Abstract
Background
Evidence of characteristics of Japanese patients with diabetes from a large-scale population is necessary. Few studies have compared glycaemic controls, complications and comorbidities between type 1 and 2 diabetic patients. This paper focuses on illustrating a clinical picture of Japanese diabetic patients and comparing glycaemic control and prognoses between type 1 and 2 diabetes using multi-institutional data.
Methods
The BioBank Japan Project enrolled adult type 1 and 2 diabetic patients between fiscal years 2003 and 2007. We have presented characteristics, controls of serum glucose, cholesterol and blood pressure, prevalence of complications and comorbidities and survival curves. We have also shown glycaemic controls according to various individual profiles of diabetic patients.
Results
A total of 558 type 1 diabetic patients and 30,834 type 2 diabetic patients participated in this study. The mean glycated haemoglobin A1c was higher in type 1 diabetes than in type 2 diabetes. In the type 1 diabetic patients, the glycated haemoglobin A1c had no consistent trend according to age and body mass index. The Kaplan–Meier estimates represented a longer survival time from baseline with type 1 diabetes than with type 2 diabetes. Compared with type 1 diabetic patients, type 2 diabetic patients had double the prevalence of macrovascular complications.
Conclusions
This work has revealed detailed plasma glucose levels of type 1 and 2 diabetic patients according to age, body mass index, blood pressure, serum cholesterol levels and smoking and drinking habits. Our data have also shown that the prognosis is worse for type 2 diabetes than for type 1 diabetes in Japan.
Keywords: Diabetes, Glycated haemoglobin A1c, Comorbidity, Smoking, Kaplan–Meier estimate
Highlights
-
•
Detailed glycaemic control data of diabetic patients are necessary.
-
•
Few studies have compared the survival times between type 1 and 2 diabetes.
-
•
Higher glycated HbA1c was observed in type 1 than in type 2 diabetes.
-
•
Data showed higher hazard ratio of mortality in type 1 than in type 2 diabetes.
-
•
Fewer macrovascular complications accompany type 1 than type 2 diabetes.
Introduction
The Japan Diabetes Society sets diabetes-treatment targets for controlling serum glucose, cholesterol, blood pressure and body mass index (BMI) in accordance with evidence.1 The guideline recommends glycated haemoglobin A1c (HbA1c) < 6.0% (42 mmol/mol) for Japanese diabetic patients to normalise serum glucose and HbA1c < 7.0% (53 mmol/mol) to prevent complications. The targets of treatment strategy include a BMI of 22 kg/m2, systolic blood pressure (SBP) of <130 mm Hg and diastolic blood pressure (DBP) of <80 mm Hg. The targeted low-density lipoprotein cholesterol (LDL-C) levels are <120 mg/dL (3.10 mmol/L) for patients without coronary artery disease and <100 mg/dL (2.59 mmol/L) for those with coronary artery disease. The targeted triglyceride level is < 150 mg/dL (1.69 mmol/L), and the targeted high-density lipoprotein cholesterol (HDL-C) level is ≥40 mg/dL (1.03 mmol/L). However, few have published a detailed picture of glycaemic control and life-style related parameters on Japanese diabetic patients.
Also, few studies have compared characteristics and mortality between type 1 and 2 diabetic patients. An Australian study has compared characteristics, glycaemic control and prevalence of complications between type 1 and 2 diabetic patients.2 In 2013, the Japan Diabetes Clinical Data Management Study Group reported mean age, BMI and HbA1c on the data of type 1 and 2 diabetic patients from many institutions.3 However, this report lacked information on complications, comorbidities and glycaemic control at various profiles for age, BMI, blood pressure, serum cholesterol level and smoking and drinking habits. Although recent data have been published about detailed profiles of Japanese type 1 diabetic patients, the data were limited to those in paediatric practice.4 Additionally, because other reports of Japanese patients were from residents in a single region, broadening applicability to real-world practice may be difficult.
This work was motivated to illustrate age, BMI, controls of serum glucose, cholesterol and blood pressure, and complications and comorbidities of Japanese type 1 and 2 diabetic patients, using large multi-regional data. In this paper, we also compared the characteristics and the survival curves between type 1 and 2 diabetic patients and aimed to understand the glycaemic control among Japanese type 1 and 2 diabetic patients in a hospital setting.
Methods
Participants
Participating diabetic patients were those registered in the BioBank Japan Project between the fiscal years of 2003 and 2007 from 66 hospitals.5 The study profiles were presented in detail elsewhere.6, 7, 8 Diabetes was diagnosed by physicians based on the criteria of The Japan Diabetes Society.9 A total of 39,697 patients were invited to the project and medically followed up. At the time of registration, we collected serum samples and medical record information. The participants majorly comprised adults aged ≥19 years. In the present study, we analysed the data of those with type 1 and 2 diabetes; and we eliminated the data of those with diabetes and glucose metabolism disorders due to other specific mechanisms and diseases (other endocrinological or metabolic diseases, systemic infectious diseases, cardiac diseases for the steroid use and amyotrophic lateral sclerosis).9 In other words, we did not analyse those with mitochondrial diabetes, maturity-onset diabetes of the young (MODY), hyper- or hypo-thyroidism, Hashimoto's thyroiditis, pheochromocytoma, Cushing disease or syndrome, acromegaly, amyloidosis, tuberculosis, atypical mycobacteriosis, systemic lupus erythematosus, rheumatoid arthritis, juvenile rheumatoid arthritis, malignant rheumatoid arthritis, dermatomyositis, polymyositis, systemic sclerosis, steroid use, myocarditis, dilated cardiomyopathy or hypertrophic cardiomyopathy.
Measurements
One of the complications of diabetes, macrovascular disease included acute myocardial infarction, stable and unstable angina pectoris, heart failure, cerebral infarction, cerebral haemorrhage, subarachnoid haemorrhage, cerebral arterial aneurysm, aortic aneurysm and peripheral arterial disease (arteriosclerosis obliterans). Microvascular disease included diabetic nephropathy, retinopathy and neuropathy. Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 at the time of registration, although the guideline for clinical practice requires continuation of this low filtration level for 3 months or more.10 History of chronic respiratory disease included asthma, chronic obstructive pulmonary disease, interstitial pneumonia, pulmonary fibrosis and pneumoconiosis. History of cancer included that of all organs. Serum HbA1c levels measured in the scale of the Japanese Diabetes Society (JDS) between the fiscal years of 2003 and 2007 were converted to those in the scales of the National Glycohemoglobin Standardization Program (NGSP) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).1, 11, 12 Serum LDL-C levels in indirect measurement were estimated in the Friedewald equation.13 The age of participants were stratified into adult (19–44 years), middle-aged (45–64 years), aged (65–79 years) and oldest (80 years or more). BMI was calculated as weight in kilograms divided by the square of height in metres, dividing the patients into the groups of less than 18.5, 18.5–22, 22–25, 25–30 (overweight) and 30 or more (obesity).
Statistical analysis
We described means [standard deviations (SDs)] of age, BMI, HbA1c and blood pressure and the distributions of smoking and drinking status, glycaemic control, macro- and microvascular complications, other comorbidities and follow-up period for the type 1 and 2 diabetic patients. Means (SDs) of glycated HbA1c levels in the strata of sex, age, BMI, blood pressure, serum cholesterol levels, cigarette smoking and alcohol drinking were also described. We depicted the Kaplan–Meier estimates14 of the patients between type 1 and 2 diabetes, statistically comparing with a log-rank test.15 We compared mortality between type 1 and 2 diabetic patients using Cox proportional hazard model after adjustment of baseline age.16 We also compared the proportions of the patients who had HbA1c under 7.0%, the treatment target for preventing diabetic complications.1 This threshold was used to consistently evaluate the proportions of the patients in whom serum glucose was better controlled. We performed all statistical analyses with SAS statistical software (version 9.3, SAS Institute, Cary, NC, USA) and drew Kaplan–Meier estimates using R statistical software (version 2.15.3, R Project for Statistical Computing, Vienna, Austria). All reported p values were 2-sided; p values of <0.05 were considered to be statistically significant.
Ethical considerations
The ethics committees of the Institute of Medical Science, The University of Tokyo, RIKEN Center for Integrative Medical Sciences and 12 cooperating medical institutions approved the protocol of this study in accordance with the ethical guidelines and regulations of the Declaration of Helsinki. The Japanese guidelines permit the use of data from medical examinations and information without patient consent if the data are anonymous. Hence, informed consent from the patients was not required for the present investigation.
Results
Type 1 and 2 diabetic patients
Table 1 summarises age, BMI, HbA1c, blood pressure and follow-up period of the type 1 diabetic patients and presents proportions of glycaemic controls, vascular complications, comorbidities and smoking and drinking statuses. Table 2 summarises these characteristics of the type 2 diabetic patients, as reported on other pages of this issue.17 In type 1 diabetes, the mean HbA1c was 8.1 (SD: 1.9)% and 65.1 (SD: 20.3) mmol/mol in men and 8.2 (SD: 1.3)% and 65.7 (SD: 14.7) mmol/mol in women. In type 2 diabetes, the mean HbA1c was 7.4 (SD: 1.4)% and 57.1 (SD: 15.4) mmol/mol in men and 7.5 (SD: 1.4)% and 58.8 (SD: 15.5) mmol/mol in women. In type 1 and 2 diabetes, the patients were the most prevalent in the categories of HbA1c<6.0% (<42 mmol/mol) and having no vascular complication in both sexes. Table 3, Table 4 report detailed HbA1c levels of the type 1 and 2 diabetic patients, respectively. In the data of both sexes, the Japanese type 1 diabetic patients were the most prevalent within the BMI range of 18.5–22 kg/m2, with blood pressures of SBP<120 mm Hg and DBP<80 mm Hg. Most of the male and female patients had serum LDL-C<120 mg/dL (3.10 mmol/L), triglyceride<150 mg/dL (1.69 mmol/L) and HDL-C<40 mg/dL (1.03 mmol/L). In type 1 diabetes, current smoker and non-drinker were the most prevalent in the male patients and never smoker and non-drinker were the most prevalent in the female patients. The Japanese type 2 diabetic patients were the most prevalent within the BMI range of 22–25 kg/m2 in men and 25–30 kg/m2 in women. Most of the male and female patients had serum LDL-C<120 mg/dL (3.10 mmol/L), triglyceride<150 mg/dL (1.69 mmol/L) and HDL-C<40 mg/dL (1.03 mmol/L). In type 2 diabetes, ex-smoker and current drinker were the most prevalent in the male patients and never smoker and non-drinker were the most prevalent in the female patients.
Table 1.
Characteristics of patients with type 1 diabetes based on Biobank Japan data.
| Characteristics | Men | Women |
|---|---|---|
| Number (%) | 304 (54.5) | 254 (45.5) |
| Age, years | 53.7 (15.0) | 52.5 (17.0) |
| Body mass index, kg/m2 | 22.2 (3.3) | 22.3 (3.6) |
| Glycated haemoglobin A1c, % and mmol/mol | 8.1 (1.9) and 65.1 (20.3) | 8.2 (1.3) and 65.7 (14.7) |
| Systolic blood pressure, mm Hg | 128.9 (19.9) | 123.9 (15.9) |
| Diastolic blood pressure, mm Hg | 73.9 (11.3) | 71.3 (9.7) |
| Smoking, never/ex-/current, no. | 87/76/141 | 183/25/46 |
| Currently drinking, no. (%) | 128 (42.1) | 42 (16.5) |
| Glycated haemoglobin, no. (%) | ||
| <6.0% (<42 mmol/mol) | 109 (35.9) | 83 (32.7) |
| 6.0–6.4% (42–47 mmol/mol) | 15 (5.0) | 8 (3.2) |
| 6.5–6.9% (48–52 mmol/mol) | 35 (11.5) | 17 (6.7) |
| 7.0–7.9% (53–63 mmol/mol) | 46 (15.1) | 56 (22.1) |
| 8.0–8.9% (64–74 mmol/mol) | 50 (16.5) | 47 (18.5) |
| ≥9.0% (≥75 mmol/mol) | 49 (16.1) | 43 (16.9) |
| Fasting plasma glucose, no. (%) | ||
| <126 mg/dL (<7.0 mmol/L) | 8 (4.7) | 9 (5.8) |
| ≥126 mg/dL (≥7.0 mmol/L) | 164 (95.4) | 145 (94.2) |
| Vascular complications, no. (%) | ||
| None | 120 (39.5) | 121 (47.6) |
| Macrovascular disease | 49 (16.1) | 38 (15.0) |
| Nephropathy | 29 (9.5) | 12 (4.7) |
| Retinopathy | 33 (10.9) | 44 (17.3) |
| Neuropathy | 73 (24.0) | 39 (15.4) |
| Comordity and past histories, no. (%) | ||
| Chronic kidney disease | 78 (25.7) | 57 (22.4) |
| Chronic Respiratory disease | 11 (3.6) | 13 (5.1) |
| Cancer | 12 (4.0) | 12 (4.7) |
| Follow-up period from baseline, years, median (interquartile range) | 8.53 (6.61–9.95) | 8.76 (7.27–10.37) |
The data are presented as mean (SD) or number (%) unless stated otherwise.
Table 2.
Characteristics of patients with type 2 diabetes in Biobank Japan data.17
| Characteristics | Men | Women |
|---|---|---|
| Number (%) | 19,830 (64.3) | 11,004 (35.7) |
| Age, years | 63.4 (11.0) | 64.3 (11.2) |
| Body mass index (kg/m2) | 24.1 (3.7) | 24.7 (4.4) |
| Glycated haemoglobin A1c (% and mmol/mol) | 7.4 (1.4) and 57.1 (15.4) | 7.5 (1.4) and 58.8 (15.5) |
| Systolic blood pressure (mm Hg) | 133.6 (17.2) | 135.0 (17.6) |
| Diastolic blood pressure, (mm Hg) | 77.5 (10.9) | 75.6 (10.9) |
| Smoking, never/ex-/current, number | 5038/8282/6510 | 8832/990/1182 |
| Currently drinking, number (%) | 9416 (47.5) | 1,94 (11.8) |
| Glycated haemoglobin, number (%) | ||
| <6.0% (<42 mmol/mol) | 7314 (36.9) | 3879 (35.3) |
| 6.0–6.4% (42–47 mmol/mol) | 2304 (11.6) | 1097 (10.0) |
| 6.5–6.9% (48–52 mmol/mol) | 2842 (14.3) | 1556 (14.1) |
| 7.0–7.9% (53–63 mmol/mol) | 3804 (19.2) | 2235 (20.3) |
| 8.0–8.9% (64–74 mmol/mol) | 2018 (10.2) | 1230 (11.2) |
| ≥9.0% (≥75 mmol/mol) | 1548 (7.8) | 1007 (9.2) |
| Fasting plasma glucose, number (%) | ||
| <126 mg/dL (<7.0 mmol/L) | 1136 (10.4) | 763 (12.3) |
| ≥126 mg/dL (≥7.0 mmol/L) | 9829 (89.6) | 5430 (87.7) |
| Vascular complications, number (%) | ||
| None | 7638 (38.5) | 4778 (43.4) |
| Macrovascular disease | 7368 (37.2) | 3152 (28.6) |
| Nephropathy | 1039 (5.2) | 578 (5.3) |
| Retinopathy | 1550 (7.8) | 1090 (9.9) |
| Neuropathy | 2235 (11.3) | 1406 (12.8) |
| Comorbidity and past histories, number (%) | ||
| Chronic kidney disease | 6236 (31.5) | 3588 (32.6) |
| History of cancer | 1910 (9.6) | 949 (8.6) |
| History of chronic respiratory disease | 805 (4.1) | 568 (5.2) |
| Follow-up period from baseline, years, median (interquartile range) | 8.03 (5.84–9.67) | 8.30 (6.47–9.88) |
The data are presented as mean (SD) or number (%) unless stated otherwise.
Table 3.
Glycated haemoglobin A1c (HbA1c) of patients with type 1 diabetes according to characteristics in BioBank Japan data. Means (standard deviations) or proportions.
| Characteristic | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| Number | HbA1c (NGSP) | HbA1c (IFCC) | HbA1c<7.0%, % | Number | HbA1c (NGSP) | HbA1c (IFCC) | HbA1c<7.0%, % | |
| Age | ||||||||
| 19–44 | 64 | 8.3 (1.7) | 67.4 (18.9) | 49.5 | 68 | 8.3 (1.4) | 67.5 (14.9) | 38.5 |
| 45–64 | 95 | 8.1 (2.0) | 65.1 (22.2) | 51.9 | 58 | 8.4 (1.3) | 67.8 (14.3) | 43.0 |
| 65–79 | 52 | 7.8 (1.7) | 61.9 (18.7) | 56.9 | 46 | 7.7 (1.2) | 61.1 (13.0) | 41.7 |
| 80+ | 2 | 8.6 (0.9) | 70.1 (10.2) | 50.0 | 6 | 7.7 (2.1) | 60.6 (23.1) | 75.0 |
| Body mass index | ||||||||
| −18.5 | 37 | 8.5 (2.2) | 69.2 (24.2) | 52.7 | 28 | 7.8 (1.4) | 61.6 (15.3) | 50.0 |
| 18.5–22 | 85 | 8.1 (1.5) | 65.0 (16.7) | 48.3 | 60 | 7.8 (1.1) | 62.0 (12.0) | 48.8 |
| 22–25 | 56 | 8.2 (2.2) | 66.0 (24.1) | 53.7 | 59 | 8.5 (1.3) | 69.3 (14.1) | 34.2 |
| 25–30 | 28 | 7.4 (1.4) | 57.8 (14.8) | 64.1 | 27 | 8.4 (1.7) | 68.1 (18.1) | 38.9 |
| 30+ | 7 | 8.2 (2.1) | 65.7 (22.8) | 37.5 | 4 | 9.6 (1.1) | 81.2 (12.4) | 33.3 |
| Blood pressure | ||||||||
| SBP<120 mm Hg and DBP<80 mm Hg | 84 | 8.4 (2.2) | 68.4 (23.7) | 45.0 | 66 | 8.0 (1.3) | 64.0 (14.0) | 46.6 |
| Else SBP<130 mm Hg and DBP<85 mm Hg | 39 | 8.0 (1.6) | 63.8 (17.9) | 56.5 | 45 | 8.3 (1.4) | 67.3 (15.8) | 38.3 |
| Else SBP<140 mm Hg and DBP<90 mm Hg | 51 | 7.9 (1.6) | 62.7 (18.0) | 48.4 | 38 | 8.0 (1.3) | 63.8 (14.1) | 43.1 |
| Else SBP<160 mm Hg and DBP<100 mm Hg | 33 | 7.9 (1.6) | 62.6 (17.6) | 62.1 | 27 | 8.4 (1.4) | 68.4 (23.7) | 37.8 |
| Else SBP<180 mm Hg and DBP<110 mm Hg | 5 | 7.3 (1.4) | 56.8 (14.8) | 77.8 | 2 | 9.8 (1.2) | 84.0 (12.6) | 33.3 |
| Else SBP≥180 mm Hg or DBP≥110 mm Hg | 1 | 9.0 | 75.1 | 50.0 | 0 | – | – | – |
| Cholesterol | ||||||||
| LDL-C<120 mg/dL (3.10 mmol/L) | 208 | 8.1 (1.9) | 65.0 (20.3) | 52.5 | 177 | 8.2 (1.4) | 65.8 (14.8) | 42.5 |
| LDL-C≥120 mg/dL (3.10 mmol/L) | 5 | 8.3 (1.9) | 67.5 (21.2) | 42.9 | 1 | 7.1 | 53.9 | 50.0 |
| TG < 150 mg/dL (1.69 mmol/L) | 201 | 8.1 (1.9) | 65.2 (20.5) | 52.3 | 171 | 8.2 (1.3) | 65.8 (14.6) | 42.0 |
| TG≥150 mg/dL (1.69 mmol/L) | 12 | 7.9 (1.7) | 63.1 (18.6) | 52.9 | 7 | 7.9 (1.7) | 62.4 (18.4) | 55.6 |
| HDL-C≥40 mg/dL (1.03 mmol/L) | 19 | 8.0 (1.6) | 64.3 (17.8) | 53.2 | 19 | 8.2 (1.6) | 66.7 (17.7) | 34.8 |
| HDL-C<40 mg/dL (1.03 mmol/L) | 194 | 8.1 (1.9) | 65.1 (20.6) | 41.7 | 159 | 8.2 (1.3) | 65.6 (14.4) | 43.3 |
| Smoking | ||||||||
| Never smoker | 55 | 7.9 (2.2) | 62.5 (23.6) | 65.5 | 128 | 8.3 (1.4) | 67.1 (15.1) | 40.4 |
| Ex-smoker | 58 | 7.8 (1.3) | 61.8 (13.8) | 43.4 | 18 | 7.8 (1.1) | 61.4 (12.5) | 48.0 |
| Current smoker | 100 | 8.4 (1.9) | 68.4 (21.2) | 48.9 | 32 | 7.9 (1.3) | 62.6 (14.0) | 47.8 |
| Alcohol drinking | ||||||||
| Non-drinker | 105 | 8.2 (2.1) | 66.4 (22.6) | 56.2 | 149 | 8.2 (1.4) | 66.5 (15.2) | 39.5 |
| Drinker | 104 | 8.0 (1.6) | 63.9 (18.0) | 46.0 | 29 | 7.8 (1.1) | 62.3 (12.5) | 49.1 |
Note that the figures and proportions are occasionally inconsistent in this table, i.e., missing data occasionally lead to differences in the numbers of patients in each stratum (profile).
Table 4.
Glycated haemoglobin A1c (HbA1c) of patients with type 2 diabetes according to characteristics in BioBank Japan data. Means (standard deviations) or proportions.
| Characteristic | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| Number | HbA1c (NGSP) | HbA1c (IFCC) | HbA1c<7.0%, % | Number | HbA1c (NGSP) | HbA1c (IFCC) | HbA1c<7.0%, % | |
| Age | ||||||||
| 19–44 | 759 | 8.0 (2.0) | 63.5 (22.1) | 60.1 | 295 | 8.1 (2.0) | 64.8 (21.5) | 57.8 |
| 45–64 | 6295 | 7.5 (1.4) | 58.2 (15.6) | 60.4 | 2876 | 7.7 (1.5) | 60.2 (15.9) | 56.9 |
| 65–79 | 6317 | 7.2 (1.3) | 55.6 (14.0) | 64.7 | 3820 | 7.4 (1.3) | 57.8 (14.5) | 59.2 |
| 80+ | 662 | 7.2 (1.2) | 54.7 (13.2) | 71.1 | 779 | 7.3 (1.4) | 55.8 (15.0) | 69.1 |
| Body mass index | ||||||||
| −18.5 | 1264 | 7.4 (1.5) | 57.4 (16.2) | 65.6 | 963 | 7.5 (1.5) | 58.5 (16.5) | 63.9 |
| 18.5–22 | 3113 | 7.3 (1.5) | 56.7 (16.0) | 65.3 | 1538 | 7.4 (1.4) | 57.4 (15.1) | 62.0 |
| 22–25 | 4825 | 7.3 (1.3) | 56.8 (14.2) | 62.3 | 2159 | 7.5 (1.3) | 58.6 (14.7) | 58.6 |
| 25–30 | 3971 | 7.4 (1.4) | 57.4 (15.1) | 61.3 | 2306 | 7.6 (1.4) | 59.7 (15.7) | 57.0 |
| 30+ | 860 | 7.6 (1.7) | 59.1 (18.4) | 58.7 | 804 | 7.6 (1.5) | 59.5 (16.1) | 57.0 |
| Blood pressure | ||||||||
| SBP<120 mm Hg and DBP<80 mm Hg | 3682 | 7.4 (1.5) | 57.6 (16.2) | 63.9 | 1949 | 7.5 (1.6) | 59.0 (16.9) | 63.1 |
| Else SBP<130 mm Hg and DBP<85 mm Hg | 2568 | 7.4 (1.5) | 57.2 (16.3) | 62.1 | 1315 | 7.5 (1.3) | 58.5 (14.7) | 58.8 |
| Else SBP<140 mm Hg and DBP<90 mm Hg | 3829 | 7.3 (1.3) | 56.8 (14.7) | 63.1 | 2136 | 7.5 (1.4) | 58.8 (15.2) | 57.1 |
| Else SBP<160 mm Hg and DBP<100 mm Hg | 3369 | 7.4 (1.3) | 57.2 (14.4) | 61.4 | 1997 | 7.5 (1.3) | 58.7 (14.6) | 57.7 |
| Else SBP<180 mm Hg and DBP<110 mm Hg | 509 | 7.3 (1.4) | 56.2 (15.3) | 65.7 | 331 | 7.5 (1.4) | 58.2 (15.3) | 61.1 |
| Else SBP≥180 mm Hg or DBP≥110 mm Hg | 76 | 7.4 (1.4) | 56.9 (14.8) | 63.6 | 42 | 7.8 (2.0) | 61.3 (21.5) | 63.4 |
| Cholesterol | ||||||||
| LDL-C<120 mg/dL (3.10 mmol/L) | 13,235 | 7.4 (1.4) | 57.1 (15.4) | 62.9 | 7210 | 7.5 (1.4) | 58.8 (15.5) | 59.1 |
| LDL-C≥120 mg/dL (3.10 mmol/L) | 798 | 7.4 (1.4) | 57.4 (14.9) | 61.7 | 560 | 7.4 (1.4) | 57.8 (14.9) | 62.9 |
| TG < 150 mg/dL (1.69 mmol/L) | 11,950 | 7.4 (1.4) | 57.1 (15.4) | 63.4 | 6676 | 7.5 (1.4) | 58.7 (15.5) | 59.7 |
| TG≥150 mg/dL (1.69 mmol/L) | 2083 | 7.4 (1.4) | 57.3 (14.8) | 59.3 | 1094 | 7.6 (1.4) | 59.3 (15.6) | 56.9 |
| HDL-C≥40 mg/dL (1.03 mmol/L) | 2595 | 7.3 (1.3) | 55.8 (13.8) | 62.1 | 1974 | 7.4 (1.3) | 57.6 (14.2) | 59.1 |
| HDL-C<40 mg/dL (1.03 mmol/L) | 11,438 | 7.4 (1.4) | 57.5 (15.7) | 63.0 | 5796 | 7.6 (1.5) | 59.2 (15.9) | 59.5 |
| Smoking | ||||||||
| Never smoker | 3522 | 7.3 (1.4) | 56.7 (15.5) | 64.4 | 6238 | 7.5 (1.4) | 58.4 (15.1) | 59.7 |
| Ex-smoker | 5997 | 7.3 (1.3) | 56.3 (14.2) | 63.7 | 726 | 7.5 (1.4) | 59.0 (15.5) | 57.3 |
| Current smoker | 4514 | 7.5 (1.5) | 58.7 (16.5) | 60.5 | 806 | 7.7 (1.6) | 61.2 (17.8) | 58.5 |
| Alcohol drinking | ||||||||
| Non-drinker | 6500 | 7.4 (1.5) | 57.7 (16.5) | 62.0 | 6560 | 7.5 (1.4) | 58.7 (15.5) | 58.8 |
| Drinker | 7132 | 7.3 (1.3) | 56.7 (14.2) | 63.1 | 1047 | 7.5 (1.4) | 59.0 (15.2) | 60.9 |
Note that the figures and proportions are occasionally inconsistent in this table, i.e., missing data occasionally lead to differences in the numbers of patients in each stratum (profile).
Survival of type 1 and 2 diabetic patients
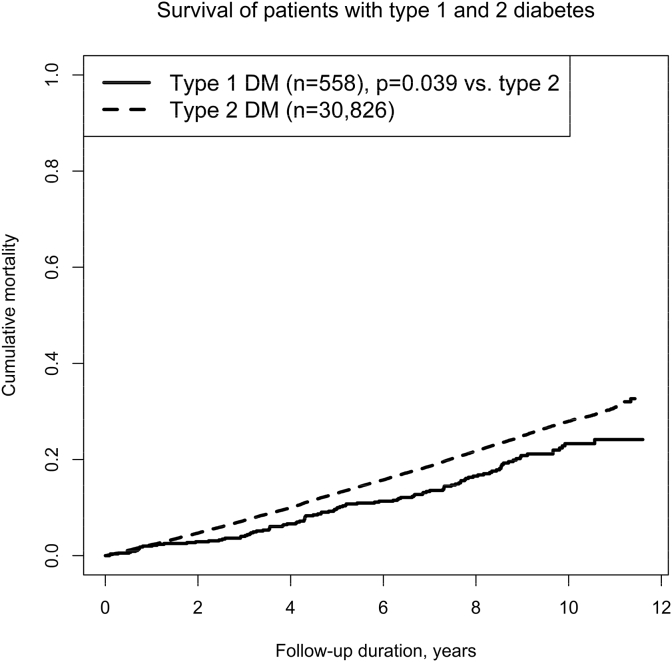
Fig. 1 shows the estimated survival curves of the type 1 and 2 diabetic patients. In the follow-up duration, 107 type 1 diabetic patients and 7207 type 2 diabetic patients died (log-rank test, p = 0.039). The multivariable analysis presents hazard ratios of 1.27 (95% confidence interval, 1.05–1.54) for type 1 diabetes vs. type 2 diabetes and 1.97 (95% confidence interval, 1.92–2.02) for 10 years of baseline age. Collectively, the crude Kaplan–Meier estimates indicate longer survival in type 1 diabetes, whereas hazard ratio indicates higher mortality in type 1 diabetes after adjustment for age.
Fig. 1.
Survival curves between Japanese patients with type 1 and 2 diabetes.
Discussion
Findings
This work has revealed detailed characteristics, complications and comorbidities of Japanese diabetic patients in a large-scaled population. The work also includes HbA1c levels in various profiles of age, BMI, blood pressure, serum cholesterol and smoking and drinking habits. In proportion to older age, the type 2 diabetic patients showed lower glycaemic control (Table 4), whereas the type 1 diabetic patients represented no trend in the glycaemic control in relation to age (Table 3). Interestingly, the glycaemic control in type 1 diabetes was higher than that in type 2 diabetes, and accordingly, the mortality in type 1 diabetes was higher after adjustment for age. In contrast, without adjustment for age, a worse prognosis in type 2 diabetes appears in the prevalence of macrovascular complications of 37.2% in men and 28.6% in women (Table 2),17 which is approximately twice of that in type 1 diabetes (Table 1). Recent Japanese evidence describes that when patients with post-pubertal diabetes were followed for 30 years, type 2 diabetes had a doubly large cumulative incidence of nephropathy compared to type 1 diabetes.18 The large prevalence of micro- and macrovascular complications in type 2 diabetes can be associated with the shorter crude survival time from baseline. Because vascular complications are major causes of death in type 2 diabetes,19 this analysis indicates that good glycaemic control and prevention of the complications has potential to extend patients' survival time.
Interpretations in the context of previous studies
We need careful interpretation of the observed figures in light of the literature. First, the fact that HbA1c was higher in type 1 diabetes than type 2 diabetes has also observed in previous Japanese3 and Swedish data.20, 21 This phenomenon is because, in type 1 diabetes, insulin secretary capacity of the pancreas is very poor, and controlling postprandial hyperglycaemia with insulin injection is more difficult than that with oral medications in type 2 diabetes.22 Second, the observed ratio of female/male type 1 diabetic patients agrees with Swedish national data20 but disagrees with Japanese consensus of female/male ratio of approximately 1.5.23, 24 The BioBank Japan Project focuses on adult patients with chronic diseases,6, 7, 8 and this focus may in part selectively-eliminate female type 1 diabetic patients who are continuously followed in paediatric practice after childhood onset.
Limitations and strengths
This work has several limitations. Primarily, the patients were not observed from the onset of diabetes. At the enrolment of the project, disease duration and treatment regimens should have been various among the patients. Because this limitation should bias the survival curves as a longitudinal analysis of this work, we could not simply conclude that life expectancy was longer in the type 1 diabetic patients than in the type 2 diabetic patients. In addition, in Japan, approximately 5% of diabetes is type 1,25 and classically, most of type 1 diabetes occurs during childhood.26 Because glycaemic control of type 1 diabetes usually becomes exacerbated in puberty,4 pre-pubertal onset type 1 diabetes is expected to cause more chance of vascular complications.27 Disease duration is also critically associated with vascular complications in type 1 diabetes.28 Hence, the inclusion of post-pubertal onset type 1 diabetes should implicitly bias the survival curve of type 1 diabetes towards longer survival time. Next, the data of this descriptive epidemiology might be relatively old. An important aspect for future studies is to determine serum glucose levels in a more recent Japanese population. Third, the participants in the middle to largest hospitals might not represent general Japanese patients. Japanese patients with mild diabetes are most likely to be followed up in internal medicine or paediatric clinics. The incompatible sex ratio in type 1 diabetes implies the existence of this limitation. The fourth limitation is the lack of details of macro- and microvascular complications. The severity of the complications is varied and critical to prognosis and patient life.
We would like to refer to several strengths of this work. First, to the best of our knowledge, this is the first work to compare characteristics, glycaemic controls and survival curves between type 1 and 2 diabetes from multi-regional institutions in Japan. Additionally, along with glycaemic controls, we could also describe the prevalence of complications, comorbidities and control of blood pressure and serum cholesterol. Second, the sample size is extra-ordinarily large. Although clinics did not participate in this project, the descriptive data would represent Japanese patients with moderate to severe diabetes.
Conclusions
This work has revealed characteristics and prevalence of complications and comorbidities in Japanese type 1 and 2 diabetic patients. The data have also shown glycaemic controls in various individual profiles in the two main types of diabetes from multi-regional data. In a Japanese hospital setting, prognosis, in terms of survival time from baseline and macrovascular complications, has been better in type 1 diabetes than that in type 2 diabetes.
Conflicts of interest
None declared.
Acknowledgements
We would like to express our gratitude to all the participants of the BioBank Japan Project and to all the medical coordinators of the cooperating hospitals for collecting samples and clinical information, as well as to Yasushi Yamashita and staff members of the BioBank Japan Project for their administrative support. We would also like to thank Doctor Kumao Toyoshima for his overall supervision of the BioBank Japan Project and Professor Shin Amemiya at the Department of Pediatrics in the Saitama Medical University and Doctor Mie Mochizuki at the Department of Pediatrics in the University of Yamanashi for their insightful advice about diabetes, which helped to dramatically improve the analyses and discussion of the manuscript.
Footnotes
Peer review under the responsibility of The Japan Epidemiological Association.
Contributor Information
Hiroshi Yokomichi, Email: hyokomichi@yamanashi.ac.jp.
BioBank Japan Cooperative Hospital Group:
Kazuo Misumi, Kiyoshi Iha, Sunao Matsubayashi, Kei Matsuura, Shiro Minami, Hitoshi Sugihara, Eitaro Kodani, Naoto Tamura, Masakazu Matsushita, Akihiko Gotoh, Satoshi Asai, Mitsuhiko Moriyama, Yasuo Takahashi, Tomoaki Fujioka, Wataru Obara, Seijiro Mori, Hideki Ito, Satoshi Nagayama, Yoshio Miki, Akihide Masumoto, Akira Yamada, Yasuko Nishizawa, Ken Kodama, Satoshi Ugi, Hiroshi Maegawa, Yukihiro Koretsune, Hideo Kusuoka, and Masao Okumura
Appendix.
Members of medical institutions cooperating on the BioBank Japan Project who co-authored this paper include Sunao Matsubayashi, Hiromasa Harada, Kazuo Misumi and Rieko Komi (Tokushukai Hospitals); Shiro Minami, Hitoshi Sugihara and Naoya Emoto (Nippon Medical School); Akio Kanazawa, Yusuke Suzuki and Yoshimune Hiratsuka (Juntendo University); Satoshi Asai, Mitsuhiko Moriyama and Yasuo Takahashi (Nihon University); Tomoaki Fujioka and Wataru Obara (Iwate Medical University); Seijiro Mori and Hideki Ito (Tokyo Metropolitan Institute of Gerontology); Satoshi Nagayama and Yoshio Miki (The Cancer Institute Hospital of JFCR); Akihide Masumoto and Akira Yamada (Aso Iizuka Hospital); Yasuko Nishizawa and Ken Kodama (Osaka Medical Center for Cancer and Cardiovascular Diseases); Satoshi Ugi and Hiroshi Maegawa (Shiga University of Medical Science); Yukihiro Koretsune and Hideki Taki (National Hospital Organization, Osaka National Hospital) and Takeshi Osawa (Fukujuji Hospital).
Funding
This work was supported by funds from the Tailor-Made Medical Treatment Program with the BioBank Japan Project from Japan Agency for Medical Research and Development (AMED) since April 2015 and the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) from April 2003 to March 2015. This work was also supported by MEXT [KAKENHI grant number: JP15K08730 and JP15K15221].
Author contributions
MK and ZY conceived the study. ZY, HY and MK designed the study. HY performed statistical analysis and wrote the manuscript. AN, TN, YK and MH researched data. All authors contributed to the discussion, and reviewed and edited the manuscript. MK and ZY are the guarantors of this work, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Tajima N., Noda M., Origasa H. Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int. 2015;6:151–187. [Google Scholar]
- 2.Eppens M.C., Craig M.E., Cusumano J. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 3.Japan Diabetes Clinical Data Management Study Group . 2015. Data of Japanese Diabetic Patients in 2013.http://jddm.jp/data/index-2013.html Accessed 22 October 2016. [Google Scholar]
- 4.Mochizuki M., Kikuchi T., Urakami T. Improvement in glycemic control through changes in insulin regimens: findings from a Japanese cohort of children and adolescents with type 1 diabetes. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12409. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y. The BioBank Japan project. Clin Adv Hematol Oncol. 2007;5:696–697. [PubMed] [Google Scholar]
- 6.Nagai A., Hirata M., Muto K. Overview of the BioBank Japan project: study design and profiles. J Epidemiol. 2017;27:S2–S8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirata M., Kamatani Y., Nagai A. Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol. 2017;27:S9–S21. doi: 10.1016/j.je.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirata M., Nagai A., Kamatani Y. Overview of BioBank Japan follow-up data in 32 diseases. J Epidemiol. 2017;27:S22–S28. doi: 10.1016/j.je.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seino Y., Nanjo K., Tajima N. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabet Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey A.S., de Jong P.E., Coresh J. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 11.Umemoto M., Raneva V., Tominaga M. Relationship between NGSP and JDS HbA1c numbers. Diabetol Int. 2015;6:77–81. [Google Scholar]
- 12.Hoelzel W., Weykamp C., Jeppsson J.-O. IFCC reference system for measurement of hemoglobin A1c in human blood and the National Standardization Schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50:166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep Part 1. 1966;50:163–170. [PubMed] [Google Scholar]
- 16.Cox D. Regression models and life tables (with discussion) J Roy Stat Soc. 1972;34:187–220. [Google Scholar]
- 17.Yokomichi H., Nagai A., Hirata M. Survival of macrovascular disease, chronic kidney disease, chronic respiratory disease, cancer and smoking in patients with type 2 diabetes: BioBank Japan cohort. J Epidemiol. 2017;27:S98–S106. doi: 10.1016/j.je.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoyama H., Okudaira M., Otani T. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–311. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 19.Seshasai S.R.K., Kaptoge S., Thompson A. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind M., Svensson A.M., Kosiborod M. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 21.Tancredi M., Rosengren A., Svensson A.-M. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Approaches to glycemic treatment. Diabetes Care. 2016;39:S52–S59. doi: 10.2337/dc16-S010. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura N., Fukuda K., Okuno A. Descriptive epidemiology of IDDM in Hokkaido, Japan: the childhood IDDM Hokkaido registry. Diabetes Care. 1998;21:1632–1636. doi: 10.2337/diacare.21.10.1632. [DOI] [PubMed] [Google Scholar]
- 24.Uchigata Y., Otani T., Takaike H. Time-course changes in clinical features of early-onset Japanese type 1 and type 2 diabetes: TWMU hospital-based study. Diabet Res Clin Pract. 2008;82:80–86. doi: 10.1016/j.diabres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto A., Nishimura R., Tajima N. Trends in the epidemiology of patients with diabetes in Japan. Jpn Med Assoc J. 2010;53:24–27. [Google Scholar]
- 26.Kawasaki E., Matsuura N., Eguchi K. Type 1 diabetes in Japan. Diabetologia. 2006;49:828–836. doi: 10.1007/s00125-006-0213-8. [DOI] [PubMed] [Google Scholar]
- 27.Diabetes Control and Complication Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT) J Pediatr. 2001;139:804–812. doi: 10.1067/mpd.2001.118887. [DOI] [PubMed] [Google Scholar]
- 28.Hirose A., Furushima D., Yamaguchi N., Kitano S., Uchigata Y. Prediction of retinopathy at 20 years after onset in younger-onset type 1 diabetes using mean metabolic memory-free HbA1c values. Diabetes Care. 2013;36:3812–3814. doi: 10.2337/dc13-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]