Abstract
Background
Prostate cancer is the sixth leading cause of cancer-related deaths in Japan. We aimed to elucidate the clinical and histopathological characteristics of patients with prostate cancer in the BioBank Japan (BBJ) project.
Methods
Four thousand, seven hundred and ninety-three patients diagnosed with prostate cancer in the BBJ project were included. Clinical and histopathological data, including causes of death, were analyzed. Relative survival (RS) rates of prostate cancer were calculated.
Results
Four thousand, one hundred and seventy-one prostate cancer patients with available histological data had adenocarcinoma. The mean age of the patients was 72.5 years. The proportion of patients who were non-smokers, non-drinkers, had a normal body mass index, did not exercise, had a normal prostate-specific antigen level, and had a family history of prostate cancer were 30.7%, 28.0%, 66.6%, 58.1%, 67.6%, and 6.5%, respectively. The proportion of patients with Stage II, III, and IV disease were 24.4%, 7.3%, and 4.4%, respectively. After limiting to patients with a time from the initial diagnosis of prostate cancer to entry into the study cohort of ≤90 days (n = 869), the 5- and 10-year RS rates were 96.3% and 100.5%, respectively, although we were unable to consider management strategies due to a plenty of data missing.
Conclusions
We provide an overview of patients with prostate cancer in the BBJ project. Our findings, coupled with those from various high throughput “omics” technologies, will contribute to the implementation of prevention interventions and medical management of prostate cancer patients.
Keywords: BioBank Japan project, Lifestyle, Prostate cancer, Survival, Tumor biomarker
Highlights
-
•
Prostate cancer represents the second leading cause of cancer incidence worldwide.
-
•
We aimed to provide an overview of patients with prostate cancer.
-
•
Based on prostate cancer histology, 99.3% had adenocarcinoma.
-
•
The 5- and 10-year relative survival rates were 96.3% and 100.5%.
-
•
Future studies will help develop preventive programs for prostate cancer.
Introduction
Prostate cancer represents the second and fourth leading cause of cancer incidence and mortality among men worldwide (1.1 M cases were recorded in 2012, resulting in 307,000 deaths).1 Prostate cancer represents the third and sixth leading cause of cancer incidence and mortality in Japan with 73,145 men diagnosed in 20122 and 11,507 prostate-cancer related deaths recorded in 2014.3 The 10-year relative survival (RS) rate of patients with localized versus metastatic disease was 99.5% versus 19.0%, respectively.4 Although prostate cancers most frequently arise in the periphery of the prostate gland, no symptom has been identified that can distinguish prostate cancer from benign prostatic hypertrophy.5 Only once the tumor has grown to compress the urethra or invade the sphincter or neurovascular bundle are symptoms apparent.6 The risk factors for prostate cancer are still largely undetermined, except for an older age and a family history of the disease.7
The BioBank Japan (BBJ) project comprises a large cohort of DNA and serum samples from approximately 200,000 patients with 47 diseases and samples. It is anticipated that the patients' clinical and histopathological information will be used in future studies. Herein, we aim to provide an overview of the patients with prostate cancer in the BBJ project.
Materials and methods
Study population
The BBJ project was launched from June 2003 to March 2008 and has been described in detail elsewhere.8, 9, 10, 11, 12 Briefly, 199,982 patients with 47 diseases were enrolled from 12 medical institutes consisting of 67 hospitals in Japan. Participants in this study were restricted to patients with an initial diagnosis of prostate cancer (n = 4793; 2.4%).
All participants provided informed written consent. The study protocol of the BBJ project was approved by the appropriate Research Ethics Committees of The Institute of Medical Science, The University of Tokyo (Tokyo, Japan), The RIKEN Yokohama Institute (Yokohama, Japan), and 12 cooperating medical institutions. Research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Data collection
Baseline clinical information was obtained from interviews using a standardized questionnaire and medical records.9, 10, 11, 12 Patient interviews included questions concerning smoking and alcohol habits, height, weight, exercise frequency, and a family history of prostate cancer. Data collected from medical records included information concerning year of birth, time from the initial diagnosis of prostate cancer to entry into the study cohort, age at the time of entry into the study cohort, histopathological features, and laboratory examinations (e.g., chemical blood biomarkers, including prostate-specific antigen [PSA] levels). Prostate cancer histology was defined according to tissue obtained at biopsy or cytological samples with unavailable data on histological type complemented by cytological samples. The clinical stage of the prostate cancer was classified according to the Japanese Classification of Prostate Cancer, third edition (2001). Pathological staging was used primarily. However, where pathological staging information was unavailable, this was complemented by clinical staging data.
Follow-up
A follow-up survival survey was implemented from 2010 to 2014. Patients' vital status and mortality data were coded according to the International Classification of Disease, tenth revision.
Statistical analyses
First, we described the distribution of time from the initial diagnosis of prostate cancer to entry into the study cohort. Second, we calculated the age-specific distribution of prostate cancer patients in the BBJ project and compared this to the Japanese Ministry of Health, Labor, and Welfare Patient Survey of 2005.13 Third, the patients' clinical and histopathological characteristics were analyzed. These included smoking (non-smoker, ex-smoker, current smoker [<20.0 or ≥20.0 pack years or unknown], or unknown) and alcohol status (non-drinker, ex-drinker, current drinker [<15.0, 15.0–29.0, or ≥30.0 g/day or unknown], or unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, or ≥30.0 kg/m2 or unknown), exercise frequency (0, 1–2, or ≥3 times/week or unknown), and a family history of prostate cancer. Serum PSA levels (<4.0, 4–9, or ≥10.0 U/mL unknown) were recorded and the clinical stage (Stage I–IV or unknown) and treatment modality (surgery, radiation therapy, or chemotherapy) were also described. Body mass index was calculated as the weight in kilograms divided by the square of the height in meters. Next, the causes of death of patients with prostate cancer were investigated. Additionally, we calculated the cumulative survival (CS) rates and RS rates of prostate cancer patients stratified according to clinical stage. To calculate survival rates, we limited our analysis to patients with a time from the initial diagnosis of prostate cancer to entry into the study cohort of ≤90 days. CS rates were determined using the Kaplan–Meier method. A reference table of survival rate data was obtained from the Cancer Registry and Statistics, Cancer Information Service, National Cancer Center, Japan14 to calculate expected survival rates. The table was based on age-specific mortality rates and Gompertz-Makeham's law in Abridged Life Tables for Japan,15 published annually by the Statistics and Information Department of The Ministry of Health, Labor, and Welfare. RS rates were calculated by dividing CS rates by the age-adjusted expected survival rates. Patients ≥100 years of age were excluded owing to a lack of data in the reference table.15 All statistical analyses were conducted using SAS software for Windows, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
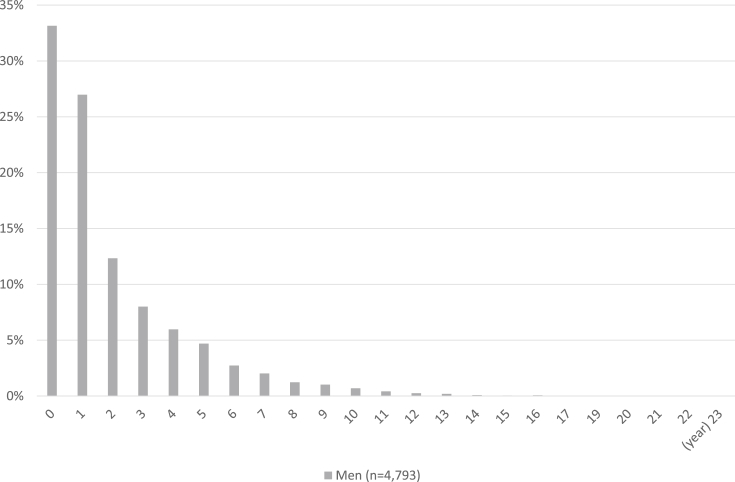
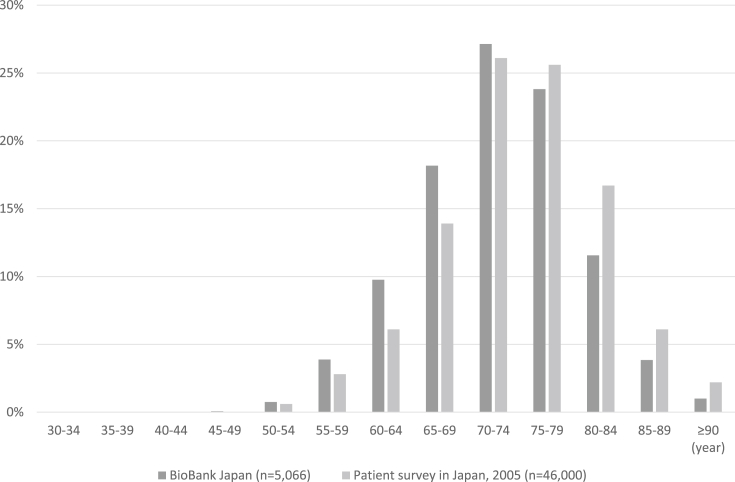
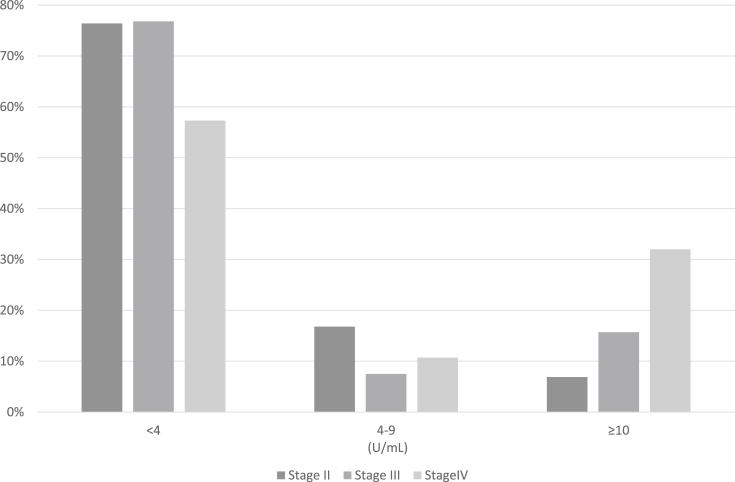
In this study, 4171 (99.3%) of 4202 prostate cancer patients with histological data available had adenocarcinoma. The distribution of time from the initial diagnosis of prostate cancer to entry into the study cohort is displayed in Fig. 1. The proportion of patients who enrolled <1, 1, 2, and ≥3 years after the initial diagnosis of prostate cancer were 33.2%, 27.0%, 12.3%, and 8.0%, respectively. The age-specific distribution of prostate cancer patients is displayed in Fig. 2. The mean age of the patients was 72.5 (range, 42–97) years. The clinical and histopathological characteristics of patients with prostate cancer in the BBJ project are summarized in Table 1. The proportion of patients who were non-smokers, non-drinkers, had a normal body mass index of 18.5–24.9 kg/m2, did not exercise, had a normal PSA level of <4.0 U/mL, and had a family history of prostate cancer were 30.7%, 28.0%, 66.6%, 58.1%, 67.6%, and 6.5%, respectively. The proportion of patients with Stage I, II, III, and IV disease were 0.0%, 24.4%, 7.3%, and 4.4%, respectively. For 63.9% of patients' information on the clinical stage of disease was unavailable. The proportion of patients who had undergone surgery for Stage II, III, and IV disease were 45.8%, 37.7%, and 16.3%, respectively. In addition, the proportion of patients who had undergone radiation therapy for Stage II, III, and IV disease were 24.0%, 36.6%, and 23.5%, respectively. Moreover, the proportion of patients who had undergone chemotherapy for Stage II, III, and IV disease were 2.2%, 6.0%, and 6.2%, respectively (Table 1). The stage-specific distribution of prostate cancer patients stratified according to PSA levels (<4.0, 4.0–9.0, or ≥10.0 U/mL) is displayed in Fig. 3. The proportion of Stage II, III, and IV patients with PSA levels of <4.0 and ≥10.0 U/mL were 76.4%, 76.8%, and 57.3% and 6.9%, 15.7%, and 32.0%, respectively.
Fig. 1.
Distribution of time from the initial diagnosis of prostate cancer to entry into the study cohort.
Fig. 2.
Age-specific distribution of prostate cancer patients in the BioBank Japan project.
Table 1.
Clinical and histopathological characteristics of study participants with prostate cancer (n = 4793) in the BioBank Japan project.
| Characteristic | Patients (n = 4793) |
|---|---|
| Smoking status, n (%) | |
| Non-smoker | 1471 (30.7) |
| Ex-smoker | 2175 (45.4) |
| Smoker | |
| <20.0 pack years | 136 (2.8) |
| ≥20.0 pack years | 574 (12.0) |
| Unknown | 289 (6.0) |
| Unknown | 148 (3.1) |
| Drinking status, n (%) | |
| Non-drinker | 1344 (28.0) |
| Ex-drinker | 606 (12.6) |
| Drinker | |
| <15.0 g/day | 1034 (21.6) |
| 15.0–30.0 g/day | 609 (12.7) |
| ≥30.0 g/day | 749 (15.6) |
| Unknown | 292 (6.1) |
| Unknown | 160 (3.3) |
| BMI, n (%) | |
| <18.5 kg/m2 | 214 (4.5) |
| 18.5–24.9 kg/m2 | 3191 (66.6) |
| 25.0–29.9 kg/m2 | 1093 (22.8) |
| ≥30.0 kg/m2 | 75 (1.6) |
| Unknown | 220 (4.6) |
| Physical exercise, n (%) | |
| None | 2784 (58.1) |
| 1–2 times/week | 193 (4.0) |
| ≥3 times/week | 1105 (23.1) |
| Unknown | 711 (14.8) |
| Family history of prostate cancer, n (%) | |
| Yes | 310 (6.5) |
| No/unknown | 4483 (93.5) |
| PSA level, n (%) | |
| <4.0 U/mL | 3241 (67.6) |
| 4.0–9.0 U/mL | 635 (13.2) |
| ≥10.0 U/mL | 761 (15.9) |
| Unknown | 156 (3.3) |
| Stage, n (%) | |
| I | 0 (0) |
| II | 1170 (24.4) |
| III | 350 (7.3) |
| IV | 209 (4.4) |
| Unknown | 3064 (63.9) |
| Surgery, n (%) | |
| Stage II | |
| Yes | 536 (45.8) |
| No | 166 (14.2) |
| Unknown | 468 (40.0) |
| Stage III | |
| Yes | 132 (37.7) |
| No | 53 (15.1) |
| Unknown | 165 (47.1) |
| Stage IV | |
| Yes | 34 (16.3) |
| No | 20 (9.6) |
| Unknown | 155 (74.2) |
| Radiation therapy, n (%) | |
| Stage II | |
| Yes | 281 (24.0) |
| No | 160 (13.7) |
| Unknown | 729 (62.3) |
| Stage III | |
| Yes | 128 (36.6) |
| No | 31 (8.9) |
| Unknown | 191 (54.6) |
| Stage IV | |
| Yes | 48 (23.5) |
| No | 17 (8.1) |
| Unknown | 144 (68.9) |
| Chemotherapy, n (%) | |
| Stage II | |
| Yes | 26 (2.2) |
| No | 254 (21.7) |
| Unknown | 890 (76.1) |
| Stage III | |
| Yes | 21 (6.0) |
| No | 66 (18.9) |
| Unknown | 263 (75.1) |
| Stage IV | |
| Yes | 13 (6.2) |
| No | 24 (11.5) |
| Unknown | 172 (82.3) |
Abbreviations: BMI, body mass index; PSA, prostate-specific antigen.
Fig. 3.
Stage-specific distribution of prostate cancer patients in the BioBank Japan project stratified according to prostate-specific antigen levels (<4.0, 4.0–9.0, or ≥10.0 U/mL).
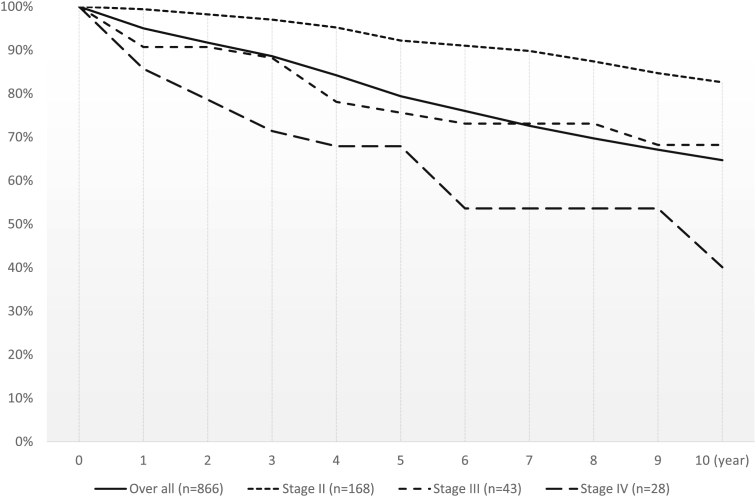
The number of cases per person-year of follow-up among all patients and the subgroup with a time from the initial diagnosis of prostate cancer to entry into the study cohort of ≤90 days were 4424 and 869, respectively. The mortality rate during the study period was approximately 36.9%. The cancer-specific mortality rate was approximately 20.6%. The CS rates of prostate cancer patients are presented in Fig. 4. The 3-, 5-, and 10-year CS rates were 88.6%, 79.7%, and 64.7%, respectively. The 5- and 10-year RS rates of all patients were 96.3% and 100.5%, respectively. The 5- and 10-year RS rates of patients with Stage II, III, and IV disease were 105.6% and 115.6%, 88.1% and 102.5%, and 79.9% and 60.5%, respectively.
Fig. 4.
Cumulative survival and relative survival rates of prostate cancer patients in the BioBank Japan project who enrolled ≤90 days after the initial diagnosis.
Discussion
In this study, we provide an overview of patients with prostate cancer in the BBJ (a large patient-based) project.
PSA levels are a useful biomarker for risk classification in patients with prostate cancer and patients with localized versus metastatic prostate cancer.16 Although measurements of PSA levels were not necessarily obtained at initial diagnosis, our findings suggest that prostate cancer patients with a higher PSA level have a more advanced clinical stage and this was consistent with a previous study. However, a recent Japanese report17 demonstrated that prostate cancer patients with a PSA level of <3.5 ng/mL at initial diagnosis had a more advanced clinical stage than prostate cancer patients with a PSA level of 3.5–10.0 ng/mL at initial diagnosis. Thus, further epidemiological studies are required to determine the risk classification of prostate cancer patients based on PSA levels at initial diagnosis.
Established risk factors for prostate cancer include an older age and a family history of the disease.7 The life expectancy at birth among Japanese men in 2013 was 80.2 years, one of the highest in the world. Moreover, the incidence and mortality rates of prostate cancer were considerably lower in Japan (22.7 and 5.0 cases/105 person-year, respectively) than in Western countries (North America, 85.7 and 9.9; Europe, 61.4 and 12.1; and Africa, 17.5 and 12.5 cases/105 person-year, respectively).18 Additionally, the proportion of patients with a family history of prostate cancer was just 4.4%. In a review article,19 the authors concluded that having a germline pathogenic variant in the breast cancer susceptibility genes BRCA1 or BRCA2 increased the risk of prostate cancer to 39.0%. Furthermore, a genome-wide association study20 identified 76 variants associated with a higher risk of developing prostate cancer, predominantly in populations of European descent compared to controls from studies in populations of European, African, Japanese, and Latin American descent. Since the association between lifestyle factors and prostate cancer risk is still unclear,21, 22, 23, 24, 25, 26, 27 to elucidate the pathogenesis of prostate cancer, the identification of genetic variants associated with prostate cancer risk among Japanese men is important.
Data from the Japanese Association of Clinical Cancer Centers28 indicated that the 5- and 10-year RS rates of prostate cancer patients was 100% and 84.4%, respectively. In our study, the 10-year RS rate of prostate cancer patients in the BBJ project was considerably higher than this. The registry also reported on the 5- and 10-year RS rates of prostate cancer patients stratified according to clinical stage. The 5- and 10-year RS rates of prostate cancer patients with Stage I, II, III, and IV disease were 100% and 93.0%, 100% and 100%, 100% and 96.0%, and 62.0% and 38.0%, respectively.28 Our findings were largely comparable to these except for the 5-year RS rate of prostate cancer patients with Stage II disease. However, since the number of patients we evaluated with Stage II disease was relatively small, we were unable to determine whether this difference had occurred by chance or was due to variations in prostate cancer prognosis between the two populations. Furthermore, management strategies (e.g., watchful waiting,29 radical prostatectomy,30 radiation therapy,31 and hormone therapy32) were chosen by the physician, according to the presence or absence of cancer metastases. Due to lack of continuity in medical record keeping in Japan, patients who attended different hospitals for treatment and follow-up after therapy were likely to have some missing information. Therefore, this is a limitation of our study. A major strength of this study is its large number of patients recruited from hospitals nationwide in Japan. This allowed us to show prevalent prostate cancer patients' characteristics as well as newly diagnosed patients' survival possibility. However, we have to pay caution that many values were missing in our manuscript.
In conclusion, we provide an overview of patients with prostate cancer in the BBJ project. We believe that our findings, coupled with those from various high throughout “omics” technologies using patient DNA and serum samples, will contribute to the implementation of prevention interventions and medical management for prostate cancer, leading to more personalized healthcare in the future.
Conflicts of interest
The authors declare they have no conflict of interest with respect to this research study and paper.
Acknowledgements
We express our gratitude to all the participants in the BioBank Japan Project. We thank all the medical coordinators of the cooperating hospitals for collecting samples and clinical information, as well as Yasushi Yamashita and staff members of the BioBank Japan Project for administrative support. We also thank Dr. Kumao Toyoshima for his overall supervision of the BioBank Japan project. This study was supported by funding from the Tailor-Made Medical Treatment with the BBJ Project from Japan Agency for Medical Research and development, AMED (since April 2015), and the Ministry of Education, Culture, Sports, Science, and Technology (from April 2003 to March 2015).
Footnotes
Peer review under responsibility of the Japan Epidemiological Association.
Contributor Information
Akiko Tamakoshi, Email: tamaa@med.hokudai.ac.jp.
BioBank Japan Cooperative Hospital Group:
Ichiro Miura, Katsuhiko Takatama, Yoshiyuki Nabeshima, Kazuo Misumi, Shiro Minami, Yukihiro Kondo, Go Kimura, Shigeo Horie, Shinichi Ohba, Shigaku Ikeda, Satoshi Asai, Mitsuhiko Moriyama, Yasuo Takahashi, Tomoaki Fujioka, Wataru Obara, Seijiro Mori, Hideki Ito, Satoshi Nagayama, Yoshio Miki, Akihide Masumoto, Akira Yamada, Yasuko Nishizawa, Ken Kodama, Keisei Okamoto, Susumu Kageyama, Yukihiro Koretsune, Yuko Nishigaki, and Tsutomu Yoshida
Appendix.
Members of medical institutions cooperating on the BioBank Japan Project who coauthored this paper include Ichiro Miura, Katsuhiko Takatama, Yoshiyuki Nabeshima and Kazuo Misumi (Tokushukai Hospitals); Shiro Minami, Yukihiro Kondo and Go Kimura (Nippon Medical School); Shigeo Horie, Shinichi Ohba and Shigaku Ikeda (Juntendo University); Satoshi Asai, Mitsuhiko Moriyama and Yasuo Takahashi (Nihon University); Tomoaki Fujioka and Wataru Obara (Iwate Medical University); Seijiro Mori and Hideki Ito (Tokyo Metropolitan Institute of Gerontology); Satoshi Nagayama and Yoshio Miki (The Cancer Institute Hospital of JFCR); Akihide Masumoto and Akira Yamada (Aso Iizuka Hospital); Yasuko Nishizawa and Ken Kodama (Osaka Medical Center for Cancer and Cardiovascular Diseases); Keisei Okamoto and Susumu Kageyama (Shiga University of Medical Science); Yukihiro Koretsune and Yuko Nishigaki (National Hospital Organization, Osaka National Hospital); and Tsutomu Yoshida (Fukujuji Hospital).
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Hori M., Matsuda T., Shibata A. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. Cancer mortality 1958–2014. http://www.ganjoho.jp/reg_stat/statistics/dl/index.html#mortality Accessed 29 July 16.
- 4.Ries L.A.G., Young J.L., Keel G.E., Eisner M.P., Lin Y.D., Horner M.-J., editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215; Bethesda, MD: 2007. http://www.seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf Available at: Accessed 3 August 16. [Google Scholar]
- 5.Hamilton W., Sharp D. Symptomatic diagnosis of prostate cancer in primary care: a structured review. Br J Gen Pract. 2004;54:617–621. [PMC free article] [PubMed] [Google Scholar]
- 6.Guess H.A. Benign prostatic hyperplasia and prostate cancer. Epidemiol Rev. 2001;23:152–158. doi: 10.1093/oxfordjournals.epirev.a000782. [DOI] [PubMed] [Google Scholar]
- 7.Brawley O.W. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y. The BioBank Japan project. Clin Adv Hematol Oncol. 2007;5:696–697. [PubMed] [Google Scholar]
- 9.Shinryo jyouhou nyuryoku sheet Ver.7.1.4. https://www.biobankjp.org/sample/pdf/list7.1.4.pdf Accessed 26 July 16.
- 10.Nagai A., Hirata M., Hirata M., Kamatani Y. Overview of the BioBank Japan project: study design and profile. J Epidemiol. 2017;27:S2–S8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata M., Nagai A., Kamatani Y. Cross-sectional analysis of BioBank Japan Clinical Data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol. 2017;27:S9–S21. doi: 10.1016/j.je.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata M., Nagai A., Kamatani Y. Overview of BioBank Japan follow-up data in 32 diseases. J Epidemiol. 2017;27:S22–S28. doi: 10.1016/j.je.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health, Labor, and Welfare, Japan Abridged life tables for Japan. Patient Surv. 2005 http://www.mhlw.go.jp/toukei/saikin/hw/kanja/05/ Accessed 26 July 16. [Google Scholar]
- 14.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. Cohort Life Table. http://www.ganjoho.jp/reg_stat/statistics/qa_words/cohort01.html Accessed 26 July 16.
- 15.Ministry of Health, Labor, and Welfare, Japan. Abridged Life Tables for Japan. http://www.mhlw.go.jp/toukei/saikin/hw/seimei/list54-57-02.html Accessed 26 July 16.
- 16.Kitagawa Y., Sawada K., Urata S. Impact of PSA levels on second-round screening for the development of prostate cancer in men with low baseline PSA levels (≤2.0 mg/ml) Anticancer Res. 2014;34:6739–6746. [PubMed] [Google Scholar]
- 17.Izumi K., Ikeda H., Maolake A. The relationship between prostate-specific antigen and TNM classification or Gleason score in prostate cancer patients with low prostate-specific antigen levels. Prostate. 2015;75:1034–1042. doi: 10.1002/pros.22985. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Yang B.X., Zhang H.T., Wang J.G., Wang H.L., Zhao X.J. Prostate cancer: an emerging threat to the health of aging men in Asia. Asian J Androl. 2011;13:574–578. doi: 10.1038/aja.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrucelli N., Daly M.B., Feldman G.L. BRCA1 and BRCA2 hereditary breast and ovarian cancer. In: Pagon R.A., Adam M.P., Ardinger H.H., editors. GeneReviews® [Internet] University of Washington, Seattle; Seattle (WA): 1998 Sep 4. pp. 1993–2016.http://www.ncbi.nlm.nih.gov/books/NBK1247/ [Updated 2013 Sep 26] Available from: Accessed 28 July 16. [PubMed] [Google Scholar]
- 20.Al Olama A.A., Kote-Jarai Z., Berndt S.I. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46:1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szymanski K.M., Wheeler D.C., Mucci L.A. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010;92:1223–1233. doi: 10.3945/ajcn.2010.29530. [DOI] [PubMed] [Google Scholar]
- 22.Lovegrove C., Ahmed K., Challacombe B., Khan M.S., Popert R., Dasgupta P. Systematic review of prostate cancer risk and association with consumption of fish and fish-oils: analysis of 495,321 participants. Int J Clin Pract. 2015;69:87–105. doi: 10.1111/ijcp.12514. [DOI] [PubMed] [Google Scholar]
- 23.Park C.H., Myung S.K., Kim T.Y., Seo H.G., Jeon Y.J., Kim Y. Coffee consumption and risk of prostate cancer: a meta-analysis of epidemiological studies. BJU Int. 2010;106:762–769. doi: 10.1111/j.1464-410X.2010.09493.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu X., Bao Z., Zou J., Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96. doi: 10.1186/1471-2407-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J., Yang B., Huang T., Yu Y., Yang J., Li D. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutr Cancer. 2011;63:663–672. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 26.Aune D., Navarro Rosenblatt D.A., Chan D.S. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 27.Xu C., Han F.F., Zeng X.T., Liu T.Z., Li S., Gao Z.Y. Fat intake is not linked to prostate cancer: a systematic review and dose-response meta-analysis. PLoS One. 2015;10:e0131747. doi: 10.1371/journal.pone.0131747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Japanese Association of Clinical Cancer Centers. Five and ten year relative survival rate in all cases in 2004–2007. http://www.gunma-cc.jp/sarukihan/seizonritu/seizonritu2007.html#10 Accessed 26 July 16.
- 29.Froehner M. Age and prostate cancer survival. JAMA. 2010;303:33–34. doi: 10.1001/jama.2009.1933. author reply 4. [DOI] [PubMed] [Google Scholar]
- 30.Vickers A.J., Bianco F.J., Serio A.M. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 31.Coates J., Souhami L., El Naqa I. Big data analytics for prostate radiotherapy. Front Oncol. 2016;6:149. doi: 10.3389/fonc.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen T.M., Pastuszak A.W. Testosterone therapy among prostate cancer survivors. Sex Med Rev. 2016 doi: 10.1016/j.sxmr.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]