Abstract
Background
Breast cancer is currently the most common type of cancer in Japanese females. Unlike most other types of cancer, breast cancer develops more frequently in middle-aged females than in elderly females.
Methods
Of all Japanese female breast cancer patients aged ≥20 years whom the BioBank Japan Project originally enrolled between 2003 and 2008, 2034 were registered within 90 days after their diagnosis. We described the lifestyle and clinical characteristics of these patients at study entry. Furthermore, we examined the effect of these characteristics on all-cause mortality.
Results
In the female patients registered within 90 days after diagnosis, the frequency of stage 0 or unclassified, stage I, II, III and IV were 11.4%, 47.9%, 37.0%, 2.9% and 0.8%, respectively. The proportion of histological types was 12.9% for non-invasive carcinoma (ductal carcinoma and lobular carcinoma), 81.0% for invasive carcinoma (papillotubular carcinoma, solid tubular carcinoma, scirrhous carcinoma and special types), 0.2% for Paget's diseases and 5.8% for others. Those positive for the estrogen and progesterone receptors accounted for 75.8% and 62.1% of all patients, respectively. Among 1860 female participants registered within 90 days, 218 participants died during 144,54 person-years of follow-up. More advanced stage, elevation of serum carcinoembryonic antigen and carbohydrate antigen 15-3 levels and absence of the estrogen receptor at study entry were crudely associated with an increased risk of all-cause mortality after adjustment for age.
Conclusions
This study showed the association of several clinical characteristics with all-cause mortality in female breast cancer patients.
Keywords: Breast cancer, Stage, Histological type, Hormone receptor, Mortality
Highlights
-
•
About 1% of Japanese female breast cancer patients were diagnosed as stage IV.
-
•
Invasive carcinoma was much more common than non-invasive carcinoma.
-
•
Papillotubular carcinoma was the most common type of invasive carcinoma.
-
•
About 75% were positive for estrogen receptor and 60% for progesterone receptor.
-
•
Some characteristics would affect all-cause mortality in breast cancer patients.
Introduction
Breast cancer is a common type of cancer in females worldwide, although the incidence rate varies by region.1, 2 The incidence of breast cancer has increased globally,1 with a remarkable increase witnessed in Japan.3 One explanation for this increasing trend in Japan may be exposure to the Westernized diet, characterized largely by its high fat content, and the subsequent development of obesity.4, 5, 6 Another explanation may be an increase in habitual alcohol consumption among middle-aged females.4, 7 In Japan, breast cancer is currently the most common type of cancer in females.8 Unlike most other types of cancer, breast cancer develops more frequently in middle-aged females than in elderly females.8
Breast cancer is a determinant of mortality in females.1, 9 According to the latest Japanese National Vital Statistics, 13,240 annual deaths are due to breast cancer in females, and breast cancer is the fifth leading cause of cancer-related death in Japanese females.10 Cancer stage and other clinical factors at diagnosis predict prognosis in breast cancer patients.11 Recently, interest has increased in hormone receptors such as estrogen receptor (ER) and progesterone receptor (PgR) with regard to the therapeutic strategies and prognostic assessment of breast cancer.12
The BioBank Japan (BBJ) Project is a large patient-based biobank designed to implement personalized medicine for common diseases such as cancer and cardiovascular disease. Since the morbidity and mortality of breast cancer are much higher in females compared with males,8, 10 it is worthwhile to examine sex-specific clinical characteristics and mortality of breast cancer patients. In this study, we attempted to investigate lifestyle and clinical characteristics of Japanese breast cancer patients and their prognosis, using the BBJ Project database.
Participants and methods
Study design and population
Details of the study design and protocol of the BBJ Project were described elsewhere.13, 14 Briefly, the project enrolled patients with any of 47 common target diseases including breast cancer between June 2003 and March 2008, from 66 hospitals consisting of 12 cooperating medical institutions throughout Japan. This project collected clinical information and biological samples from participants annually until March 2013, regardless of whether the patients were newly diagnosed or treated cases. This project also followed up participants who had at least one of 32 diseases until 2014. The study protocol of the BBJ Project was approved by the research ethics committees of the Institute of Medical Science, the University of Tokyo, RIKEN Yokohama Institute and the 12 cooperating medical institutions. Written informed consent was obtained from all participants.
The BBJ Project originally enrolled 6336 breast cancer patients (46 males and 6290 females). In this report, we focused on the 6290 female participants; we excluded the 46 male participants because of the small number of participants. Of the 6290 female participants, 138 were excluded due to being aged less than 20 years having no data on smoking and alcohol drinking habits (n = 2) or missing data on the time from their diagnosis of breast cancer to study entry (n = 136). Accordingly, we described the overview of lifestyle factors and clinical profiles of the remaining 6152 female breast cancer patients. To describe the characteristics of possible newly diagnosed breast cancer patients, we selected 2034 female participants who were registered in the BBJ Project within 90 days after their diagnosis of breast cancer. Of these 2034 participants, 174 were excluded due to their refusal to participate in the follow-up survey (n = 173) or loss of follow-up (n = 1). Thus, 1860 female participants were included in the survival analyses to examine the prognosis and its risk factors in breast cancer patients.
Data collection
Clinical data were collected at study entry via interviews and medical records.15 The data included age at study entry, time from the diagnosis of breast cancer to study entry, height, weight, smoking habits, alcohol intake, past history, family history and clinical information at study entry including laboratory examination data (e.g., blood chemical markers and imaging data). Body mass index was calculated as weight in kilograms divided by height in meters squared. The blood chemical marker used in this report were serum carcinoembryonic antigen (CEA) level, carbohydrate antigen 15-3 (CA15-3) level and ER and PgR statuses. The stage of breast cancer was classified according to the Japanese Classification of Breast Cancer, the 15th edition (2004). In this report, the histological type of breast cancer was determined primarily based on the findings in surgically resected tissues, while missing histological data were complemented with the findings from biopsy or cytological samples.
Follow-up survey
Follow-up survival survey was conducted until 2014 to determine whether the status of each participant was alive, relocated, unidentified, or dead by confirming their residence cards. The new addresses of participants who relocated were also recorded in the next survival survey. The date of death was also recorded for deceased participants. The outcome assessed in this study was death due to all causes. Details of the follow-up survey were described elsewhere.14, 16
Statistical analysis
We performed a descriptive analysis of lifestyle and clinical characteristics of female breast cancer patients. The characteristics of interest were age at study entry, body mass index, smoking and drinking habits, comorbidities of mastopathy, family history of breast cancer, stage, histological type, serum CEA and CA15-3 levels and ER and PgR statuses. The frequency of each characteristic was calculated in female study participants after excluding those with missing data. This analysis was performed in all participants and participants who were registered in the BBJ Project within 90 days after their diagnosis of breast cancer. The same analysis was conducted after stratifying the participants by age at study entry (20–59 versus ≥60 years old) or invasive status. The non-invasive type group included ductal carcinoma and lobular carcinoma, while invasive type group included papillotubular carcinoma, solid tubular carcinoma, scirrhous carcinoma and special types.
We evaluated the prognosis of breast cancer in female participants registered within 90 days after their diagnosis. We calculated the 5-year relative survival rate by dividing the 5-year cumulative survival rate by the sex- and age-adjusted expected survival rate in our eligible female breast cancer patients. The 5-year cumulative survival rate was calculated, using the Kaplan–Meier method. The expected survival rate was calculated, using a survival-rate table of a reference Japanese cohort from the Cancer Registry and Statistics, Cancer Information Service, National Cancer Center, Japan,17 which was based on sex- and age-specific mortality rates and Gompertz–Makeham's law in abridged life tables, published annually by the Statistics and Information Department of the Ministry of Health, Labour and Welfare, Japan.18
We examined the impact of lifestyle and clinical characteristics on all-cause mortality in breast cancer patients registered within 90 days after their diagnosis. We used a Cox proportional hazards model to estimate the hazard ratios and 95% confidence intervals (CIs) for mortality for each characteristic category, with one category set as the reference. The model was stratified by institution to account for variability in baseline hazards among the institutions. The model incorporated age at study entry (years as a continuous variable) and the entry year (2003, 2004, 2005, 2006, 2007 and 2008, setting year 2003 as the reference) in addition to each characteristic. We examined the association between BMI and mortality using a continuous variable, as well as the categorical variable. In this analysis, we excluded the participants with missing data or the group of small number of participants.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All p values were two-tailed, and the significance level was set at p < 0.05.
Results
Characteristics of all participants with breast cancer
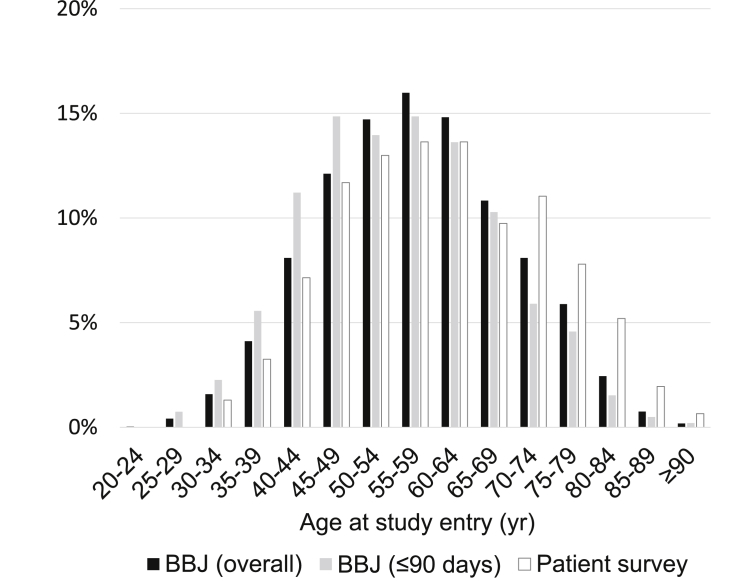
Among 6152 female breast cancer patients aged ≥20 years at the enrollment, the mean (standard deviation) age at study entry was 57.6 (11.9) years. The distribution of the participants according to age at study entry is illustrated in Fig. 1; age distribution was normal, with 55–59 years representing the peak age. The distribution of the participants according to the time from the diagnosis to study entry was as follows: 33.1% for ≤90 days, 19.9% for 91–365 days, 28.0% for 1–4 years, 12.6% for 5–9 years, 4.3% for 10–14 years, 1.3% for 15–19 years, 0.9% for ≥20 years. Table 1 summarizes other characteristics of the female breast cancer patients. Over 10% of female participants had a family history of breast cancer. Among the participants with stage information, approximately 40% were classified as stage I at study entry, while 2% were classified as stage IV. Invasive carcinoma was much more common than the non-invasive type. Of the invasive types, papillotubular carcinoma was the most common histological type followed by scirrhous carcinoma, solid tubular carcinoma and special types. Less than 10% had a serum CEA level of 5 ng/mL or higher, while approximately 5% had a serum CA15-3 level of 27 U/mL or higher. Approximately 70% were positive for ER and approximately 60% for PgR.
Fig. 1.
Distribution of the female breast cancer patients according to age at study entry. Bars colored in black, gray and white represent patients in the BioBank Japan (BBJ) Project: ( ) the overall participants and (
) the overall participants and ( ) those registered within 90 days after their diagnosis and (
) those registered within 90 days after their diagnosis and ( ) patients in the Patient Survey in Japan, 2005,19 respectively.
) patients in the Patient Survey in Japan, 2005,19 respectively.
Table 1.
Characteristics of the 6152 female breast cancer patients in the Biobank Japan Project. Data are also presented for the 2034 female breast cancer patients who were registered within 90 days after diagnosis and for patients grouped according to age at entry.
| Overall participants |
Those registered within 90 days after diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 6152) | Total (n = 2034) | Age at study entry |
||||||
| 20–59 yr (n = 1290) |
≥60 yr (n = 744) |
|||||||
| n | % | n | % | n | % | n | % | |
| Body mass index | ||||||||
| <18.5 kg/m2 | 535 | 9.0 | 189 | 9.4 | 145 | 11.4 | 44 | 6.0 |
| 18.5– 24.9 kg/m2 | 4071 | 68.4 | 1387 | 69.2 | 926 | 72.7 | 461 | 63.2 |
| ≥25.0 kg/m2 | 1347 | 22.6 | 427 | 21.3 | 203 | 15.9 | 224 | 30.7 |
| Unknown | 199 | 31 | 16 | 15 | ||||
| Smoking habit | ||||||||
| Never | 4759 | 78.3 | 1546 | 76.2 | 910 | 70.8 | 636 | 85.7 |
| Ever (current/former) | 1316 | 21.7 | 482 | 23.8 | 376 | 29.2 | 106 | 14.3 |
| Unknown | 77 | 6 | 4 | 2 | ||||
| Alcohol drinking habit | ||||||||
| Never | 3855 | 63.6 | 1176 | 58.1 | 640 | 49.8 | 536 | 72.3 |
| Ever (current/former) | 2207 | 36.4 | 849 | 41.9 | 644 | 50.2 | 205 | 27.7 |
| Unknown | 90 | 9 | 6 | 3 | ||||
| History of mastopathy | ||||||||
| Absence | 6083 | 98.9 | 2009 | 98.8 | 1272 | 98.6 | 737 | 99.1 |
| Presence | 69 | 1.1 | 25 | 1.2 | 18 | 1.4 | 7 | 0.9 |
| Family history of breast cancer | ||||||||
| Absence | 5459 | 88.7 | 1789 | 88.0 | 1142 | 88.5 | 647 | 87.0 |
| Presence | 693 | 11.3 | 245 | 12.0 | 148 | 11.5 | 97 | 13.0 |
| Stage | ||||||||
| 0 | 200 | 6.8 | 144 | 10.9 | 110 | 12.5 | 34 | 7.8 |
| Unclassified | 12 | 0.4 | 7 | 0.5 | 7 | 0.8 | 0 | 0.0 |
| I | 1188 | 40.2 | 633 | 47.9 | 434 | 49.2 | 199 | 45.4 |
| IIA | 911 | 30.8 | 413 | 31.3 | 258 | 29.2 | 155 | 35.4 |
| IIB | 382 | 12.9 | 76 | 5.8 | 43 | 4.9 | 33 | 7.5 |
| IIIA | 94 | 3.2 | 19 | 1.4 | 15 | 1.7 | 4 | 0.9 |
| IIIB | 86 | 2.9 | 15 | 1.1 | 6 | 0.7 | 9 | 2.1 |
| IIIC | 25 | 0.8 | 4 | 0.3 | 3 | 0.3 | 1 | 0.2 |
| IV | 59 | 2.0 | 10 | 0.8 | 7 | 0.8 | 3 | 0.7 |
| Unknown | 3195 | 713 | 407 | 306 | ||||
| Histological type | ||||||||
| Non-invasive | ||||||||
| Ductal carcinoma | 430 | 7.9 | 236 | 12.4 | 177 | 14.4 | 59 | 8.7 |
| Lobular carcinoma | 34 | 0.6 | 10 | 0.5 | 6 | 0.5 | 4 | 0.6 |
| Invasive | ||||||||
| Papillotubular carcinoma | 2081 | 38.2 | 596 | 31.3 | 382 | 31.1 | 214 | 31.7 |
| Solid tubular carcinoma | 798 | 14.7 | 262 | 13.8 | 160 | 13.0 | 102 | 15.1 |
| Scirrhous carcinoma | 1466 | 26.9 | 556 | 29.2 | 358 | 29.1 | 198 | 29.3 |
| Special type | 327 | 6.0 | 130 | 6.8 | 72 | 5.9 | 58 | 8.6 |
| Paget's disease | 12 | 0.2 | 4 | 0.2 | 1 | 0.1 | 3 | 0.4 |
| Others | 295 | 5.4 | 111 | 5.8 | 74 | 6.0 | 37 | 5.5 |
| Unknown | 709 | 129 | 60 | 69 | ||||
| Serum CEA level | ||||||||
| <5 ng/mL | 5437 | 92.9 | 1868 | 95.0 | 1198 | 96.1 | 670 | 93.1 |
| ≥5 ng/mL | 417 | 7.1 | 98 | 5.0 | 48 | 3.9 | 50 | 6.9 |
| Unknown | 298 | 68 | 44 | 24 | ||||
| Serum CA15-3 level | ||||||||
| <27 U/mL | 5436 | 95.1 | 1897 | 97.2 | 1208 | 97.7 | 689 | 96.2 |
| ≥27 U/mL | 283 | 4.9 | 55 | 2.8 | 28 | 2.3 | 27 | 3.8 |
| Unknown | 433 | 82 | 54 | 28 | ||||
| Estrogen receptor status | ||||||||
| Positive | 3110 | 71.7 | 1253 | 75.8 | 823 | 77.5 | 430 | 72.6 |
| Negative | 1226 | 28.3 | 401 | 24.2 | 239 | 22.5 | 162 | 27.4 |
| Unknown | 1816 | 380 | 228 | 152 | ||||
| Progesterone receptor status | ||||||||
| Positive | 2568 | 60.3 | 1021 | 62.1 | 704 | 66.6 | 317 | 54.1 |
| Negative | 1692 | 39.7 | 622 | 37.9 | 353 | 33.4 | 269 | 45.9 |
| Unknown | 1892 | 391 | 233 | 158 | ||||
Abbreviations: CA15-3, carbohydrate antigen 15-3; CEA, carcinoembryonic antigen.
Characteristics of participants registered within 90 days after diagnosis
Of all female breast cancer patients aged ≥20 years, 2034 female participants were registered within 90 days after the diagnosis of breast cancer. The mean age (standard deviation) at study entry was 55.3 (12.0) years. Age distribution of these participants was shifted to younger as compared to the overall participants (Fig. 1). The frequency of ever drinker was higher in those registered within 90 days compared with the overall participants (Table 1). The frequency of stage 0 or I was higher in those registered within 90 days compared with the overall participants, with the lower frequency of stage II or III. As compared with the overall participants, the proportion of ductal carcinoma was higher in those registered within 90 days, while the proportion of papillotubular carcinoma was lower in those registered within 90 days. Other characteristics were similar for those registered within 90 days and the overall participants.
When these female participants were stratified by age at study entry, most of the clinical characteristics were similar for younger (aged 59 years or less) and older (aged 60 years or more) groups. The frequency of obesity was higher in the older age group compared with the younger age group, while the frequencies of ever smoker, alcohol drinker and PgR positivity were lower in the older, than younger, age group (Table 1).
When these female participants were stratified by invasive status, most of the clinical characteristics were similar for the non-invasive and invasive types (Table 2). Non-invasive carcinoma was more prevalent at a younger age, compared with the invasive type. Over half of the non-invasive cases were classified as stage 0, while approximately 90% of the invasive cases were classified as stage I or II. Those with invasive carcinoma had a higher frequency of obesity and a lower frequency of PgR positivity, compared with the non-invasive cases.
Table 2.
Characteristics of female breast cancer patients registered in the Biobank Japan Project within 90 days after diagnosis grouped according to invasive status.
| Invasive status |
||||
|---|---|---|---|---|
| Non-invasive type (n = 246) |
Invasive type (n = 1544) |
|||
| n | % | n | % | |
| Age at study entry | ||||
| 20–29 yr | 1 | 0.4 | 9 | 0.6 |
| 30–39 yr | 29 | 11.8 | 111 | 7.2 |
| 40–49 yr | 80 | 32.5 | 399 | 25.8 |
| 50–59 yr | 73 | 29.7 | 453 | 29.3 |
| 60–69 yr | 51 | 20.7 | 367 | 23.8 |
| 70–79 yr | 11 | 4.5 | 170 | 11.0 |
| 80–89 yr | 1 | 0.4 | 31 | 2.0 |
| ≥90 yr | 0 | 0.0 | 4 | 0.3 |
| Body mass index | ||||
| <18.5 kg/m2 | 31 | 12.7 | 134 | 8.8 |
| 18.5–24.9 kg/m2 | 175 | 71.7 | 1046 | 68.9 |
| ≥25.0 kg/m2 | 38 | 15.6 | 339 | 22.3 |
| Unknown | 2 | 25 | ||
| Smoking habit | ||||
| Never | 192 | 78.0 | 1174 | 76.3 |
| Ever (current/former) | 54 | 22.0 | 364 | 23.7 |
| Unknown | 0 | 6 | ||
| Alcohol drinking habit | ||||
| Never | 142 | 57.7 | 891 | 58.0 |
| Ever (current/former) | 104 | 42.3 | 644 | 42.0 |
| Unknown | 0 | 9 | ||
| History of mastopathy | ||||
| Absence | 243 | 98.8 | 1526 | 98.8 |
| Presence | 3 | 1.2 | 18 | 1.2 |
| Family history of breast cancer | ||||
| Absence | 219 | 89.0 | 1353 | 87.6 |
| Presence | 27 | 11.0 | 191 | 12.4 |
| Stage | ||||
| 0 | 99 | 51.3 | 37 | 3.6 |
| Unclassified | 4 | 2.1 | 3 | 0.3 |
| I | 57 | 29.5 | 532 | 51.7 |
| IIA | 31 | 16.1 | 348 | 33.8 |
| IIB | 1 | 0.5 | 69 | 6.7 |
| IIIA | 1 | 0.5 | 15 | 1.5 |
| IIIB | 0 | 0.0 | 15 | 1.5 |
| IIIC | 0 | 0.0 | 4 | 0.4 |
| IV | 0 | 0.0 | 7 | 0.7 |
| Unknown | 53 | 514 | ||
| Serum CEA level | ||||
| <5 ng/mL | 233 | 96.3 | 1424 | 95.3 |
| ≥5 ng/mL | 9 | 3.7 | 70 | 4.7 |
| Unknown | 4 | 50 | ||
| Serum CA15-3 level | ||||
| <27 U/mL | 236 | 98.7 | 1449 | 97.3 |
| ≥27 U/mL | 3 | 1.3 | 40 | 2.7 |
| Unknown | 7 | 55 | ||
| Estrogen receptor status | ||||
| Positive | 154 | 74.4 | 1032 | 76.3 |
| Negative | 53 | 25.6 | 321 | 23.7 |
| Unknown | 39 | 191 | ||
| Progesterone receptor status | ||||
| Positive | 138 | 67.0 | 826 | 61.5 |
| Negative | 68 | 33.0 | 518 | 38.5 |
| Unknown | 40 | 200 | ||
Abbreviations: CA15-3, carbohydrate antigen 15-3; CEA, carcinoembryonic antigen.
Prognosis of participants registered within 90 days after diagnosis
Among the 1860 female participants involved in the survival survey, the mean (standard deviation) follow-up period was 7.8 (2.0) years in a total of 14,454 person-years of follow-up. During the follow-up period, 218 deaths were identified in the total period and 120 were identified during the 5 years of follow-up. Consequently, the 5-year cumulative survival rate was 93.5% (95% CI, 92.4–94.6). The 5-year relative survival rate was 96.2% (95% CI, 95.0–97.3).
Table 3 shows which clinical characteristics will affect all-cause mortality in female breast cancer patients. The risk of all-cause mortality increased with increasing age at study entry. In addition, more advanced stage, elevation of serum CEA and CA15-3 levels and absence of ER at study entry were crudely associated with an increased risk of all-cause mortality after adjustment for age at study entry and entry year (Table 3).
Table 3.
Age-adjusted hazard ratios for all-cause mortality in female breast cancer patients registered in the Biobank Japan Project within 90 days after diagnosis grouped according to characteristics, after a mean follow-up period of 7.8 years.
| Those registered within 90 days after diagnosis |
|||||
|---|---|---|---|---|---|
| Participants | Cases | PYFU | Age-adjusted HR (95% CI) | ||
| Age at study entry | |||||
| Additional 1 yr increase | 1.04 | (1.03–1.05) | |||
| Body mass index | |||||
| <18.5 kg/m2 | 174 | 17 | 1323 | 1.03 | (0.56–1.88) |
| 18.5–24.9 kg/m2 | 1271 | 141 | 9932 | 1.00 | Reference |
| ≥25.0 kg/m2 | 386 | 48 | 3006 | 0.79 | (0.53–1.18) |
| Additional 1 kg/m2 increase | 0.98 | (0.94–1.02) | |||
| Smoking habit | |||||
| Never | 1416 | 172 | 10981 | 1.00 | Reference |
| Ever (current/former) | 439 | 46 | 3425 | 0.97 | (0.64–1.46) |
| Alcohol drinking habit | |||||
| Never | 1075 | 130 | 8248 | 1.00 | Reference |
| Ever (current/former) | 777 | 85 | 6146 | 1.06 | (0.75–1.52) |
| Stage | |||||
| 0 or unclassified | 138 | 3 | 1051 | 0.65 | (0.08–5.28) |
| I | 582 | 38 | 4622 | 1.00 | Reference |
| II | 444 | 50 | 3528 | 1.28 | (0.64–2.56) |
| III | 33 | 8 | 237 | 16.06 | (3.13–82.49) |
| IV | 10 | 6 | 39 | 45.83 | (10.83–193.89) |
| Invasive status | |||||
| Non-invasive | 226 | 10 | 1785 | 1.00 | Reference |
| Invasive | 1414 | 182 | 11036 | 2.09 | (0.91–4.77) |
| Serum CEA level | |||||
| <5 ng/mL | 1726 | 173 | 13527 | 1.00 | Reference |
| ≥5 ng/mL | 90 | 35 | 570 | 3.92 | (2.56–6.00) |
| Serum CA15-3 level | |||||
| <27 U/mL | 1753 | 181 | 13753 | 1.00 | Reference |
| ≥27 U/mL | 51 | 27 | 243 | 5.16 | (3.28–8.13) |
| Estrogen receptor status | |||||
| Positive | 1149 | 104 | 9110 | 1.00 | Reference |
| Negative | 369 | 55 | 2795 | 1.55 | (1.01–2.39) |
| Progesterone receptor status | |||||
| Positive | 934 | 80 | 7399 | 1.00 | Reference |
| Negative | 576 | 77 | 4437 | 1.31 | (0.86–1.98) |
Abbreviations: CA15-3, carbohydrate antigen 15-3; CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; PYFU, person-years of follow-up. The hazard ratios were calculated using a Cox proportional hazards regression model stratified by institution and adjusted for age at study entry and entry year.
Discussion
In this report, we described the lifestyle and clinical characteristics of Japanese female breast cancer patients involved in the BBJ Project. As compared to the results of female breast cancer patients in the Patient Survey in Japan, 2005 (Fig. 1),19 our registry contained a greater proportion of younger age distribution. Contrary to expectation, those registered within 90 days had a higher frequency of stage 0 and I and a lower frequency of stage II and III, compared with the overall participants. These unreasonable results may have been due to missing data on stage and/or a possible selection bias. ER- and PgR-negative cases accounted for approximately 25% and 40% of those registered within 90 days after diagnosis, respectively, and were crudely associated with poorer prognosis.
The Japanese Breast Cancer Society (JBCS) Registry reported that the frequencies of stage 0, I, II, III and IV were 9.5%, 38.2%, 41.0%, 8.5% and 2.9%, respectively.11 In addition, the JBCS Registry reported that the proportions of ER and PgR positivity were 75.7% and 62.3% among those registered in 2005, respectively.11 Furthermore, with respect to histological type of breast cancer, the JBCS Registry reported proportions of 82.9%, 8.2% and 8.9% for invasive carcinoma, non-invasive ductal carcinoma and remaining types (including non-invasive lobular carcinoma and special and other types), respectively, among breast cancer patients registered in 2004.20 Our results were in accordance with the results of this relevant registry.
The Japanese Association of Clinical Cancer Centers registered 21,322 female breast cancer patients between 2004 and 2007, and reported that the frequency of each cancer stage was 42.2% for stage I, 42.9% for stage II, 10.0% for stage III, 4.1% for stage IV and 0.8% for others or unknown.21 Our stage frequencies are comparable to those of that registry. The Japanese Association of Clinical Cancer Centers reported a 5-year relative survival rate of 92.9%.21 As expected from the similarities in stage frequency between the corresponding registry and our registry, our 5-year relative survival rate of female breast cancer patients was similar.
The JBCS Registry further demonstrated that more advanced stage was associated with poorer prognosis in breast cancer patients after a median follow-up period of 60.0 months.11 A negative ER status tended to be associated with poorer prognosis, compared with a positive ER status, although prognosis was compared with respect to both ER and human epidermal growth factor receptor 2 statuses.11 PgR negativity also tended to be associated with poorer prognosis, compared with PgR positivity, although this comparison was conducted only in ER-positive cases.11 The effectiveness of endocrine therapy may explain better prognosis in ER- or PgR-positive cases compared with the respective negative cases.22 Our results were somewhat consistent with the results of that survival survey, although the association was not significant between PgR status and mortality in our patients. Along with patient characteristics evaluated in the JCBS Registry, our survival analysis also identified similar predictive factors for all-cause mortality in female breast cancer patients. In addition to cancer stage and hormone receptor status, we observed that elevation of serum CEA and CA15-3 levels was also crudely associated with poorer prognosis after adjustment for age and study entry year.
The strength of the present study was the enrollment of female breast cancer patients from many participating hospitals nationwide. On the other hand, the present study has several limitations. First, the survival analysis was conducted among cases registered within 90 days after their diagnosis, not definite incident cases. There might have been a bias resulting from selecting survival patients in our prognostic assessment. Therefore, the true prognosis of breast cancer may be worse than we observed. Second, we identified factors predicting all-cause mortality in breast cancer patients after adjustment only for age and entry year. These predictive factors may be interrelated, while other factors such as performance status and treatment may confound the associations we observed. Therefore, caution should be taken in interpreting our results. Finally, there were missing data on some variables in the BBJ Project database. The main reason for missing data was that the data were collected mostly from medical records in which limited clinical information was available. Another possible reason for missing data on cancer stage was that the data were collected through medical records regarding each component of the TNM classification (included in the Japanese Classification of Breast Cancer, the 15th edition (2004)), but not stage. Furthermore, some cancer patients admitted participating hospitals for only therapeutic procedures or follow-up after being diagnosed at other hospitals. The lack of data on stage may explain the unreasonable results regarding the frequency of stage among the overall participants and those registered within 90 days after diagnosis. However, it is unclear whether these reasons for missing data had a crucial effect on our results.
In conclusion, we evaluated the characteristics of female breast cancer patients in detail, and found that they are in excellent agreement with other nationwide registries of breast cancer patients. In the survival analysis, we found that some characteristics at study entry would be crudely associated with all-cause mortality in Japanese female breast cancer patients. Due to the potentially high generalizability of the breast cancer patients in the BBJ Project, this project can be expected to provide reliable and valuable evidence on breast cancer.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
We express our gratitude to all the participants in the BioBank Japan Project. We thank all the medical coordinators of the cooperating hospitals for collecting samples and clinical information, as well as Yasushi Yamashita and staff members of the BioBank Japan Project for administrative support. We also thank Dr. Kumao Toyoshima for his overall supervision of the BioBank Japan project. This study was supported by funding from the Tailor-Made Medical Treatment with the BBJ Project from Japan Agency for Medical Research and development, AMED (since April 2015), and the Ministry of Education, Culture, Sports, Science, and Technology (from April 2003 to March 2015).
Footnotes
Peer review under the responsibility of The Japan Epidemiological Association
Contributor Information
Akiko Tamakoshi, Email: tamaa@med.hokudai.ac.jp.
BioBank Japan Cooperative Hospital Group:
Rai Shimoyama, Koichi Maekawa, Kiyoshi Kaneko, Hiromasa Harada, Shiro Minami, Hiroyuki Takei, Mitsue Saito, Yasuhisa Terao, Satoru Takeda, Satoshi Asai, Mitsuhiko Moriyama, Yasuo Takahashi, Tomoaki Fujioka, Wataru Obara, Seijiro Mori, Hideki Ito, Satoshi Nagayama, Yoshio Miki, Akihide Masumoto, Akira Yamada, Yasuko Nishizawa, Ken Kodama, Hajime Abe, Tomoharu Shimizu, Yukihiro Koretsune, Norikazu Masuda, and Yasutaka Takeda
Appendix.
Author list for BioBank Japan Cooperative Hospital Group
Members of medical institutions cooperating on the BioBank Japan Project who coauthored this paper include Rai Shimoyama, Koichi Maekawa, Kiyoshi Kaneko and Hiromasa Harada (Tokushukai Hospitals); Shiro Minami and Hiroyuki Takei (Nippon Medical School); Mitsue Saito, Yasuhisa Terao and Satoru Takeda (Juntendo University); Satoshi Asai, Mitsuhiko Moriyama and Yasuo Takahashi (Nihon University); Tomoaki Fujioka and Wataru Obara (Iwate Medical University); Seijiro Mori and Hideki Ito (Tokyo Metropolitan Institute of Gerontology); Satoshi Nagayama and Yoshio Miki (The Cancer Institute Hospital of JFCR); Akihide Masumoto and Akira Yamada (Aso Iizuka Hospital); Yasuko Nishizawa and Ken Kodama (Osaka Medical Center for Cancer and Cardiovascular Diseases); Hajime Abe and Tomoharu Shimizu (Shiga University of Medical Science); Yukihiro Koretsune and Norikazu Masuda (National Hospital Organization, Osaka National Hospital); and Yasutaka Takeda (Fukujuji Hospital).
References
- 1.Global Burden of Disease Cancer Collaboration The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Katanoda K., Hori M., Matsuda T. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol. 2015;45:390–401. doi: 10.1093/jjco/hyv002. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health, Labour and Welfare, Japan . 2014. National Health and Nutrition Survey in Japan.http://www.e-stat.go.jp/SG1/estat/GL08020103.do?_toGL08020103_&listID=000001151595&disp=Other&requestSender=dsearch Accessed 25 July 2016 [in Japanese] [Google Scholar]
- 5.Iwasaki M., Otani T., Inoue M., Sasazuki S., Tsugane S., Japan Public Health Center-Based Prospective Study Group Body size and risk for breast cancer in relation to estrogen and progesterone receptor status in Japan. Ann Epidemiol. 2007;17:304–312. doi: 10.1016/j.annepidem.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki R., Iwasaki M., Inoue M., Japan Public Health Center-based Prospective Study Group Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status–the Japan Public Health Center-Based Prospective Study. Int J Cancer. 2011;129:1214–1224. doi: 10.1002/ijc.25744. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R., Iwasaki M., Inoue M., Japan Public Health Center-based Prospective Study Group Alcohol consumption-associated breast cancer incidence and potential effect modifiers: the Japan Public Health Center-based Prospective Study. Int J Cancer. 2010;127:685–695. doi: 10.1002/ijc.25079. [DOI] [PubMed] [Google Scholar]
- 8.Hori M., Matsuda T., Shibata A., Katanoda K., Sobue T., Nishimoto H., Japan Cancer Surveillance Research Group Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 9.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Health, Labour, and Welfare, Japan. National Vital Statistics in 2014. http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei14/index.html. Accessed 25 July 2016 [in Japanese].
- 11.Anan K., Fukui N., Kinoshita T. Comprehensive prognostic report of the Japanese Breast Cancer Society Registry in 2005. Breast Cancer. 2016;23:50–61. doi: 10.1007/s12282-015-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horii R., Honma N., Ogiya A. The Japanese Breast Cancer Society clinical practice guidelines for pathological diagnosis of breast cancer, 2015 edition. Breast Cancer. 2016;23:391–399. doi: 10.1007/s12282-016-0675-6. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y. The BioBank Japan project. Clin Adv Hematol Oncol. 2007;5:696–697. [PubMed] [Google Scholar]
- 14.Nagai A., Hirata M., Kamatani Y. Overview of the BioBank Japan Project: study design and profile. J Epidemiol. 2017;27:S2–S8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirata M., Kamatani Y., Nagai A. Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol. 2017;27:S9–S21. doi: 10.1016/j.je.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata M., Nagai A., Kamatani Y. Overview of BioBank Japan follow-up data in 32 diseases. J Epidemiol. 2017;27:S22–S28. doi: 10.1016/j.je.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Registry and Statistics, Cancer Information Service, National Cancer Center, Japan. Cohort Life Table. http://ganjoho.jp/reg_stat/statistics/qa_words/cohort01.html. Accessed 25 July 2016 [in Japanese].
- 18.Ministry of Health, Labour and Welfare, Japan. Abridged Life Tables for Japan. http://www.mhlw.go.jp/toukei/saikin/hw/seimei/list54-57-02.html. Accessed 25 July 2016 [in Japanese].
- 19.Ministry of Health, Labour and Welfare, Japan. Patient Survey in 2005. http://www.e-stat.go.jp/SG1/estat/List.do?lid=000001047095. Accessed 25 July 2016 [in Japanese].
- 20.Kurebayashi J., Miyoshi Y., Ishikawa T. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: based on the breast cancer registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer. 2015;22:235–244. doi: 10.1007/s12282-015-0599-6. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Association of Clinical Cancer Centers. Five-Year Relative Survival Rate in All Cases in 2004–2007. http://www.gunma-cc.jp/sarukihan/seizonritu/seizonritu2007.html#10. Accessed 25 July 2016 [in Japanese].
- 22.Barnes D.M., Hanby A.M. Oestrogen and progesterone receptors in breast cancer: past, present and future. Histopathology. 2001;38:271–274. doi: 10.1046/j.1365-2559.2001.01060.x. [DOI] [PubMed] [Google Scholar]