B, Lao U, Nourigat C, Flowers DA, Cullen S, Jorstad NL, Yang Y, Glaskova L, Vingeau S, Kozlitina J, Yetman MJ, Jankowsky JL, Reichardt SD, Reichardt HM, Gartner J, Bartolomei MS, Fang M, Loeb K, Keene CD, Bernstein I, Goodell M, Brat DJ, Huppke P, Neul JL, Bedalov A & Pieper AA (2015). Wild‐type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521, E1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang X, Zhao L, Ma W, Rodriguez IR, Fariss RN & Wong WT (2014). Macroglia‐microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J Neurosci 34, 3793–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS & Colonna M (2012). IL‐34 is a tissue‐restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Yang H & Schlichter LC (2008). Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci 28, 1316–1328. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Schuldiner O, Perrino J, Larsen C & Luo L (2004). Glia engulf degenerating axons during developmental axon pruning. Curr Biol 14, 678–684. [DOI] [PubMed] [Google Scholar]
- Wehrspaun CC, Haerty W & Ponting CP (2015). Microglia recapitulate a hematopoietic master regulator network in the aging human frontal cortex. Neurobiol Aging 36, 2443.e2449–2443.e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieghofer P, Knobeloch KP & Prinz M (2015). Genetic targeting of microglia. Glia 63, 1–22. [DOI] [PubMed] [Google Scholar]
- Williamson LL, McKenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, Parker W & Bilbo SD (2015). Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early‐life infection. Brain Behav Immun 51, 14–28. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF & Godbout JP (2014). Re‐establishment of anxiety in stress‐sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT (2013). Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dissing‐Olesen L, MacVicar BA & Stevens B (2015). Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol 36, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu L, He S, Wu Y, Jin W, Yu T, Qu JY & Wen Z (2015). Temporal‐spatial resolution fate mapping reveals distinct origins for embryonic and adult microglia in zebrafish. Dev Cell 34, 632–641. [DOI] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss‐Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E & Jung S (2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D & Gross CT (2014). Deficient neuron‐microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17, 400–406. [DOI] [PubMed] [Google Scholar]
- Zusso M, Methot L, Lo R, Greenhalgh AD, David S & Stifani S (2012). Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. J Neurosci 32, 11285–11298. [DOI] [PMC free article] [PubMed] [Google Scholar]


