Abstract
Background
Several studies have evaluated associations between the characteristics of patients with esophageal and gastric cancer and survival, but these associations remain unclear. We described the distribution of demographic and lifestyle factors among patients with esophageal and gastric cancer in Japan, and investigated their potential effects on survival.
Methods
Between 2003 and 2007, 24- to 95-year-old Japanese patients with esophageal and gastric cancer were enrolled in the BioBank Japan Project. The analysis included 365 patients with esophageal squamous cell carcinoma (ESCC) and 1574 patients with gastric cancer. Hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality were estimated using medical institution-stratified Cox proportional hazards models.
Results
During follow-up, 213 patients with ESCC (median follow-up, 4.4 years) and 603 patients with gastric cancer (median follow-up, 6.1 years) died. Among patients with ESCC, the mortality risk was higher in ever drinkers versus never drinkers (multivariable HR = 2.37, 95% CI: 1.24, 4.53). Among patients with gastric cancer, the mortality risk was higher in underweight patients versus patients of normal weight (multivariable HR = 1.66, 95% CI: 1.34, 2.05). Compared to patients with gastric cancer with no physical exercise habit, those who exercised ≥3 times/week had a lower mortality risk (multivariate HR = 0.75, 95% CI = 0.61, 0.93). However, lack of stage in many cases was a limitation.
Conclusions
Among patients with ESCC, alcohol drinkers have a poor prognosis. Patients with gastric cancer who are underweight also have a poor prognosis, whereas patients with physical exercise habits have a good prognosis.
Keywords: Esophageal cancer, Gastric cancer, Survival, Cohort study, Japan
Highlights
-
•
Among ESCC patients, alcohol drinkers had a poor prognosis.
-
•
Underweight gastric cancer patients had a poor prognosis.
-
•
Gastric cancer patients who exercised had a good prognosis.
-
•
No association between esophageal or gastric cancer and smoking was observed.
Introduction
Esophageal cancer is the seventh most common type of cancer and the sixth most common cause of death from cancer worldwide.1 Esophageal cancer is classified into two main histological types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EA). The incidence of each type differs depending on race and geographical region. EA is increasing in Western countries, whereas ESCC is the dominant type of esophageal cancer in East Asian countries such as China, Korea, and Japan.2 Gastric cancer is the fifth most common type of cancer and the third most common cause of death from cancer worldwide.1 Established risk factors for esophageal cancer include tobacco smoking, heavy alcohol drinking, and frequent consumption of high-temperature beverages.3 Risk factors for gastric cancer include smoking,4 high salt intake,5 and infection by Helicobacter pylori.6, 7 In addition, gastroesophageal reflux disease and the reflux-related condition Barrett's esophagus are known risk factors for esophageal cancer, because the esophagus is connected to the cardia of the stomach.8, 9 Thus, esophageal and gastric cancer should be investigated together.
Some studies have reported that male sex, increased age, weight loss, smoking and alcohol drinking decrease survival in patients with esophageal cancer,10, 11 but other studies revealed no significant association between smoking and alcohol drinking and esophageal cancer.12, 13 In patients with gastric cancer, smoking has been shown to decrease survival,14 but other studies revealed no significant association.12, 13, 15 Tobacco smoking remains a popular lifestyle choice among many East Asian males,16 despite it being an established risk factor for multiple cancers in the general population. Moreover, evidence for associations between demographic and lifestyle factors and the prognosis of esophageal and gastric cancer in Japan is scarce.
The objective of this study was to describe the distribution of demographic and lifestyle factors among patients with esophageal and gastric cancer registered in the BioBank Japan (BBJ) project. In addition, we investigated the potential effect of demographic and lifestyle factors on survival in patients with esophageal and gastric cancer.
Material and methods
Study population
Between 2003 and 2007, patients with any of 47 target common diseases were enrolled in the BBJ at 66 hospitals, which comprised 12 cooperating medical institutions, located throughout Japan. Details of the study design have been described elsewhere.17, 18, 19 We included participants whose disease duration could be calculated from the date of diagnosis of esophageal and/or gastric cancer and the date of registration for this study. In the present study, 1258 patients with esophageal cancer and 5597 patients with gastric cancer were included at baseline. Of these patients, 1162 patients with esophageal cancer and 5103 patients with gastric cancer completed follow-up. When we performed the analysis for prognosis, new patients who entered the study ≤90 days after diagnosis were included. Among patients with esophageal cancer, patients who entered this study >90 days after diagnosis (n = 702), patients with a histology other than ESCC (n = 93), and patients whose smoking history and/or alcohol drinking history were missing (n = 2) were excluded from the survival analysis. Because ESCC is the major histologic type of esophageal cancer in Asian countries, including Japan,2 we focused on ESCC herein. Among patients with gastric cancer, patients for whom >90 days passed between diagnosis and study entrance (n = 3513) and patients for whom smoking and alcohol drinking histories were missing (n = 16) were excluded from the survival analysis. Patients whose smoking and alcohol drinking histories were missing were excluded because these are significant risk factors for ESCC and gastric cancer in the general population. A total of 365 patients with ESCC and 1574 patients with gastric cancer were included in the survival analysis. The study design was reviewed and approved by the Ethics Committees of all participating institutions. Written informed consent was obtained from all participants.
Data collection
Baseline clinical information was collected through medical records and interviews using a standardized questionnaire. Interview items included smoking and alcohol drinking habits, height, weight, and frequency of physical exercise. Information collected from medical records included birth year and sex. In this study, esophageal and gastric cancer histology was determined from excised tissue specimens, and missing histological data were complemented by biopsy or cytological specimens. Esophageal and gastric cancer stages were classified according to the Japanese Classification of Esophageal Cancer, ninth edition (1999) and the Japanese Classification of Gastric Carcinoma, twelfth edition (1993).
Follow-up surveys
A survival follow-up survey was implemented from 2010 to 2014 for patient vital statistics. Information about death using the 10th revision of the International Classification of Disease codes was collected from the Vital Statistics of the Statistics and Information Department of the Ministry of Health, Labour and Welfare, Japan.20
Statistical analysis
To calculate expected survival rates, a survival rate table of a Japanese reference cohort was obtained from the Cancer Registry and Statistics, Cancer Information Service, National Cancer Center, Japan.21 The survival rate table was based on sex- and age-specific mortality rates and Gompertz-Makeham's law in Abridged Life Tables, which is annually published by the Statistics and Information Department of the Ministry of Health, Labour and Welfare, Japan.22 Relative survival rates were calculated by dividing cumulative survival rates by expected sex- and age-adjusted survival rates. Patients ≥100 years old were excluded due to a lack of data in the reference life table. We compared the 5-year relative survival rates of esophageal and gastric cancer patients in this study to data from the Japanese Association of Clinical Cancer Centers (cases diagnosed from 2004 to 2007).23
Univariate and multivariate hazard ratios (HRs) and 95% confidence intervals (CI) of demographic and lifestyle factor variables for mortality risk were evaluated using medical institution-stratified Cox proportional hazards model. The following variables were included in the multivariate models: sex, age (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, or ≥80 years), year of diagnosis (2003, 2004, 2005, 2006, 2007, or 2008), body mass index (BMI) (<18.5, 18.5–24.9, 25–29.9, ≥30.0 kg/m2, or unknown), smoking history (never or ever smoker), alcohol drinking history (never or ever drinker), physical exercise (no habit, 1–2 times/week, ≥3 times/week, or unknown), and stage (0, I, II, III, IVa, IVb, or unknown for ESCC, and Ia, Ib, II, IIIa, IIIb, IVa, IVb, or unknown for gastric cancer). All statistical analyses were performed using the SAS statistical package for Windows (version 9.4, SAS). Differences were considered statistically significant at p < 0.05.
Results
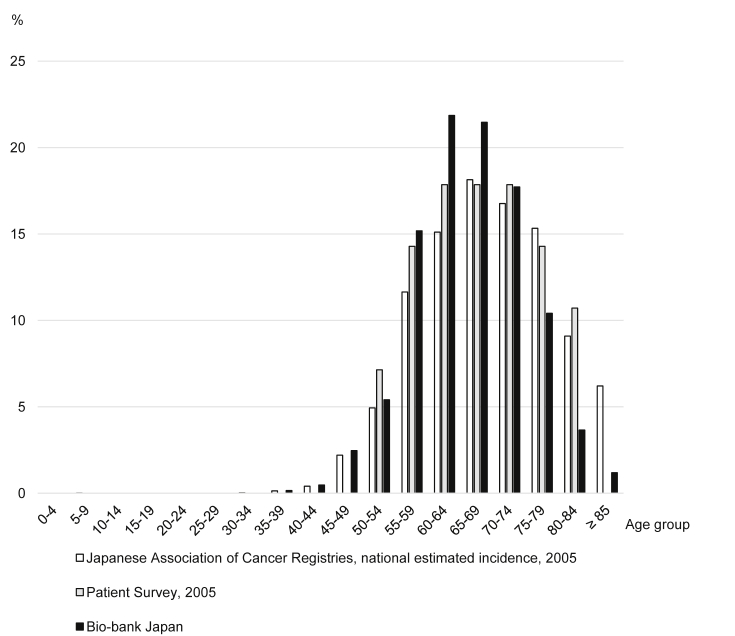
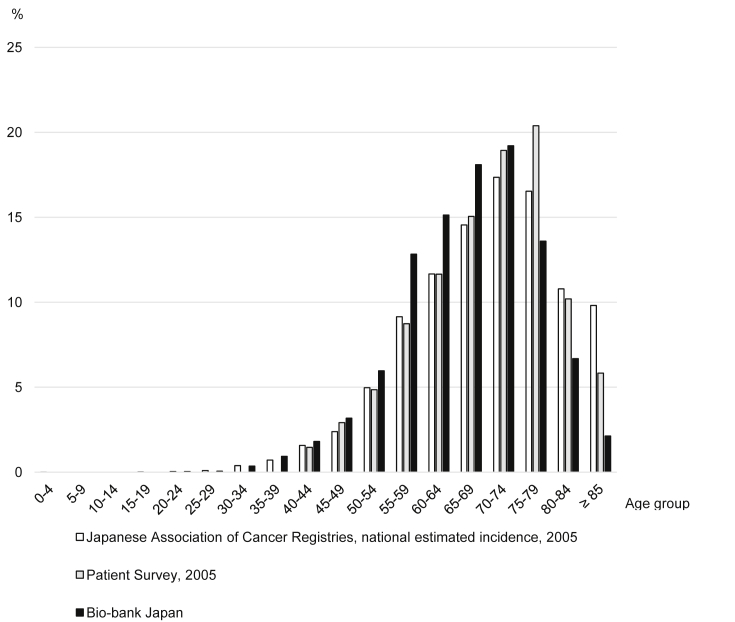
The proportions of patients by age group according to the BBJ, the Japanese Association of Cancer Registries,24 and the Patient Survey25 are shown in Fig. 1 for esophageal cancer and Fig. 2 for gastric cancer. Compared to the Japanese Association of Cancer Registries and the Patient Survey, which were performed in Japan, the proportion of patients with esophageal and gastric cancer age 55–69 years in the BBJ was about 4% higher within each 5-year age group, whereas the proportion of patients ≥75 years was about 5% lower.
Fig. 1.
Proportion of patients with esophageal cancer by age group.
Fig. 2.
Proportion of patients with gastric cancer by age group.
Baseline demographic and lifestyle factors of patients with esophageal and gastric cancer are shown in Table 1. Among patients with esophageal and gastric cancer, patients were more likely to be male (esophageal cancer: 86.5%; gastric cancer: 73.2%), age 60–69 or 70–79 years (esophageal cancer: 43.3% and 28.1%, respectively; gastric cancer: 33.2% and 32.8%, respectively), have a BMI of 18.5–24.9 kg/m2 (esophageal cancer: 64.3%; gastric cancer: 69.1%), be ever smokers (esophageal cancer: 82.8%; gastric cancer: 66.3%), be ever drinkers (esophageal cancer: 86.6%; gastric cancer: 62.8%), and have no physical exercise habit (esophageal cancer: 73.9%; gastric cancer: 72.7%). For patients with esophageal cancer, almost all had ESCC histology (89.9%), and among cases for which the stage was known, stage II (29.9%) and III (25.8%) disease was most common. For patients with gastric cancer, tubular adenocarcinoma was the most common histology (61.5%), and among patients for whom the stage was known, stage Ia disease was most common (48.0%).
Table 1.
Demographic and lifestyle factors of patients with esophageal and gastric cancer at baseline in the Biobank Japan Project.
| Esophageal cancer (n = 1258) |
Gastric cancer (n = 5597) |
|||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| Sex | ||||
| Male | 1088 | (86.5) | 4095 | (73.2) |
| Female | 170 | (13.5) | 1502 | (26.8) |
| Age range, y | ||||
| 20–29 | 0 | (0.0) | 5 | (0.1) |
| 30–39 | 2 | (0.2) | 72 | (1.3) |
| 40–49 | 37 | (2.9) | 279 | (5.0) |
| 50–59 | 259 | (20.6) | 1052 | (18.8) |
| 60–69 | 545 | (43.3) | 1860 | (33.2) |
| 70–79 | 354 | (28.1) | 1836 | (32.8) |
| ≥80 | 61 | (4.9) | 493 | (8.8) |
| BMI range, kg/m2 | ||||
| <18.5 | 347 | (28.4) | 1124 | (21.0) |
| 18.5–24.9 | 786 | (64.3) | 3694 | (69.1) |
| 25–29.9 | 85 | (7.0) | 486 | (9.1) |
| ≥30 | 4 | (0.3) | 41 | (0.8) |
| Unknown | 36 | – | 252 | – |
| Smoking history | ||||
| Never smoker | 214 | (17.2) | 1861 | (33.7) |
| Ever smoker | 1027 | (82.8) | 3659 | (66.3) |
| Unknown | 17 | – | 77 | – |
| Alcohol drinking history | ||||
| Never drinker | 166 | (13.4) | 2048 | (37.2) |
| Ever drinker | 1074 | (86.6) | 3453 | (62.8) |
| Unknown | 18 | – | 96 | – |
| Physical exercise | ||||
| No habit | 822 | (73.9) | 3639 | (72.7) |
| 1–2 times/week | 41 | (3.7) | 238 | (4.8) |
| ≥3 times/week | 249 | (22.4) | 1129 | (22.6) |
| Unknown | 146 | – | 591 | – |
| Year of diagnosis | ||||
| −2000 | 232 | (18.4) | 1463 | (26.1) |
| 2001 | 65 | (5.2) | 323 | (5.8) |
| 2002 | 85 | (6.8) | 437 | (7.8) |
| 2003 | 128 | (10.2) | 669 | (12.0) |
| 2004 | 183 | (14.6) | 767 | (13.7) |
| 2005 | 172 | (13.7) | 737 | (13.2) |
| 2006 | 184 | (14.6) | 697 | (12.5) |
| 2007 | 199 | (15.8) | 483 | (8.6) |
| 2008 | 10 | (0.8) | 21 | (0.4) |
| Histology of esophageal cancer | ||||
| Squamous cell carcinoma | 971 | (89.9) | ||
| Adenocarcinoma | 73 | (6.8) | ||
| Adenosquamous carcinoma | 13 | (1.2) | ||
| Adenoid cystic carcinoma | 1 | (0.1) | ||
| Basaloid cell carcinoma | 2 | (0.2) | ||
| Anaplastic carcinoma | 6 | (0.6) | ||
| Other cancers | 14 | (1.3) | ||
| Unknown | 178 | – | ||
| Histology of gastric cancer | ||||
| Papillary adenocarcinoma | 93 | (1.9) | ||
| Tubular adenocarcinoma | 2988 | (61.5) | ||
| Poorly differentiated adenocarcinoma | 884 | (18.2) | ||
| Signet-ring cell carcinoma | 620 | (12.8) | ||
| Mucinous adenocarcinoma | 80 | (1.6) | ||
| Special type | 18 | (0.4) | ||
| Other cancers | 179 | (3.7) | ||
| Unknown | 735 | – | ||
| Stage of esophageal cancer | ||||
| 0 | 40 | (10.1) | ||
| I | 70 | (17.7) | ||
| II | 118 | (29.9) | ||
| III | 102 | (25.8) | ||
| IVa | 40 | (10.1) | ||
| IVb | 25 | (6.3) | ||
| Unknown | 863 | – | ||
| Stage of gastric cancer | ||||
| Ia | 689 | (48.0) | ||
| Ib | 227 | (15.8) | ||
| II | 168 | (11.7) | ||
| IIIa | 142 | (9.9) | ||
| IIIb | 65 | (4.5) | ||
| IVa | 66 | (4.6) | ||
| IVb | 77 | (5.4) | ||
| Unknown | 4163 | – | ||
Table 2 shows the 5-year relative survival rate of patients with esophageal and gastric cancer. Relative survival rates of all patients and patients who participated in the study for ≤90 days after diagnosis are shown. The 5-year relative survival rate of patients for whom ≤90 days passed from diagnosis to study enrollment was 49.6% and 75.7% for esophageal and gastric cancer, respectively.
Table 2.
Five-year relative survival rate of patients with esophageal and gastric cancer.
| No. of patients | Follow-up rate (%) | Relative survival rate (%) | ||
|---|---|---|---|---|
| Esophageal cancer | Biobank Japan (total) | 1158 | 97.5 | 59.3 |
| Biobank Japana | 460 | 96.7 | 49.6 | |
| Japanese Association of Clinical Cancer Centers | 6109 | 95.1 | 42.4 | |
| Gastric cancer | Biobank Japan (total) | 5094 | 97.6 | 82.1 |
| Biobank Japana | 1590 | 97.4 | 75.7 | |
| Japanese Association of Clinical Cancer Centers | 23,690 | 93.5 | 73.0 | |
Patients who entered the study ≤90 days after diagnosis.
For patients with ESCC who participated in the study for ≤90 days after diagnosis, the median follow-up period was 4.4 years. During 1605 person-years, there were 213 deaths. The HRs and 95% CIs for mortality according to demographic and lifestyle factors among patients with ESCC are shown in Table 3. Compared to patients aged 50–59 years, patients ≥80 years had an increased risk of mortality after adjusting for other variables (multivariate HR = 2.79, 95% CI = 1.34, 5.80). With respect to alcohol drinking, the multivariate HR for mortality in ever drinkers was 2.37 (95% CI = 1.24, 4.53) compared to that of never drinkers. No significant association was observed for smoking history.
Table 3.
HRs and 95% CIs for mortality according to demographic and lifestyle factors among patients with ESCC in the Biobank Japan Project (n = 365).
| Person-years | No. of deaths | Univariate model |
Multivariate modela |
|||
|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | |||
| Sex | ||||||
| Male | 1344 | 181 | 1.05 | (0.71,1.55) | 0.70 | (0.42,1.17) |
| Female | 260 | 32 | 1.00 | 1.00 | ||
| Age range, years | ||||||
| 30–39 | 19 | 0 | NA | NA | ||
| 40–49 | 56 | 10 | 1.53 | (0.77,3.04) | 1.56 | (0.77,3.15) |
| 50–59 | 408 | 51 | 1.00 | 1.00 | ||
| 60–69 | 784 | 91 | 0.96 | (0.68,1.36) | 0.84 | (0.58,1.20) |
| 70–79 | 307 | 50 | 1.14 | (0.76,1.70) | 1.19 | (0.78,1.81) |
| ≥80 | 31 | 11 | 2.18 | (1.11,4.29)∗ | 2.79 | (1.34,5.80)∗∗ |
| Year of diagnosis | ||||||
| 2003 | 101 | 17 | 1.00 | 1.00 | ||
| 2004 | 266 | 46 | 1.03 | (0.57,1.85) | 1.44 | (0.76,2.70) |
| 2005 | 331 | 33 | 0.63 | (0.34,1.20) | 0.94 | (0.47,1.86) |
| 2006 | 365 | 50 | 0.83 | (0.45,1.52) | 1.23 | (0.64,2.38) |
| 2007 | 495 | 65 | 0.75 | (0.42,1.34) | 1.15 | (0.61,2.17) |
| 2008 | 45 | 2 | 0.27 | (0.06,1.19) | 0.41 | (0.09,1.86) |
| BMI range, kg/m2 | ||||||
| <18.5 | 343 | 52 | 1.17 | (0.85,1.61) | 0.90 | (0.63,1.28) |
| 18.5–24.9 | 1107 | 145 | 1.00 | 1.00 | ||
| 25–29.9 | 133 | 14 | 0.84 | (0.48,1.47) | 0.93 | (0.52,1.67) |
| ≥30 | 10 | 0 | NA | NA | ||
| Unknown | 12 | 2 | 1.00 | (0.24,4.15) | 0.92 | (0.20,4.23) |
| Smoking history | ||||||
| Never smoker | 274 | 36 | 1.00 | 1.00 | ||
| Ever smoker | 1330 | 177 | 0.97 | (0.67,1.40) | 0.97 | (0.62,1.50) |
| Alcohol drinking history | ||||||
| Never drinker | 183 | 17 | 1.00 | 1.00 | ||
| Ever drinker | 1422 | 196 | 1.43 | (0.86,2.36) | 2.37 | (1.24,4.53)∗∗ |
| Physical exercise | ||||||
| No habit | 1053 | 144 | 1.00 | 1.00 | ||
| 1–2 times/week | 102 | 6 | 0.46 | (0.20,1.06) | 0.36 | (0.15,0.86) |
| ≥3 times/week | 338 | 44 | 0.93 | (0.66,1.32) | 0.96 | (0.67,1.39) |
| Unknown | 111 | 19 | 1.21 | (0.74,1.97) | 1.26 | (0.76,2.11) |
All analyses were stratified by medical institution.
∗p < 0.05, ∗∗p < 0.01.
Multivariate HRs were adjusted for sex, age, year of diagnosis, BMI, smoking history, alcohol drinking history, physical exercise and stage.
In gastric cancer patients who participated in the study for ≤90 days after diagnosis, the median follow-up period was 6.1 years. During 9620 person-years, there were 603 deaths. The HRs and 95% CIs for mortality according to demographic and lifestyle factors among patients with gastric cancer are shown in Table 4. For males, the multivariate HR for mortality was 1.42 (95% CI = 1.11, 1.81) compared to females. Compared to patients aged 50–59 years, younger patients had a decreased risk of mortality (40–49 years: multivariate HR = 0.55, 95% CI = 0.34, 0.90), and older patients had an increased risk of mortality (70–79 years: multivariate HR = 1.94, 95% CI = 1.53, 2.46; ≥80 years: multivariate HR = 3.50, 95% CI = 2.52, 4.87). Multivariate HR for mortality in patients with a BMI <18.5 kg/m2 was 1.66 (95% CI = 1.34, 2.05) compared to patients with a BMI 18.5–24.9. Compared to patients who had no physical exercise habit, patients who exercised ≥3 times/week had a decreased risk of mortality (multivariate HR = 0.75, 95% CI = 0.61, 0.93).
Table 4.
HRs and 95% CIs for mortality according to demographic and lifestyle factors among patients with gastric cancer in the Biobank Japan Project (n = 1574).
| Person-years | No. of deaths | Univariate model |
Multivariate modela |
|||
|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | |||
| Sex | ||||||
| Male | 6910 | 481 | 1.48 | (1.21,1.81)∗∗∗ | 1.42 | (1.11,1.81)∗∗ |
| Female | 2710 | 122 | 1.00 | 1.00 | ||
| Age range, years | ||||||
| 20–29 | 18 | 1 | 1.35 | (0.19,9.73) | 1.55 | (0.21,11.50) |
| 30–39 | 204 | 5 | 0.64 | (0.26,1.57) | 0.72 | (0.29,1.78) |
| 40–49 | 946 | 20 | 0.53 | (0.33,0.86)∗∗ | 0.55 | (0.34,0.90)∗ |
| 50–59 | 2474 | 109 | 1.00 | 1.00 | ||
| 60–69 | 3235 | 175 | 1.19 | (0.94,1.51) | 1.16 | (0.91,1.48) |
| 70–79 | 2394 | 233 | 2.00 | (1.59,2.52)∗∗∗ | 1.94 | (1.53,2.46)∗∗∗ |
| ≥80 | 348 | 60 | 3.33 | (2.42,4.58)∗∗∗ | 3.50 | (2.52,4.87)∗∗∗ |
| Year of diagnosis | ||||||
| 2003 | 998 | 60 | 1.00 | 1.00 | ||
| 2004 | 1918 | 131 | 1.04 | (0.77,1.42) | 0.93 | (0.68,1.28) |
| 2005 | 2415 | 147 | 0.89 | (0.66,1.21) | 0.89 | (0.64,1.22) |
| 2006 | 2346 | 136 | 0.89 | (0.65,1.21) | 0.80 | (0.58,1.12) |
| 2007 | 1858 | 121 | 0.95 | (0.69,1.30) | 0.89 | (0.64,1.25) |
| 2008 | 85 | 8 | 1.36 | (0.64,2.87) | 1.13 | (0.53,2.41) |
| BMI range, kg/m2 | ||||||
| <18.5 | 1145 | 121 | 1.65 | (1.34,2.02)∗∗∗ | 1.66 | (1.34,2.05)∗∗∗ |
| 18.5–24.9 | 6885 | 398 | 1.00 | 1.00 | ||
| 25–29.9 | 1280 | 62 | 0.82 | (0.63,1.08) | 0.86 | (0.65,1.13) |
| ≥30 | 115 | 3 | 0.43 | (0.14,1.33) | 0.59 | (0.19,1.87) |
| Unknown | 195 | 19 | 1.47 | (0.92,2.35) | 1.21 | (0.75,1.95) |
| Smoking history | ||||||
| Never smoker | 3215 | 184 | 1.00 | 1.00 | ||
| Ever smoker | 6405 | 419 | 1.14 | (0.96,1.36) | 0.96 | (0.78,1.18) |
| Alcohol drinking history | ||||||
| Never drinker | 3563 | 224 | 1.00 | 1.00 | ||
| Ever drinker | 6057 | 379 | 1.06 | (0.90,1.25) | 1.11 | (0.92,1.34) |
| Physical exercise | ||||||
| No habit | 6392 | 430 | 1.00 | 1.00 | ||
| 1–2 times/week | 516 | 17 | 0.56 | (0.35,0.92)∗ | 0.70 | (0.43,1.15) |
| ≥3 times/week | 2105 | 113 | 0.82 | (0.66,1.01) | 0.75 | (0.61,0.93)∗∗ |
| Unknown | 607 | 43 | 1.09 | (0.78,1.52) | 1.08 | (0.77,1.53) |
All analyses were stratified by medical institution.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Multivariate HRs were adjusted for sex, age, year of diagnosis, BMI, smoking history, alcohol drinking history, physical exercise and stage.
Discussion
We have described the distribution of demographic and lifestyle factors among patients with esophageal and gastric cancer in Japan. Patients with ESCC experienced shorter survival due to aging and alcohol drinking. Among patients with gastric cancer, those who were older and/or underweight experienced shorter survival, while those with a physical exercise habit lived longer.
The results of the present study demonstrated a relatively similar age distribution compared to other surveys performed in Japan, although slight differences existed. The 5-year relative survival rate of all patients in this study was higher than that of patients in the Japanese Association of Clinical Cancer Centers. However, patients for whom ≤90 days passed from diagnosis to study entry showed a similar 5-year relative survival rate to that of patients in the Japanese Association of Clinical Cancer Centers (42.4% and 73.0% for esophageal and gastric cancer, respectively).23 It was possible to reduce the bias for the number of years of study registration by including only patients who participated in the study for ≤90 days after diagnosis.
The present survival results for patients with ESCC were consistent with those of previous studies.10, 11, 26 Among ESCC patients in a cohort study in China, alcohol drinkers were more likely to experience poor survival compared to nondrinkers (HR = 1.372, 95% CI = 1.2, 1.6).11 In another Chinese cohort study, patents with esophageal cancer (ESCC or EA) who were ever drinkers also experienced poor survival (HR = 1.22, 95% CI = 1.06, 1.41), and the study demonstrated a dose-response relationship between alcohol consumption and survival.26 However, studies conducted in Western countries showed no significant association between alcohol drinking and mortality in ESCC and EA patients.12, 13, 15 The frequencies of EA and ESCC are similar in Western countries, whereas ESCC is the dominant type of esophageal cancer in East Asian countries.2 Differences in the relative proportions in esophageal cancer types between Asian and Western populations likely contribute to the difference in factors associated with survival.
The present observation that underweight gastric cancer patients experience poor survival is similar to that of several other studies.27, 28 In the Japanese population, lower BMI has been observed to be associated with an increased risk of mortality among gastric cancer patients, with a linear inverse association.27 Some studies have reported that being overweight has no effect on long-term survival among patients with gastric cancer,29, 30 while another study in Japan indicated better prognoses in overweight patients.31 Given these observations, the present findings remain inconsistent. Further studies are required to investigate the association between BMI and long-term survival in gastric cancer. In the present study, gastric cancer patients who had physical exercise habits experienced better survival. However, the results of a previous study conducted in Sweden indicated no significant association between physical exercise and long-term survival in gastric cancer.12 A meta-analysis of seven cohort and nine case-control studies demonstrated that physical exercise is associated with a reduced risk of gastric cancer in the general population.32 As few previous reports have examined physical exercise habits, it is necessary to further assess the association between physical exercise and gastric cancer prognosis.
We observed no significant difference in survival of ESCC and gastric cancer patients between never and ever smokers. Some studies have reported that smoking decreases survival in patients with ESCC10, 11 and gastric cancer,14 but other studies revealed no significant association.26, 33 Although smoking is an established risk factor for both esophageal and gastric cancer in the general population,3, 4 whether or not it influences patient prognosis remains unclear.
A strength of the present cohort study is that it involved prospective observation of a large number of patients who were recruited nationwide in Japan. However, the lack of stage information for many patients might have affected the multivariate analyses.
In conclusion, we found that among Japanese patients with esophageal and gastric cancer, patients were more likely to be male, older, of normal weight, be ever smokers, be ever drinkers, and have no physical exercise habit. The present findings suggest that patients with ESCC experience decreased survival due to alcohol consumption. Gastric cancer patients who are underweight also have a poor prognosis, whereas patients with physical exercise habits have a good prognosis. Further studies are required to clarify the impact of demographic and lifestyle factors on long-term survival for esophageal and gastric cancer in different populations and to confirm the underlying mechanisms of these findings.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Acknowledgements
We express our gratitude to all of the participants in the BioBank Japan Project. We thank all of the medical coordinators of the cooperating hospitals for collecting samples and clinical information, as well as Yasushi Yamashita and staff members of the BioBank Japan Project for administrative support. We also thank Dr. Kumao Toyoshima for his overall supervision of the BioBank Japan Project. This study was supported by funding from the Tailor-Made Medical Treatment with the BBJ Project from Japan Agency for Medical Research and development, AMED (since April 2015), and the Ministry of Education, Culture, Sports, Science, and Technology (from April 2003 to March 2015).
Footnotes
Peer review under the responsibility of The Japan Epidemiological Association.
Appendix. Author list for BioBank Japan Cooperative Hospital Group
Members of medical institutions cooperating in the BioBank Japan Project who coauthored this paper include Rai Shimoyama, Shinichiro Makimoto, Hiromasa Harada and Tomoaki Fujikawa (Tokushukai Hospitals); Shiro Minami, Eiji Uchida and Masao Miyashita (Nippon Medical School); Yoshiaki Kajiyama, Natsumi Tomita and Akihito Nagahara (Juntendo University); Satoshi Asai, Mitsuhiko Moriyama and Yasuo Takahashi (Nihon University); Tomoaki Fujioka and Wataru Obara (Iwate Medical University); Seijiro Mori and Hideki Ito (Tokyo Metropolitan Institute of Gerontology); Satoshi Nagayama and Yoshio Miki (The Cancer Institute Hospital of JFCR); Akihide Masumoto and Akira Yamada (Aso Iizuka Hospital); Yasuko Nishizawa and Ken Kodama (Osaka Medical Center for Cancer and Cardiovascular Diseases); Hiromitsu Ban and Satoshi Murata (Shiga University of Medical Science); Yukihiro Koretsune and Motohiro Hirao (National Hospital Organization, Osaka National Hospital); and Hideo Ogata (Fukujuji Hospital).
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer J Int Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H.Z., Jin G.F., Shen H.B. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31(6):281–286. doi: 10.5732/cjc.011.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamangar F., Chow W.H., Abnet C.C., Dawsey S.M. Environmental causes of esophageal cancer. Gastroenterol Clin N Am. 2009;38(1):27–57. doi: 10.1016/j.gtc.2009.01.004. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladeiras-Lopes R., Pereira A.K., Nogueira A. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control CCC. 2008;19(7):689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund and American Institute for Cancer Research . American Institute for Cancer Research; Washington (DC): 2007. Food N, Physical Activity, and the Prevention of Cancer: a Global Perspective; p. 265. [Google Scholar]
- 6.Group HaCC Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasazuki S., Inoue M., Iwasaki M. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case-control study. Cancer Epidemiol Biomark Prev: A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(7):1341–1347. doi: 10.1158/1055-9965.EPI-05-0901. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54(suppl 1):i1–i5. doi: 10.1136/gut.2004.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagergren J., Bergstrom R., Lindgren A., Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl J Med. 1999;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S.S., Yang H., Luo K.J. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer. 2013;109(11):2894–2903. doi: 10.1038/bjc.2013.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y., Cao X., Wen J. Smoking affects treatment outcome in patients with resected esophageal squamous cell carcinoma who received chemotherapy. PloS One. 2015;10(4):e0123246. doi: 10.1371/journal.pone.0123246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundelof M., Lagergren J., Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer (Oxford, England: 1990) 2008;44(11):1566–1571. doi: 10.1016/j.ejca.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Trivers K.F., De Roos A.J., Gammon M.D. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol: The Official Clinical Practice Journal of the American Gastroenterological Association. 2005;3(3):225–230. doi: 10.1016/s1542-3565(04)00613-5. [DOI] [PubMed] [Google Scholar]
- 14.Han M.A., Kim Y.W., Choi I.J. Association of smoking history with cancer recurrence and survival in stage III-IV male gastric cancer patients. Cancer Epidemiol Biomark Prev: A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(10):1805–1812. doi: 10.1158/1055-9965.EPI-13-0385. [DOI] [PubMed] [Google Scholar]
- 15.Ferronha I., Castro C., Carreira H. Prediagnosis lifestyle exposures and survival of gastric cancer patients: a cohort study from Portugal. Br J Cancer. 2012;107(3):537–543. doi: 10.1038/bjc.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization for Economic Cooperation and Development . 2014. OECD Health Statistics. [Google Scholar]
- 17.Nagai A., Hirata M., Kamatani Y. Overview of the BioBank Japan project: study design and profile. J Epidemiol. 2017;27:S2–S8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirata M., Kamatani Y., Nagai A. Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol. 2017;27:S9–S21. doi: 10.1016/j.je.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirata M., Nagai A., Kamatani Y. Overview of BioBank Japan follow-up data in 32 diseases. J Epidemiol. 2017;27:S22–S28. doi: 10.1016/j.je.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vital Statistics [homepage on the Internet]. Ministry of Health, Labour and Welfare, Japan; Accessed August 5 2016. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei14/dl/10_h6.pdf [in Japanese].
- 21.Cohort Life Table [homepage on the Internet]. Cancer Registry and Statistics, Cancer Information Service, National Cancer Center, Japan; Accessed August 5 2016. Available from: http://ganjoho.jp/reg_stat/statistics/qa_words/cohort01.html [in Japanese].
- 22.Abridged Life Tables for Japan [homepage on the Internet]. Ministry of Health, Labour and Welfare, Japan; Accessed August 5 2016. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/seimei/list54-57-02.html [in Japanese].
- 23.Survival statistics of Japanese association of Clinical Cancer Centers, Cancer survival rates at Japanese Association of Clinical Cancer Centers [homepage on the Internet]. Japanese association of Clinical Cancer Centers; Accessed August 5 2016. Available from: http://www.gunma-cc.jp/sarukihan/seizonritu/seizonritu2007.html [in Japanese].
- 24.Hori M., Matsuda T., Shibata A., Katanoda K., Sobue T., Nishimoto H. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45(9):884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 25.Ministry of Health, Labour and Welfare . 2005. Patient Survey. [Google Scholar]
- 26.Huang Q., Luo K., Yang H. Impact of alcohol consumption on survival in patients with esophageal carcinoma: a large cohort with long-term follow-up. Cancer Sci. 2014;105(12):1638–1646. doi: 10.1111/cas.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minami Y., Kawai M., Fujiya T. Family history, body mass index and survival in Japanese patients with stomach cancer: a prospective study. Int J Cancer J Int Cancer. 2015;136(2):411–424. doi: 10.1002/ijc.29001. [DOI] [PubMed] [Google Scholar]
- 28.Nozoe T., Kohno M., Iguchi T. Analysis of the impact of the body mass index in patients with gastric carcinoma. Surg Today. 2012;42(10):945–949. doi: 10.1007/s00595-012-0183-z. [DOI] [PubMed] [Google Scholar]
- 29.Oh S.J., Hyung W.J., Li C. Effect of being overweight on postoperative morbidity and long-term surgical outcomes in proximal gastric carcinoma. J Gastroenterol Hepatol. 2009;24(3):475–479. doi: 10.1111/j.1440-1746.2008.05704.x. [DOI] [PubMed] [Google Scholar]
- 30.Ojima T., Iwahashi M., Nakamori M. Influence of overweight on patients with gastric cancer after undergoing curative gastrectomy: an analysis of 689 consecutive cases managed by a single center. Arch Surg (Chicago, Ill: 1960) 2009;144(4):351–358. doi: 10.1001/archsurg.2009.20. discussion 8. [DOI] [PubMed] [Google Scholar]
- 31.Tokunaga M., Hiki N., Fukunaga T., Ohyama S., Yamaguchi T., Nakajima T. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol. 2009;16(12):3245–3251. doi: 10.1245/s10434-009-0645-8. [DOI] [PubMed] [Google Scholar]
- 32.Singh S., Edakkanambeth Varayil J., Devanna S., Murad M.H., Iyer P.G. Physical activity is associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Cancer Prev Res (Philadelphia, Pa) 2014;7(1):12–22. doi: 10.1158/1940-6207.CAPR-13-0282. [DOI] [PubMed] [Google Scholar]
- 33.Ferronha I., Bastos A., Lunet N. Prediagnosis lifestyle exposures and survival of patients with gastric cancer: systematic review and meta-analysis. Eur J Cancer Prev: The Official Journal of the European Cancer Prevention Organisation (ECP) 2012;21(5):449–452. doi: 10.1097/CEJ.0b013e32834fdb1b. [DOI] [PubMed] [Google Scholar]