Abstract
Objectives:
The AspireAssist System (AspireAssist) is an endoscopic weight loss device that is comprised of an endoscopically placed percutaneous gastrostomy tube and an external device to facilitate drainage of about 30% of the calories consumed in a meal, in conjunction with lifestyle (diet and exercise) counseling.
Methods:
In this 52-week clinical trial, 207 participants with a body-mass index (BMI) of 35.0–55.0 kg/m2 were randomly assigned in a 2:1 ratio to treatment with AspireAssist plus Lifestyle Counseling (n=137; mean BMI was 42.2±5.1 kg/m2) or Lifestyle Counseling alone (n=70; mean BMI was 40.9±3.9 kg/m2). The co-primary end points were mean percent excess weight loss and the proportion of participants who achieved at least a 25% excess weight loss.
Results:
At 52 weeks, participants in the AspireAssist group, on a modified intent-to-treat basis, had lost a mean (±s.d.) of 31.5±26.7% of their excess body weight (12.1±9.6% total body weight), whereas those in the Lifestyle Counseling group had lost a mean of 9.8±15.5% of their excess body weight (3.5±6.0% total body weight) (P<0.001). A total of 58.6% of participants in the AspireAssist group and 15.3% of participants in the Lifestyle Counseling group lost at least 25% of their excess body weight (P<0.001). The most frequently reported adverse events were abdominal pain and discomfort in the perioperative period and peristomal granulation tissue and peristomal irritation in the postoperative period. Serious adverse events were reported in 3.6% of participants in the AspireAssist group.
Conclusions:
The AspireAssist System was associated with greater weight loss than Lifestyle Counseling alone.
Introduction
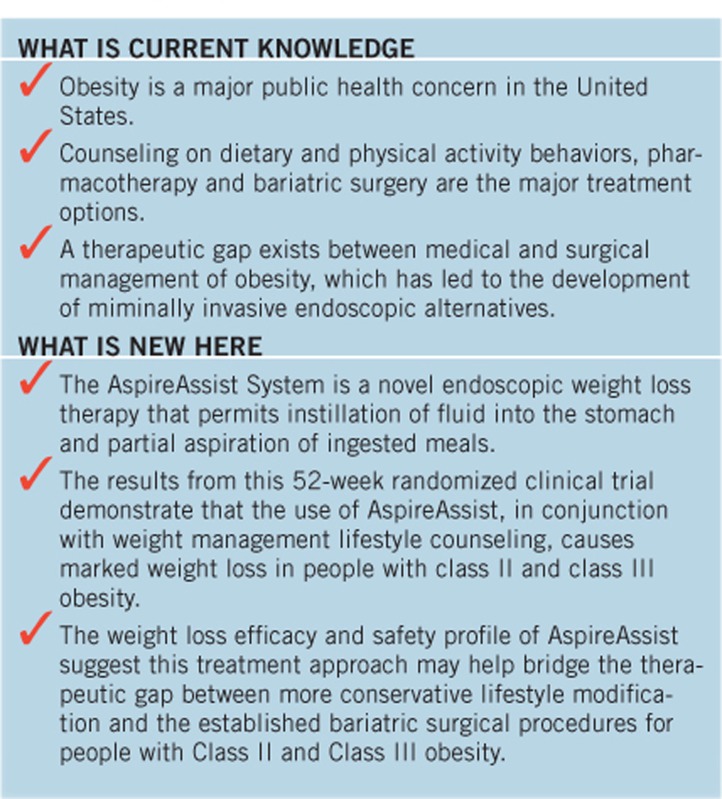
Obesity is a major public health problem in the United States and throughout most of the world due to its high prevalence and adverse effects on health, quality of life, and health-care costs (1). The overall goal of all obesity therapies is to reduce body fat mass by consuming less energy than expended, and then to maintain a targeted healthier and lower body weight via a reduced-calorie diet. Accordingly, portion control of food intake is an important tenant of obesity treatment. Currently, counseling on dietary and physical activity behaviors, pharmacotherapy, and bariatric surgery are the major treatment options. These approaches cause weight loss primarily by decreasing energy intake, while some surgical procedures also work by inducing calorie malabsorption (2). The ability to achieve successful weight loss increases progressively from lifestyle counseling to pharmacotherapy to surgical intervention; however, the availability and utilization of these therapies remain limited with less than 2% of people who qualify for either pharmacotherapy or surgical intervention receiving those therapies (3, 4). This limited acceptance and low utilization rate is partially due to the invasive and irreversible nature of surgery and its potential complications, the limited effectiveness of non-surgical treatments (5), and high costs. These issues have led to increasing interest in developing endoscopic obesity therapies for patients who have not had successful weight loss with conservative therapies, but who do not qualify for, or desire, more invasive bariatric surgery.
The AspireAssist System (AspireAssist) is a novel endoscopic weight loss therapy comprising an endoscopically placed percutaneous gastrostomy tube (A-tube), a skin port, and a separate accessory device. The system permits instillation of fluid into the stomach and partial aspiration of ingested meals. This therapy is used in conjunction with lifestyle counseling aimed to reduce energy intake and increase physical activity. A 1-year pilot study, conducted in a small number of obese participants at a single center in the United States, found that the AspireAssist caused a threefold greater weight loss than lifestyle counseling alone (6).
The present report describes the Pivotal Aspiration Therapy with Adjusted Lifestyle (PATHWAY) Study, a 1-year multicenter, randomized, controlled trial designed to evaluate the efficacy and safety of AspireAssist for weight management in persons who have obesity.
Methods
Study overview
This study was a 52-week randomized controlled trial conducted at 10 sites in the United States from 13 November 2012 until 17 June 2015 under the guidelines of the Declaration of Helsinki (registered at ClinicalTrials.gov (NCT01766037); the study protocol is available in the Supplementary Appendix online). All participants provided written informed consent before participating in this study, which was approved by the Institutional Review Board at each site. All authors were involved in the preparation of the manuscript, agreed to submit it for publication, and assumed responsibility for the accuracy and completeness of the data and the data analyses. The sponsor, Aspire Bariatrics (King of Prussia, PA), performed the statistical analyses and provided editorial assistance in preparing this manuscript.
Study participants
Key eligibility criteria were age 21–65 years old and a body mass index (BMI; the weight in kilograms divided by the square of the height in meters) of 35.0–55.0 kg/m2. Key exclusion criteria were history of gastrointestinal disease or previous abdominal surgery that would increase the risk of endoscopic A-tube placement, previous bariatric surgery, chronic abdominal pain, serious cardiovascular disease (including acute coronary syndrome or New York Heart Association class III or IV heart failure), use of medications that cause clinically significant weight gain or loss, or a history of major depressive or other severe psychiatric disorders. In addition, potential participants were excluded if they had a history of an eating disorder (binge eating disorder, bulimia nervosa or night eating syndrome) or evidence of an eating disorder evaluated by using the Questionnaire on Eating and Weight Patterns-Revised (7) and by conducting an Eating Disorder Examination (8), which provide a self-reported measure and an interview-based assessment of binge eating, purging and disordered attitudes and behaviors related to eating, body-shape, and weight. Details of the eligibility and exclusion criteria are provided in Supplementary eTable S1 in the Supplementary Appendix.
Study design
Eligible participants were randomized in a 2:1 ratio to 52 weeks of treatment with AspireAssist (Aspiration Therapy plus Lifestyle Counseling) or Lifestyle Counseling alone, respectively, by using a Web-based system provided by the sponsor. Participants in the AspireAssist were allowed to continue in the study for an additional 48 months if they lost and maintained at least 10% of their baseline body weight. Allocation occurred in blocks of random sizes to avoid temporal bias, and each site was randomized independently.
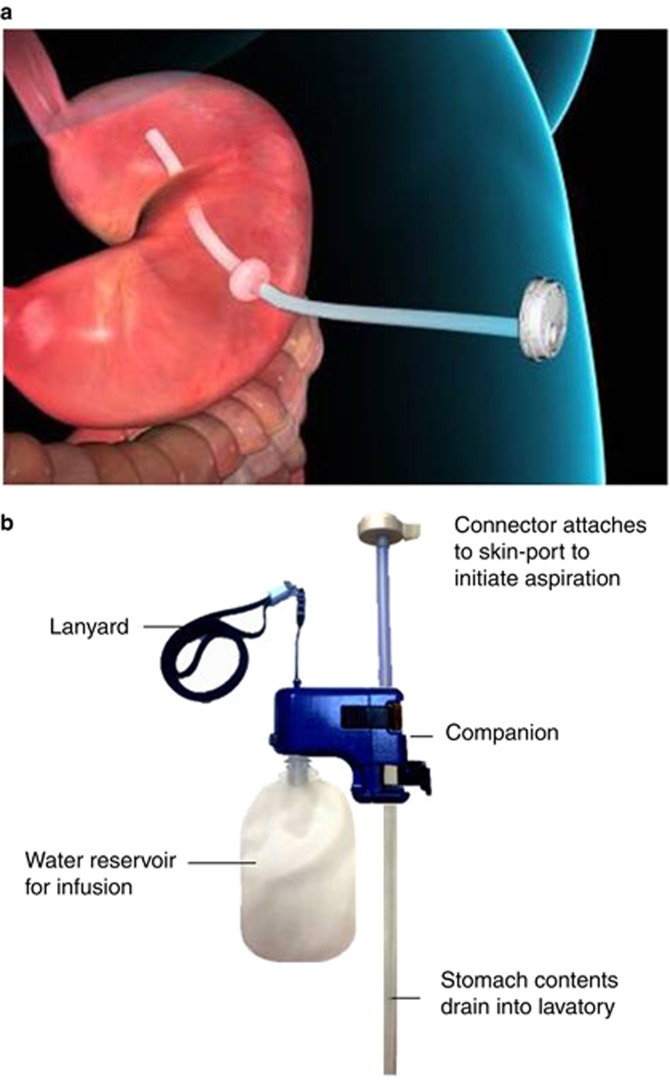
Participants randomized to therapy with AspireAssist underwent endoscopic placement of a specially designed gastrostomy tube, known as the A-tube, which has a 15-cm fenestrated intragastric portion to allow aspiration of gastric contents. The endoscopic procedure is analogous to the placement of a percutaneous endoscopic gastrostomy tube (9) (details of the procedure are provided in the Supplementary Appendix), which is typically performed on an outpatient basis and takes approximately 15–20 min to complete. After the gastrostomy matured, at approximately 10–14 days following A-tube placement, at the week-0 visit, the proximal end of the A-tube was cut to within 1 cm of the abdominal wall and attached to a Skin-Port. Participants were then trained on how to aspirate after meals and instructed to chew very thoroughly, to avoid A-tube blockage, and to aspirate about 20 min after each of three main meals daily. The components of the AspireAssist device and the aspiration procedure are shown in Figure 1. The aspiration process involves flushing food particles out of the stomach and through the A-Tube by infusing water into the stomach from the reservoir and then reversing the flow by opening the clamp on the Companion component to allow gastric contents to drain out of the stomach into a lavatory. This process is repeated (typically 3–8 infusions) until food particles are no longer seen in the aspirate. The aspiration process usually takes 10–15 min to perform. The counter mechanism within the Connector counts down by 1 count, from 115 initial counts, each time the Connector opens the Skin Port valve. When the Counter reaches “0” counts (usually after ~5 weeks of therapy), the Connector can no longer open the Skin Port valve, preventing additional aspiration procedures without being seen by the research team to obtain a new Connector.
Figure 1.
(a,b) AspireAssist A-Tube with Skin-Port (a) and External Device (b). Individual components of the AspireAssist device are labeled. After most of the ingested meal has been converted into a chyme by the stomach (about 20 min after meal ingestion), the Connector is attached to the Skin-Port, which opens the closed Skin-Port valve. Gastric contents then spontaneously flow out of the stomach through the drain tube into a toilet bowl. Remaining food particles are flushed out of the stomach and through the A-tube by flipping the lever on the Companion to allow water to be infused into the stomach from the reservoir and then reversing the flow to allow gastric contents to drain out of the stomach.
Participants in both treatment groups completed a 10-session behavioral and diet education weight loss program (details provided in the Supplementary Appendix) delivered to participants over 52 weeks. Participants in both treatment groups were seen by the study team for medical monitoring, lifestyle counseling, and blood tests at weeks 0, 2, 6, 10, 14, 20, 24, 28, 32, 36, 40, 44, 48, and 52. An assessment of eating behaviors (assessed by the Questionnaire on Eating and Weight Patterns-Revised and the Eating Disorder Examination) was made at baseline and at weeks 28 and 52, in both treatment groups, and an additional assessment at week 14 in the AspireAssist group.
Study end points
The overall purpose of the trial was to evaluate the safety and efficacy of AspireAssist. There were two prespecified co-primary end points. The first co-primary end point was mean percent excess weight loss (% EWL) at 52 weeks, defined as absolute weight loss divided by baseline excess weight (based on an ideal body weight at a BMI=25 kg/m2) multiplied by 100; success was defined as at least a 10% difference in %EWL between the AspireAssist and Lifestyle Counseling groups. The second co-primary end point was the proportion of participants who achieved at least a 25% EWL at 52 weeks; success was defined as having at least 50% of the AspireAssist group achieve at least 25% EWL. The co-primary end points were established after discussion with the Food and Drug Administration, and were based on typical end points for obesity devices at that time (10, 11, 12, 13, 14). Key secondary end points included change in percent total body weight from baseline, proportion of participants who achieved a reduction in total body weight of 10% or more, percent change in systolic and diastolic blood pressures, change in glycated hemoglobin, percent change from baseline in serum lipids (triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol), and change in the quality of life (assessed by using the Impact of Weight on Quality of Life questionnaire (15), which has scores that range from 0 to 100, with higher scores indicating a better quality of life). Procedural success was defined as percent of endoscopic attempts to place the A-tube that were successful. The safety end points included the incidence of procedure-related, device-related, and therapy-related adverse events.
Statistical analyses
There were two primary effectiveness objectives in this study. The first was to determine if the %EWL in the AspireAssist group was 10% better than the %EWL in the Lifestyle Counseling group. For this first co-primary end point, we estimated that 87 participants in the AspireAssist group and 42 participants in the Lifestyle Counseling group would yield a 90% power to detect a 10% difference in % EWL between groups at a 5% significance level. The second objective was to determine if the percentage of patients in the AspireAssist group who had %EWL of at least 25% was greater than 50%. For the second co-primary end point, we estimated that 85 participants in the AspireAssist group would yield a power of 90% to demonstrate that at least 50% of the AspireAssist group would achieve at least a 25% EWL at a 5% significance level. There was no adjustment in the significance level for multiple end points.
According to the prespecified analysis plan, all safety and efficacy analyses were conducted on the modified intent-to-treat (mITT) population. For the AspireAssist participants, the mITT population is defined as all participants who underwent attempted endoscopic placement of the A-Tube, and for the Lifestyle Counseling participants, as all participants who attended any Lifestyle Therapy session. Hence participants that dropped out after randomization but before receiving any treatment were not included in the mITT population.
The first and second co-primary effectiveness analyses were performed on the mITT population in which missing weight data for all time points were imputed by using a multiple imputation algorithm. For the analyses, the results of all imputations were analyzed via the protocol methods of a two sample t-test, for the first co-primary end point, and one-sample binomial test, for the second co-primary end point, and then compiled in PROC MIANALYZE to determine the final P value. In the multiple imputation model, data from all 111 mITT AspireAssist participants and 59 out of 60 mITT Lifestyle Counseling participants (1 Lifestyle Counseling subject only attended the first study visit and hence there were no data to impute for further visits) were used.
Secondary effectiveness analyses were performed by using the per-protocol population and the mixed effects linear model to confirm the robustness of the results. In the per protocol population, no value is imputed for missed data. Since the 52-week data are primary outcome and only the patients who have completed that time point are included in the per protocol population, no consistent cohort was tabulated across all the time points. In the per protocol model, data from all 31 Control participants and all 82 AT participants who completed their 52-week visit were included.
No differences in any participant characteristics were identified in those who dropped out from those who remained in the study. Accordingly, the assumption of the data is Missing at Random (MAR) is valid for this study. Further, since the least-squares means that are derived from a mixed effects model are unbiased with respect to this missing data pattern, the data were also analyzed by using a repeated measures mixed effects model and the least-squares means for the AspireAssist and Lifestyle Counseling groups estimated along with the difference. These results provide unbiased estimates of the primary end point results. Data from all 31 Lifestyle Counseling participants and all 82 AspireAssist participants who completed their 52-week visit were included.
A sensitivity analysis was conducted using two different imputation methods on the mITT population. The first imputation method is the Last Observation Carried Forward to impute the missing values. All missing post treatment data were imputed using the last available observation, including the first time point post treatment. The second imputation method is the Return to Baseline method. All missing post treatment data were imputed using the baseline observation. This includes the first time point post treatment.
Results
Trial population
A total of 207 participants were randomized in a 2:1 fashion: 137 to AspireAssist and 70 to Lifestyle Counseling (see Supplementary eFigure S1 in the Supplementary Appendix). Baseline characteristics were not different between groups (Table 1). Among those randomized, 26 AspireAssist and 10 Lifestyle Counseling participants withdrew before beginning the study so 111 in the AspireAssist group and 60 in the Lifestyle Counseling group enrolled in the study (Table 1). Six of the 26 AspireAssist participants withdrew, or were withdrawn, for medical reasons (see Supplementary eFigure S1 in the Supplementary Appendix). Excluding those subjects withdrawn for medical reasons, the pre-enrollment withdrawal rate of the AspireAssist group (14.5%) was approximately the same as the withdrawal rate of the Lifestyle Counseling group (14.2%).
Table 1. Baseline characteristics of the study groupsa.
|
AspireAssist |
Lifestyle Counseling |
|||
|---|---|---|---|---|
| Modified intention-to-treat analysis | Completer analysis | Modified intention-to-treat analysis | Completer analysis | |
| N | 111 | 82 | 60 | 31 |
| Sex—n (%) | ||||
| Male | 15 (13.5) | 14 (17.1) | 7 (11.7) | 4 (12.9) |
| Female | 96 (86.5) | 68 (82.9) | 53 (88.3) | 27 (87.1) |
| Age—years | 42.4±10.0 | 43.5±10.2 | 46.8±11.6 | 47.6±11.4 |
| Race or ethnic group—n (%)b | ||||
| White, non-Hispanic | 63 (56.8) | 51 (62.2) | 31 (51.7) | 16 (51.6) |
| Black or African American | 33 (29.7) | 21 (25.6) | 17 (28.3) | 10 (32.3) |
| Hispanic or Latino | 11 (9.9) | 8 (9.8) | 11 (18.3) | 4 (12.9) |
| Other | 4 (3.6) | 2 (2.4) | 1 (1.7) | 1 (3.2) |
| Weight—kg | 116.9±21.2 | 119.1±21.0 | 112.8±16.1 | 114.7±14.8 |
| Body mass index—kg/m2 c | 42.0±5.1 | 42.4±5.0 | 40.9±3.9 | 41.3±4.0 |
| Blood pressure—mm Hg | ||||
| Systolic | 124.4±13.6 | 124.2±13.3 | 127.3±15.0 | 124.5±13.4 |
| Diastolic | 78.9±8.9 | 78.8±8.1 | 78.1±8.8 | 73.1±6.8 |
| Cholesterol—mg/dl | ||||
| Total | 194.3±38.2 | 193.8±37.4 | 198.8±36.0 | 199.3±28.4 |
| LDL | 115.8±33.1 | 115.4±32.8 | 119.0±31.0 | 119.2±31.8 |
| HDL | 52.7±13.3 | 52.2±14.4 | 51.8±12.3 | 53.8±12.6 |
| Triglycerides—mg/dl | 136.1±77.9 | 140.8±81.7 | 125.3±77.3 | 133.8±53.7 |
| Fasting glucose—mg/dl | 92.9±20.2 | 93.5±23.1 | 94.3±20.3 | 93.7±11.5 |
| Glycated hemoglobin—% | 5.7±0.6 | 5.7±0.5 | 5.8±0.6 | 5.7±0.5 |
| Diabetes—n (%)d | 3 (2.7) | 2 (2.4) | 8 (13.3) | 3 (9.7) |
| Dyslipidemia—n (%)d | 86 (77.5) | 68 (82.9) | 51 (85.0) | 27 (87.1) |
| Hypertension—n (%)d | 46 (41.4) | 37 (45.1) | 24 (40.0) | 8 (25.8) |
Plus–minus values are observed means±s.d. There were no statistically significant differences between the two groups for any characteristic for either the mITT or the completer populations. To convert values for glucose to millimoles per liter, multiply by 0.05551. To convert values for cholesterol to millimoles per liter, multiply by 0.0259. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Race and ethnic group were self-reported.
The body mass index is the weight in kilograms divided by the square of the height in meters.
The diagnoses of diabetes, dyslipidemia, and hypertension were based on self-reported medical history and medical evaluation at screening.
After enrollment, 29 AspireAssist and 29 Lifestyle Counseling participants subsequently withdrew from the study. Therefore, 82 AspireAssist (74% of those enrolled) and 31 Lifestyle Counseling participants (52% of those enrolled) completed the entire 52-week study. The major reasons cited for post-enrollment withdrawals in the AspireAssist group were lack of time/motivation (17 participants) or moving out of state (5 participants), and the major reasons cited in the Lifestyle Counseling group were not identified (10 participants) and lack of time (6 participants) (see Supplementary eFigure S1 in the Supplementary Appendix). One participant in the AspireAssist group (0.9%) withdrew from the study owing to adverse events while no Lifestyle Counseling withdrew owing to adverse events; the withdrawal in the AspireAssist group was due to peristomal irritation. A smaller percentage of participants in the AspireAssist group than in the Lifestyle Counseling group withdrew from the study owing to ineffective therapy (0.9% (1 of 111 participants) vs. 5% (3 of 60)).
Procedural success
Successful endoscopic placement of the A-tube was achieved in 97% of attempts (111 of 114 endoscopies performed in 112 participants). Endoscopy was aborted before A-tube placement in three participants: one because of inability to trans-illuminate through the abdomen; and two participants with contraindications (one suspected gastric varices and another discovery of previous Roux-en-Y gastric bypass surgery by a participant who failed to disclose such prior surgery during screening). Mean procedure time was 16±7 min and mean recovery time post-procedure was 106±48 min. Approximately 80% of the procedures were performed under conscious sedation, and the remaining 20% were performed under general anesthesia with endotracheal intubation, at the discretion of the anesthesiology team.
Weight loss
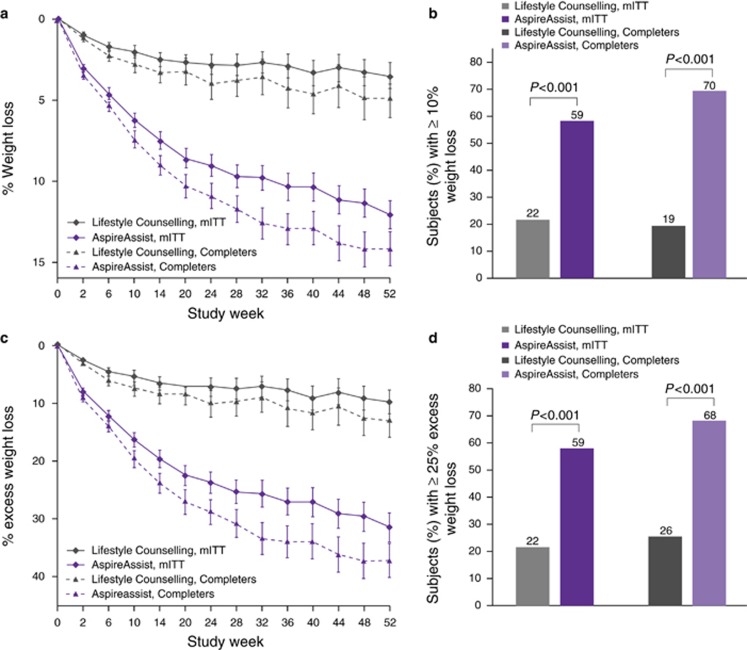
At 52 weeks, based on an mITT analysis, mean percent body weight loss at 52 weeks was12.1±9.6% (14.2±9.8% for completers only) in the AspireAssist group and 3.5±6.0% (4.9±7.0% for completers only) in the Lifestyle Counseling group (Figure 2a). The difference in the mean percent body weight loss between the two groups was 8.6% (95% CI: 6.2–10.9). A greater proportion of participants in the AspireAssist group than in the Lifestyle Counseling group lost 10% or more of their initial body weight (58.6% vs. 22.0% in the mITT analysis and 69.5% vs. 19.4% in the completers only analysis) (Figure 2b). Mean weight loss of the mITT population was 14.2±11.3 kg in the AspireAssist group and 4.1±7.2 kg in the Lifestyle Counseling group (see Supplementary eFigure S2).
Figure 2.
Effect of AspireAssist on excess weight loss and percentage weight loss. Mean percentage body weight loss (a) at each study visit is shown, according to study group, for the modified intention-to-treat population (with multiple imputation for missing values) and for those who completed the entire study. The proportion of participants who lost 10% or more of their total body weight at 52 weeks (b) is shown for the modified intention-to-treat population and for those who completed 52 weeks. I bars (a,c) indicate s.e. Mean percent excess weight loss (c) at each study visit is shown, according to study group, for the modified intention-to-treat population (with multiple imputation for missing values) and for those who completed the entire study. The proportion of participants who lost 25% or more of their excess weight at 52 weeks (d) is shown for the modified intention-to-treat population and for those who completed 52 weeks.
Based on an mITT analysis, participants in the AspireAssist group had lost a mean (±s.d.) of 31.5±26.7% of their excess body weight (37.2±27.5% for completers only), whereas those in the Lifestyle Counseling group had lost a mean of 9.8±15.5% of their excess body weight (13.0±17.6% for completers only) (Figure 2c). The difference in %EWL achieved between groups was 21.7% (95% CI 15.3, 28.1), which was greater than the 10% threshold needed to achieve the a priori definition of success (P=0.008). A greater proportion of participants in the AspireAssist group than in the Lifestyle Counseling group lost at least 25% of their excess body weight (58.6% vs. 22.0% in a mITT analysis and 68.3% vs. 25.8% in a completers only analysis) (Figure 2d). Several sensitivity analyses confirmed the beneficial effect of AspireAssist in the co-primary end points (see Supplementary eTables S2 and S3 in the Supplementary Appendix).
Early responsiveness with the AspireAssist, defined as 5% or more body weight loss at week 14, was predictive of weight loss at week 52. The early responders who completed the study (76% of AspireAssist completers) lost 17.2±8.7% body weight at 52 weeks, while participants who were not early responders but completed the study lost 4.9±6.3% body weight, which nearly the same as the weight loss observed by the completers in the Lifestyle Counseling group (see Supplementary eFigure S3). There were no significant differences in demographic and baseline characteristics of the early responders vs. early non-responders.
Cardiometabolic risk factors and quality of life
For the AspireAssist group, at week 52 compared with baseline, clinically significant improvement was seen in HbA1C (−0.36% relative to 5.7% baseline, P<0.0001), triglycerides (−9.9%, P=0.02), and high-density lipoprotein cholesterol (+8.1%, P=0.0001), while modest improvement was seen in systolic blood pressure (−1.2%, P=0.38), diastolic blood pressure (−2.6%, P=0.06), low-density lipoprotein cholesterol (−4.2%, P=0.06), and total cholesterol (−2.5%, P=0.07). For the Lifestyle group, at week 52 compared with baseline, moderate improvement was seen in HbA1C (−0.22% relative to 5.7% baseline, P<0.0001), while modest or no improvement was seen in triglycerides (+0.1%, P=0.62), high-density lipoprotein cholesterol (+1.7%, P=0.55), systolic blood pressure (−2.5%, P=0.17), diastolic blood pressure (+0.5%, P=0.83), low-density lipoprotein cholesterol (−1.8%, P=0.72), and total cholesterol (−2.5%, P=0.28). The differences in improvement from week 52 relative to baseline with the aforementioned parameters between the AspireAssist group and the Lifestyle Counseling group were not statistically significant (see Supplementary eTable S4 in Supplementary Appendix), except for glycated hemoglobin. It should be noted that medications to treat hypertension, dyslipidemia, and diabetes were not fixed throughout the study: the participants' primary physicians changed such medications as they saw fit. The Impact of Weight on Quality of Life score increased in both treatment groups, across all five measures (physical function, self-esteem, sexual life, public distress, and work) with the AspireAssist group showing a greater increase in total Impact of Weight on Quality of Life score than the Lifestyle Counseling group (P=0.03) (see Supplementary eTable S4).
Electrolytes and minerals
Mean plasma electrolytes (potassium, sodium, chloride, carbon dioxide), calcium and magnesium concentrations at 52 weeks of therapy were not different than those at baseline in both the AspireAssist and Lifestyle Counseling groups (see Supplementary eTable S5 in the Supplementary Appendix). However, hypokalemia (ranging from 3.2 to 3.7 mEq/l) was reported as an adverse event by the study site investigators in four participants, and was successfully treated with oral potassium supplementation.
At week 52 of therapy, no participant in the AspireAssist group developed hip or whole-body T-Scores of ≤−2.5 s.d. below the normal peak values. The hip T-score decreased slightly (1.08±1.15 at baseline to 0.97±1.19 at 52 weeks, P=0.1) in AspireAssist subjects, consistent with the expected reduction in bone mineral density that occurs with weight loss (16). Spine bone mineral density in AspireAssist participants was greater at the week 52 than at baseline, an artifact of the Skin Port sitting directly over the spine area being evaluated and influencing the reading.
Eating behaviors
Exclusion criteria from participating in the study included diagnosed bulimia or binge eating disorder based on DSM IV criteria or night eating syndrome as diagnosed by Eating Disorders Examination. At baseline, five participants (three AspireAssist and two Lifestyle Counseling) demonstrated evidence of binge eating behavior when evaluated by using the Questionnaire on Eating and Weight Patterns-Revised, but not when evaluated by using the more reliable Eating Disorders Examination, so these participants were not excluded for participating in the study. One participant in the Lifestyle Counseling group, who showed no evidence of binge-eating behaviors at screening, demonstrated, at week 28, evidence of binge eating, when evaluated by using the both the Questionnaire on Eating and Weight Patterns-Revised and the Eating Disorders Examination, and hence was withdrawn from the study. One participant in the AspireAssist group demonstrated, at weeks 14 and 28 (as well at screening), evidence of binge eating behavior when evaluated by using the Questionnaire on Eating and Weight Patterns-Revised, but not by using the Eating Disorders Examination, and hence was allowed to continue in the study. No evidence of bulimia in any subject was detected by using either the Questionnaire on Eating and Weight Patterns-Revised or the Eating Disorders Examination at baseline, or at weeks 14, 28, or 52.
The frequency of use of the AspireAssist was tracked roughly by the use of the Connector, which counts down by one count from an initial 115 counts, each time a participant connected the AspireAssist. The remaining Connector counts were recorded at each study visit, as were the issuance of each new Connector. Mean and median daily usage, as recorded by Connector counts, were 2.5 and 2.4 times, respectively, from week 1 to week 14, 2.3 and 2.2 times, respectively, from week 14 to week 28, and from week 28 to week 40, and 2.2 and 2.0 times, from week 40 to week 52. There was no evidence of any subject overusing the device (e.g., more than ~3.0 times per day).
Adverse events
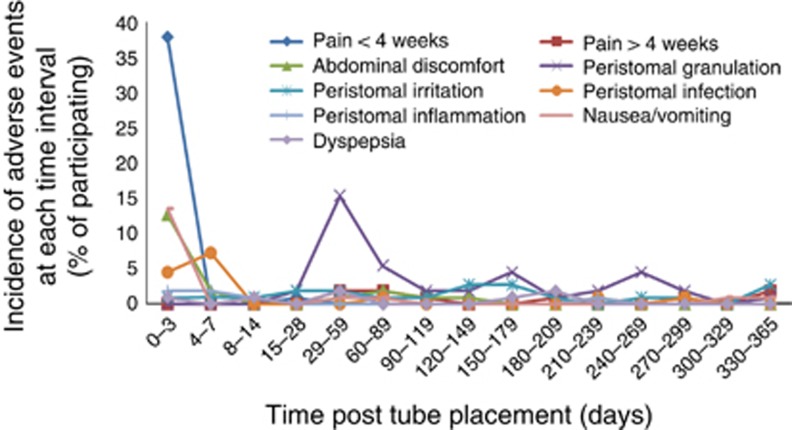
Approximately 90% of the study-related adverse events (SAEs) in the AspireAssist group were those known to be associated with percutaneous endoscopic gastrostomy tubes (Table 2), and approximately half of all SAEs occurred within the first 7 days after A-tube placement (Figure 3 and Table 2). The development of peristomal granulation tissue occurred later, at 1–2 months after A-tube placement. Most adverse events resolved spontaneously or with standard medical therapy, such as oral analgesics for abdominal pain, oral antibiotics for suspected or documented peristomal infection, and topical silver nitrate for granulation tissue. A listing of all adverse events is provided in Supplementary eTable S6 in the Supplementary Appendix. Five serious SAEs, occurred in four participants in the AspireAssist group (Table 2); no study-related SAEs occurred in the Lifestyle Counseling group. One of the SAEs was mild peritonitis, which occurred 2 days after A-Tube placement and was treated with intravenous antibiotics and analgesics during a 2-day hospitalization. Two of the SAEs were associated with severe abdominal pain that occurred in the same participant within the first 3 days after A-tube placement; each time the participant was hospitalized overnight and treated with pain medication. Another SAE was associated with abdominal pain caused by a pre-pyloric ulcer that occurred 53 weeks after A-tube placement, judged to have been caused by mucosal contact with the intragastric portion of the A-tube which had rotated toward the pylorus from its usual position in the fundus. The final SAE was a product malfunction that required outpatient endoscopy to replace the A-tube. The three perioperative SAEs completely resolved with medical therapy, while the two postoperative SAEs resolved by removal of the A-Tube in one case and replacement of the A-Tube in the other.
Table 2. Adverse events occurring in 5% or more of participants and serious adverse events in the AspireAssist group (N=111), and time period in which the event occurred (i) <7 days (perioperative) and (ii) >7days (postoperative) of A-tube placement.
| Adverse events | No. of participants (%) | No. of participants, perioperative | No. of participants, postoperative |
|---|---|---|---|
| Peristomal granulation tissue | 45 (40.5%) | 0 | 45 |
| Abdominal pain within 4 weeks after A-tube placementa | 42 (37.8%) | 41 | 1 |
| Nausea/vomiting | 19 (17.1%) | 15 | 4 |
| Peristomal irritation | 19 (17.1%) | 2 | 17 |
| Intermittent abdominal discomfort | 18 (16.2%) | 16 | 2 |
| Possible or definite peristomal bacterial infection | 15 (13.5%) | 13 | 2 |
| Abdominal pain 4 weeks or more after A-tube placementa | 9 (8.1%) | 0 | 9 |
| Dyspepsia (acid reflux, heartburn, hiccups, belching) | 7 (6.3%) | 1 | 6 |
| Peristomal inflammation | 6 (5.4%) | 4 | 2 |
| Serious adverse events | |||
| Severe abdominal pain | 1 (0.9%) | 1 | |
| Peritonitis | 1 (0.9%) | 1 | |
| Pre-pyloric ulcer | 1 (0.9%) | 1 | |
| A-tube replacement because of Skin-Port malfunction | 1 (0.9%) | 1 | |
Defined as abdominal pain not relieved with standard oral analgesic therapy.
Figure 3.
Occurrence of adverse events in the AspireAssist group. Time course of the adverse advents that occurred at a prevalence rate 5% or more during the 52-week study in the AspireAssist group.
Tube replacement
There were four A-Tubes that had to be replaced because of A-Tube deterioration in the study: three because of fungal growth and one because of punctures of an unknown origin. No A-Tube had to be removed because of clogs.
Discussion
Obesity continues to have an increasing impact on a global scale. Lifestyle modification and surgical procedures are important components to a successful management strategy, but additional options are needed to help address this worsening epidemic. The results from this 52-week randomized clinical trial demonstrate that the use of AspireAssist, in conjunction with weight management lifestyle counseling, causes marked weight loss in people with class II and class III obesity. Treatment with AspireAssist was superior to treatment with lifestyle counseling alone and met the prespecified criteria for success for both of the study's co-primary end points: (1) % EWL in the AspireAssist group was 22% greater than the %EWL achieved in the Lifestyle Counseling only group, which was more than the 10% prespecified criterion; and (2) 59% of the AspireAssist group lost at least 25% of their excess body weight, which was more than the 50% prespecified criterion. These results are consistent with the results from a previous trial (6), and thereby further supports the efficacy of this intervention.
The AspireAssist group saw clinically significant improvement in glycated hemoglobin, high-density lipoprotein cholesterol, triglycerides, and modest improvement in blood pressure, low density lipoprotein, and low-density lipoprotein cholesterol. The treatment difference between the AspireAssist group and the Lifestyle group was statistically significant for glycated hemoglobin, but was only modest for blood pressure and lipids. As the study was sized to show statistically significant treatment difference for weight loss, but not for cardiometabolic parameters, the modest treatment difference observed in blood pressure and lipids is not surprising. Further, although some subjects were being treated for hypertension (29 AspireAssist/20 Lifestyle), dyslipidemia (13 AspireAssist/12 Lifestyle), and diabetes (3 AspireAssist/6 Lifestyle), only a small fraction of participants had abnormal baseline blood pressure, lipid, or glycated hemoglobin levels, reducing the likelihood of seeing a large treatment effect in either group. A possible confounder in this analysis are changes made in medications to treat these cardiometabolic conditions by the participants primary care physicians.
The Food and Drug Administration has recently approved two intragastric balloons (IGBs) for weight loss (13, 14). The AspireAssist and the IGBs are similar in that they can be easily placed and removed in a short endoscopic procedure; however, the AspireAssist allows long duration of use while IGBs are intended for only temporary use (6 months). Weight loss of participants with the AspireAssist follows a similar trajectory those with IGBs for the first 6 months; however, after IGB removal at 6 months, the IGB participants showed moderate weight gain between 6 and 12 months while AspireAssist participants continued to lose weight, resulting in 12.1% TBL for AspireAssist participants at 12-months, vs. 7.6 and 4.8% TBL for the Orbera and ReShape Duo balloons, respectively (13, 14). Finally, IGBs are targeted for less-obese patients (30–40 kg/m2), while the AspireAssist is targeted for more obese patients (35–55 kg/m2).
The AspireAssist induces weight loss by direct removal of ingested food from the stomach before it passes into the small intestine, where it is further digested and absorbed. After meal consumption, solid food is triturated into small particles before it can pass through the pylorus (17). This lag phase between meal consumption and gastric emptying provides an opportunity to remove a portion of ingested food, which has become a homogenized liquid chyme, from the stomach before it is absorbed. Nevertheless, a previous study found that the aspiration process is inefficient and removes less than 30% of ingested calories, when all instructions are adhered to precisely (6). Although there are no data on the percent of daily caloric intake aspirated in this study, the actual percent is similarly expected to have been less than 30%, on average. Additionally, connector count data showed that the device was used for approximately 2.5 sessions per day over the first 14 weeks and approximately 2 sessions per day thereafter. This suggests that participants consumed, on average, one or more meals and/or snacks per day after which the AspireAssist was not used. In light of these above factors, and the weight loss realized in this study, it is possible that mechanisms beyond caloric loss due to gastric aspiration are contributing to the overall weight loss (6). Some likely secondary mechanisms may be due to proper device use necessitating adoption of standard dietary principles, including thorough and complete mastication of food, drinking water at meals, and better meal planning. Other potential mechanisms are less well defined.
The results from this study also provide insights into the factors involved in the regulation of food intake in people who have obesity. Despite the removal of a portion of ingested calories in the AspireAssist group, there was no evidence of a compensatory increase in food intake during or between meals to compensate for the reduction in energy intake. This observation implicates central reward pathways and chronic lifestyle behaviors in driving food consumption. Therefore, in addition to the neuroendocrine response to calorie restriction (18), liking and wanting food, habitual eating habits, and social influences are likely major contributors to the high rate of recidivism observed after initial weight loss (19), and need to be addressed to achieve successful long-term weight management. As such, the system could be viewed as providing portion control at the level of the stomach and as a tool to better ensure compliance with low-calorie diet therapy (e.g., approximately 750 kcal/day would be removed if all meals are aspirated in a person who normally consumes a 2,500 kcal/day diet), as recommended by several major medical and scientific societies (20, 21).
Our data demonstrate that the increased abdominal girth in people with obesity does not prohibit successful and safe percutaneous endoscopic gastrostomy tube placement. The procedure was aborted in only one of 111 participants (<1%) because of an inability to trans-illuminate through the abdominal wall during endoscopy. The success we observed in our participants is consistent with the experience in placing percutaneous endoscopic gastrostomy tubes reported by other groups in patients who are obese with BMI values up to 70 kg/m2 (22, 23).
The durability of the AspireAssist A-Tube appears to be significantly greater than PEG Tubes, with less than 5% of AspireAssist subjects requiring A-tube replacements in the first year. Possible reasons for this greater durability are its low profile and larger diameter.
The adverse events associated with the use of AspireAssist were primarily those known to be associated with percutaneous endoscopic gastrostomy tubes (24), and resolved spontaneously or with standard medical therapy. Only one participant in the AspireAssist group withdrew from the study because of an adverse event. Of the 29 subjects who had their A-Tubes removed before the end of the 52-week study, one had a persistent fistula, which was successfully treated with endoscopic clip placement, whereas the fistulae in the remaining 28 subjects healed spontaneously. No clinically meaningful changes in plasma electrolyte, calcium, phosphorous, or magnesium concentrations were detected.
A potential concern with use of this device is that it might promote eating disorders, in particular bulimia and binge-eating; however, people with pre-existing eating disorders (binge-eating, bulimia nervosa, and night-eating syndrome) were excluded from this study. Bulimia nervosa is a psychological disorder involving distortion of body image and an obsessive desire to lose weight, in which bouts of bingeing (eating vast amounts of food, typically rapidly and mindlessly) are followed by depression and self-induced vomiting, purging, or fasting. There was no evidence of participants with the AspireAssist developing bulimia, binge-eating syndrome, or other adverse effects eating behaviors, as evaluated by a comprehensive interview-based psychological evaluation (i.e. Eating Disorder Examination) conducted at weeks 14, 28, and 52. Further, use of the device would seem to mitigate against binge-eating and hence bulimia. Thorough mastication is required for successful use of the device. If patients fails to chew thoroughly and eat slowly, larger food particles will clog the tube, preventing aspiration. Sullivan et al. (6), in an earlier study, found that participants with the AspireAssist developed greater cognitive dietary restraint (deliberate control of food intake), less disinhibition (loss of control of food intake), and less perceived hunger (awareness and susceptibility to hunger.) Another concern was that the therapy might give patients a license to overeat since they could later aspirate some of the contents of their meal. There was no evidence of this occurring either. In fact, the use of AspireAssist reinforces eating behaviors that are commonly recommended for weight management, including adherence to a structured meal plan, eating slowly and chewing food thoroughly, and drinking plenty of water with meals (1), because these behaviors facilitate successful postprandial aspiration.
This study has several limitations. First, although this was a randomized controlled trial, participants could not be blinded as to treatment group because of the nature of the therapy. However, all other aspects of the study protocol, such as weight management counseling and study visits, were the same in the in the AspireAssist and Lifestyle Counseling groups to minimize any additional potential influences on our outcome measures. Second, it is possible that bias was introduced into the study by the high number of pre-enrollment withdrawals (~14% in each treatment group) and post-enrollment withdrawals (26% in the AspireAssist group and 48% in the Lifestyle Counseling group), which is a common problem in weight loss intervention studies (25, 26). However, the baseline and demographic characteristics of the randomized, enrolled, and completer populations were analyzed for homogeneity and were not different in the AspireAssist and Lifestyle Counseling groups. The consistency of study results by using different statistical analyses further indicate that withdrawals did not bias the results. Third, this report includes only one-year results, and hence does not provide longer term safety and efficacy of the AspireAssist therapy. However, ~90% of the adverse events associated with AspireAssist are related to the A-tube, with ~50% occurring within the first week of implantation. The placement and management of the A-tube is similar to percutaneous endoscopic gastrostomy tubes, which have been used in clinical practice for more than 35 years, so the short-term and long-term complications of this device are already well known (27). Finally, our study population contained a high percentage of female participants, which is a common problem of weight loss studies (10, 11, 12, 13, 14, 25, 26). Therefore, our results might not necessarily apply to men with obesity.
In conclusion, the use of AspireAssist causes considerable weight loss and is more effective than intensive lifestyle modification alone in the treatment of obesity. The system is designed for the long-term treatment of obesity and necessitates regular monitoring, both aspects of which are important for treatment of a chronic disease. The placement procedure is the same as that used for percutaneous endoscopic gastrostomy tube placement and can be performed in an outpatient setting. It can also be removed if it is later decided to discontinue therapy, and does not cause anatomical changes that would preclude future bariatric surgery. The weight loss efficacy and safety profile of AspireAssist suggest this treatment approach may help bridge the therapeutic gap between more conservative lifestyle modification and the established bariatric surgical procedures for people with Class II and Class III obesity.
Study Highlights
Footnotes
Supplementary Material is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Christopher C. Thompson, MD, MSc, FASGE, FACG.
Specific author contributions: All authors were involved in the preparation of the manuscript, agreed to submit it for publication, and assumed responsibility for the accuracy and completeness of the data and the data analyses. The sponsor, Aspire Bariatrics (King of Prussia, PA, USA), performed the statistical analyses and provided editorial assistance in preparing this manuscript. Study supervision, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content: Christopher C. Thompson; study supervision and critical revision of the manuscript for important intellectual content: Louis J. Aronne; subject supervision: Barham K. Abu Dayyeh, Adam C. Stein, Michel Kahaleh, Marvin Ryou, Christopher Huang, Daniel D. Tran, Joseph P. Glaser, John A. Martin, Francis A. Farraye, Samuel B. Ho, and Nitin Kumar; study supervision: Robert Kushner, Shelby Sullivan, Alan B. Schorr, Anastassia Amaro, Caroline M. Apovian, Terrence Fullum, Amir Zarrinpar, Michael D. Jensen, Steven Edmundowicz, J. Matthew Bohning, Gregory Ginsberg, and David L. Jaffe; acquisition of data: Donna Harakal, Meredith Young, Catherine E. Thomas, Alpana P. Shukla, Michele B. Ryan, and Miki Haas; study concept and design, analysis and interpretation of data, assistance in preparing the manuscript: Heidi Goldsmith and Jennifer McCrea.
Financial support: This trial was sponsored in full by Aspire Bariatrics, Inc., King of Prussia, PA, USA. C.C.T., L.J.A., R.K., S.S., A.B.S., A.A., C.M.A., T.F., A.Z., M.D.J., S.E., J.M.B., G.G., and D.L.J. received institutional research support from Aspire Bariatrics for this clinical trial.
Potential competing interests: Heidi Goldsmith and Jennifer McCrea are employees of Aspire Bariatrics. The remaining authors declare no potential competing interests.
Supplementary Material
References
- Klein S, Wadden T, Sugerman HJ. American Gastroenterological Association Technical Review: clinical issues in obesity. Gastroenterology 2002;123:882–932. [DOI] [PubMed] [Google Scholar]
- Li W, Baraboi ED, Cluny NL et al. Malabsorption plays a major role in the effects of the biliopancreatic diversion with duodenal switch on energy metabolism in rats. Surg Obes Relat Dis 2015;11:356–66. [DOI] [PubMed] [Google Scholar]
- Xia Y, Kelton CM, Guo JJ et al. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity 2015;23:1721–8. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Oien D. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013;23:427–36. [DOI] [PubMed] [Google Scholar]
- Langeveld M, DeVries JH. The long-term effect of energy restricted diets for treating obesity. Obesity 2015;23:1529–38. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Stein R, Jonnalagadda S et al. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology 2013;145:1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Yanovski SZ, Marcus MD. The Questionnaire on Eating and Weight Patterns—Revised (QEWP-R). New York State Psychiatric Institute: New York. 1993. [Google Scholar]
- Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, (eds). Binge Eating: Nature, Assessment, and Treatment 12th edn Guilford: New York. 1993. pp. 317–60. [Google Scholar]
- Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R et al. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol 2014;20:7739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food & Drug Administration Summary of Safety and Effectiveness, LAP-BAND® Adjustable Gastric Banding (LAGB®) System, P00008. 2001.Accessible at: http://www.accessdata.fda.gov/cdrh_docs/pdf/P000008b.pdf.
- Food & Drug Administration Summary of Safety and Effectiveness, REALIZE™ Adjustable Gastric Band, P070009. 2007. Accessible at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM302775.pdf.
- Food & Drug Administration Summary of Safety and Effectiveness, MAESTRO® Rechargeable System, P130019. 2014. Accessible at: http://www.accessdata.fda.gov/cdrh_docs/pdf13/P130019b.pdf.
- Food & Drug Administration Summary of Safety and Effectiveness, Reshape™ Integrated Dual Balloon System, P140012. 2015. Accessible at http://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf.
- Food & Drug Administration Summary of Safety and Effectiveness, Orbera™ Intragastric Balloon System, P140008. 2015. Accessible at http://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf.
- Kolotkin RL, Crosby RD, Kosloski KD et al. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–111. [DOI] [PubMed] [Google Scholar]
- Chao D, Espeland MA, Farmer D et al. Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc 2000;48:753–9. [DOI] [PubMed] [Google Scholar]
- Abell TL, Camilleri M, Donohoe K et alAmerican Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 2008;103:753–63. [DOI] [PubMed] [Google Scholar]
- Sumithran P, Prendergast LA, Delbridge E et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–1604. [DOI] [PubMed] [Google Scholar]
- Kramer FM, Jeffery RW, Forster JL et al. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. Int J Obes 1989;13:123–36. [PubMed] [Google Scholar]
- National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The Evidence Report. Obes Res 1998;6 (Suppl 2): 51S–209S. [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- Wiggins TF, Garrow DA, DeLegge MH. Evaluation of percutaneous endoscopic feeding tube placement in obese patients. Nutr Clin Pract. 2009;24:723–7. [DOI] [PubMed] [Google Scholar]
- Bochicchio GV, Guzzo JL, Scalea TM. Percutaneous endoscopic gastrostomy in the supermorbidly obese patient. JSLS 2006;10:409–13. [PMC free article] [PubMed] [Google Scholar]
- Itkin M, DeLegge MH, Fang JC et alSociety of Interventional RadiologyAmerican Gastroenterological Association InstituteCanadian Interventional Radiological AssociationCardiovascular and Interventional Radiological Society of Europe. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology 2011;141:742–65. [DOI] [PubMed] [Google Scholar]
- Allison DB, Gadde KM, Garvey WT et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity 2011;20:330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Weissman NJ, Anderson CM et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010;363:245–56. [DOI] [PubMed] [Google Scholar]
- Nicholson FB, Korman MG, Richardson MA. Percutaneous endoscopic gastrostomy: a review of indications, complications and outcome. J Gastroenterol Hepatol 2000;15:21–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.