My Neurolab colleagues and I studied autonomic mechanisms of healthy subjects on Earth and in space, and in two articles (Eckberg et al. 2016 a, b ) drew several conclusions, including this one: that at usual breathing frequencies (∼0.25 Hz), R‐R interval changes occur too soon after systolic pressure changes to be mediated by vagal baroreflex mechanisms.
Karemaker & DeBoer (2017) challenged this conclusion on three bases. (1) They simulated blood pressure fluctuations with a 0.25 Hz sinusoid and calculated latencies between the peaks of the waveforms and the intervals between waveforms (presumably) offset by 0.6 s. Their calculated phase angles were −54 deg (latency of 1.35 s) for 0.1 Hz oscillations, and 0 deg (latency of 0 s) for 0.25 Hz oscillations. (2) They cited evidence that baroreflex slopes calculated after pressor injections yield higher correlation coefficients when each systolic pressure is correlated with the R‐R interval in which it occurs, rather than the next. (3) They speculated that the longer latencies calculated at 0.1 Hz reflect sympathetic stimulation, which shifts pressure pulse to P wave intervals.
We thank Karemaker and DeBoer for their careful reading of our article, and for their thoughtful comments. Their challenge focuses on several interrelated aspects of the physiology we studied: respiratory sinus arrhythmia; sinoatrial node responses to individual, or trains of successive, experimental or spontaneous baroreceptor stimuli; and the kinetics of sinoatrial node responses to noradrenaline and acetylcholine.
Data derived from animal research indicate that about 72% of the total baroreflex latency reflects the kinetics of sinoatrial node responses to released acetylcholine (Eckberg & Sleight, 1992). A human study published 40 years ago (Eckberg, 1976) delineates the time course of sinoatrial node responses to baroreflex inhibition. Carotid baroreceptors were stimulated by precise, highly reproducible 60 mmHg, 0.58 s neck suction pulses, timed to sweep entire R‐R intervals.
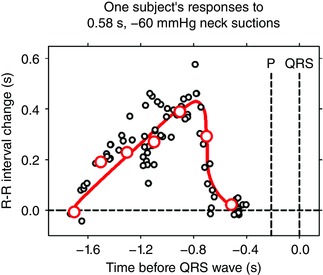
Figure 1 shows the responses of one subject to ∼73 individual applications of neck suction. The author assumed that each stimulus provokes equal releases of acetylcholine and that, therefore, the variability of responses reflects changing sinoatrial membrane properties. Since during brief held expiration R‐R intervals are nearly constant, responses to baroreceptor stimuli can be plotted as functions of their timing before the next P waves. These data document an absolute latency between the onset of stimulation and P waves of ∼0.54 s, and QRS complexes of ∼0.7 s, and illustrate the time course of sinoatrial responses to released acetylcholine. Phase angles are functions of the kinetics of sinoatrial node responses; it is unclear how baroreflex responses can be generated when calculated (and measured, Eckberg et al. 2016 a) phase angles are zero (no delay between pressure changes and P waves).
Figure 1. Sinoatrial node responses of one supine subject to precise 0.58 s. 60 mm Hg neck suctions. Since R‐R intervals fluctuate only minimally during early held‐expiration, responses were plotted as functions of the time before the P wave and QRS complexes would have occurred. Adapted, with permission from J Physiol, of Figure 2 (Eckberg 1976).
Another of our findings was that, unlike baroreflex relations calculated at lower (0.05 and 0.1 Hz) breathing frequencies, pressure–R‐R interval relations calculated at respiratory frequencies have highly variable phase angles and coherences. Other experimental interventions, including lower body suction (Blaber et al. 1995), mechanical ventilation (Koh et al. 1998), and upright tilt (Cooke et al. 1999), also provoke qualitatively different response patterns at respiratory frequencies (P wave changes may lead, as well as follow, arterial pulses). It is unclear why, if R‐R interval fluctuations do in fact reflect baroreflex physiology, the mode of breathing and other interventions so dramatically alter the timing and coherence between stimuli and putative baroreflex responses.
If respiratory‐frequency R‐R intervals reflect ongoing baroreflex buffering of pressure changes, those pressure changes should be increased when R‐R interval buffering is abolished. The opposite occurs: fixed‐rate atrial pacing reduces respiratory‐frequency arterial pressure fluctuations (Taylor & Eckberg, 1996). This indicates that R‐R intervals drive arterial pressure fluctuations, rather than the reverse. Moreover, if respiratory sinus arrhythmia reflects the ongoing baroreflex buffering of pressure changes, this fact should be documented by information transfer analysis. Stankovski et al. (2013) found significant information transfer between breathing and systolic pressure and R‐R interval oscillations at respiratory frequencies, but no significant information transfer between systolic pressure and R‐R interval oscillations.
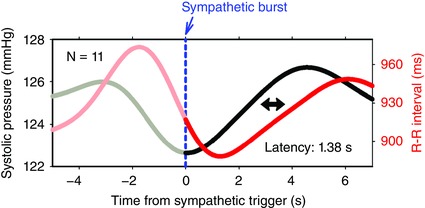
After intravenous injections of pressor drugs, successive R‐R intervals are longer than their predecessors; therefore, each pressure pulse falls farther in advance of successive P waves. Pickering et al. (1972) examined the first R‐R interval prolongations that occur after pressor injections and reported that the best correlations are found with the next interval after the pressure pulse, a latency of 1.6 s. In healthy volunteers who have very infrequent muscle sympathetic bursts, bursts sequentially trigger increases of pressure, and, after a latency of 1.38 s, increases of R‐R intervals (Diedrich et al. 2013). Fig. 2 shows the median systolic pressure and R‐R interval responses of 11 subjects to isolated sympathetic bursts. The calculated latency is close to the one we report (Eckberg et al. 2016 a), the one measured from the first prolonged R‐R interval after pressor injections (Pickering et al. 1972), and the latency calculated from 0.1 Hz simulated data by Karemaker & DeBoer (2017).
Figure 2. Average systolic pressure and R‐R interval changes of 11 subjects after large, isolated muscle sympathetic bursts, adapted from Figure 4. Diedrich et al. (2013).
Although it is true that sympathetic stimulation of the sinoatrial node takes more time to occur than vagal inhibition, sympathetic influences are not limited to 0.1 Hz, but are distributed widely over breathing frequencies (Taylor et al. 2001). We (J. B. Hoag, W. H. Cooke, P. T. Clemson, A. Stefanovska & D. L. Eckberg, unpublished observations) analysed haemodynamic responses of supine subjects breathing at 0.25 Hz. Our findings do not support the speculation of Karemaker & DeBoer (2017) that long baroreflex latencies at 0.1 Hz reflect sympathetic opposition to released acetylcholine. In our subjects, the median systolic pressure to P wave interval was 0.54 s after saline and 0.55 s after sympathetic blockade with a large dose of propranolol (P = 0.71, paired t test).
In our article (Eckberg et al. 2016 a), we were careful to own the possibility that subjects who breathe at less than the usual breathing frequency, 0.25 Hz, or those who have very long R‐R intervals, might experience baroreflex‐mediated slowing at their breathing frequencies. Although we cannot say conclusively that a baroreflex mechanism never occurs at usual breathing frequencies, our data, and the data discussed above, indicate that a baroreflex mechanism is highly unlikely. If respiratory sinus arrhythmia is not baroreflex mediated, what physiology explains it? The answer may be obscured by the expression ‘feed‐forward’: the physiological fact is that each breath alters vagal‐cardiac membrane properties (Gilbey et al. 1984) and thereby imposes a respiratory rhythm upon vagal‐cardiac nerve traffic. This central gating process (Eckberg, 2003) is sufficient to explain respiratory‐frequency R‐R interval fluctuations.
Additional information
Competing interests
None declared.
Linked articles This is a reply to a Letter to the Editor by Karemaker & DeBoer. To read the Letter to the Editor, visit http://dx.doi.org/10.1113/JP273766.
References
- Blaber AP, Yamamoto Y & Hughson RL (1995). Change in phase relationship between SBP and R‐R interval during lower body negative pressure. Am J Physiol 268, H1688–H1693. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO & Eckberg DL (1999). Human responses to upright tilt: a window on central autonomic integration. J Physiol 517, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich A, Crossman AA, Beightol LA, Tahvanainen KUO, Kuusela TA, Ertl AC & Eckberg DL (2013). Baroreflex physiology studied in healthy subjects with very infrequent muscle sympathetic bursts. J Appl Physiol 114, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL (1976). Temporal response patterns of the human sinus node to brief carotid baroreceptor stimuli. J Physiol 258, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL (2003). The human respiratory gate. J Physiol 548, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Cooke WH, Diedrich A, Biaggioni I, Buckey JC Jr, Pawelczyk JA, Ertl AC, Cox JF, Kuusela TA, Tahvanainen KUO, Mano T, Iwase S, Baisch FJ, Levine BD, Adams‐Huet B, Robertson D & Blomqvist CG (2016. a). Respiratory modulation of human autonomic function on Earth. J Physiol 594, 5611–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Diedrich A, Cooke WH, Biaggioni I, Buckey JC Jr, Pawelczyk JA, Ertl AC, Cox JF, Kuusela TA, Tahvanainen KUO, Mano T, Iwase S, Baisch FJ, Levine BD, Adams‐Huet B, Robertson D & Blomqvist CG (2016. b). Respiratory modulation of human autonomic function: long‐term neuroplasticity in space. J Physiol 594, 5629–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL & Sleight P (1992). Human Baroreflexes in Health and Disease, p. 256 Clarendon Press, Oxford. [Google Scholar]
- Gilbey MP, Jordan D, Richter DW & Spyer KM (1984). Synaptic mechanisms involved in the inspiratory modulation of vagal cardio‐inhibitory neurones in the cat. J Physiol 356, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karemaker J & DeBoer R (2017). Vagal baroreflex latency in circulatory control. J Physiol 595, 2197–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J, Brown TE, Beightol LA & Eckberg DL (1998). Contributions of tidal lung inflation to human R‐R interval and arterial pressure fluctuations. J Auton Nerv Syst 68, 89–95. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Gribbin B & Sleight P (1972). Comparison of the reflex heart rate response to rising and falling arterial pressure in man. Cardiovasc Res 6, 277–283. [DOI] [PubMed] [Google Scholar]
- Stankovski T, Cooke WH, Rudas L, Stefanovska A & Eckberg DL (2013). Time‐frequency methods and voluntary ramped‐frequency breathing: a powerful combination for exploration of human neurophysiological mechanisms: J Appl Physiol 115, 1806–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA & Eckberg DL (1996). Fundamental relations between short‐term RR interval and arterial pressure oscillations in humans. Circulation 93, 1527–1532. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H & Eckberg DL (2001). Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal‐cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol 280, H2804–H2814. [DOI] [PubMed] [Google Scholar]


