Abstract
Isocyanide-based multicomponent reactions (IMCR) are by far the most versatile reactions that can construct relatively complex molecules by one-pot synthesis. More importantly, the development of post IMCR modifications significantly improves the scaffold’s diversity. Here, we describe the use of N-Boc protected hydrazine together with α-amino acid derived isocyanides in the Ugi tetrazole reaction and its post cyclization under both acidic and basic conditions. The cyclization in acidic conditions was conducted in a one pot fashion, which give 7-aminotetrazolopyrazinone (6) and tetrazolotriazepinone (7) cyclic products. The post cyclization under basic condition could selectively afford Boc-protected 7-aminotetrazolopyrazinone (8) products in yield of 38–87%.
Keywords: isocyanide-based multicomponent reactions, hydrazine, post cyclization, tetrazole, one-pot synthesis, scaffolds diversity, European Lead Factory
Introduction
Small molecules have always been of great interest in medicinal chemistry and chemical genetics. They can provide oral leads for drug discovery while maintaining favorable cost-of-goods or can function as probes to illuminate the mechanism of the biological processes in the living systems.1−4 Such small molecule modulators are most commonly identified by high throughput screening of large compound collections. The European Lead Factory (ELF) supported by the Innovative Medicines Initiative (IMI) is aiming to bring together 500 000 high-quality compounds library from both academia and industry as the starting point for innovative drug discovery.5 A general consensus has emerged that library diversity, instead of library size, is a crucial consideration. Multicomponent reaction (MCR) chemistry is playing an important part in generating highly diverse compound libraries as it is capable of assembling novel molecular scaffolds and generating complex functionalization motifs in just a few steps on a short time scale.6−8 Furthermore, the exploitation of MCR-product postcyclization has also become a very popular strategy to generate complex skeletons.9−13
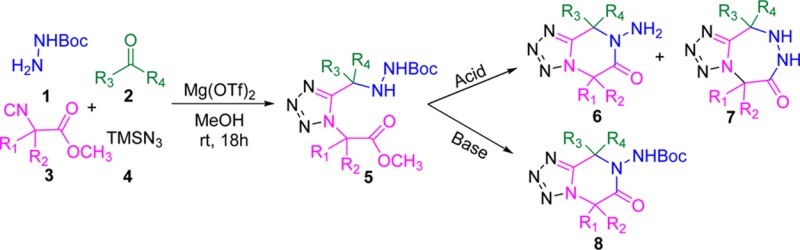
Ugi/de-Boc/cyclize (UDC) chemistry has been successfully used in Hulme group.14 Recently, the application of the mono-Boc protected hydrazine in the Ugi-tetrazole synthesis has been first explored in our lab.15 Herein, we show how α-amino acid derived isocyanides together with hydrazine can be used to obtain bicyclic scaffolds. We envisioned that both 6-membered (6) and 7-membered (7) products could be formed under the acidic conditions. Optimization of the protocol, with a particular emphasis to selective obtainment of 6-membered rings, as well as an application of the resulting compounds for further diversification, are described.
Results and Discussion
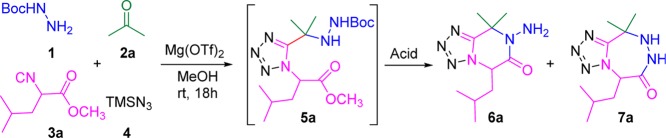
The reaction of Boc-hydrazine 1 with acetone 2a, isocyanide 3a, and trimethylsilyl azide 4 was performed in methanol at ambient temperature for 18 h which afforded a moderate yield of 5a (45%) (Scheme 1). It is well-known that Lewis acids could help the activation of the Schiff base and its formation which can be the rate-limiting step in the Ugi-4CR.16 Hence, we initiated our work by screening various Lewis acids such as CF3SO3Li, CF3SO3Ag, CF3SO3K, (CF3SO3)2Mg, (CF3SO3)2Zn, ZnCl2, SnCl2, (CF3SO3)3In, and (CF3SO3)3Al as the catalyst in the Ugi-4CR reaction (Table 1).
Scheme 1. Synthesis of Ugi-Adduct (5a).
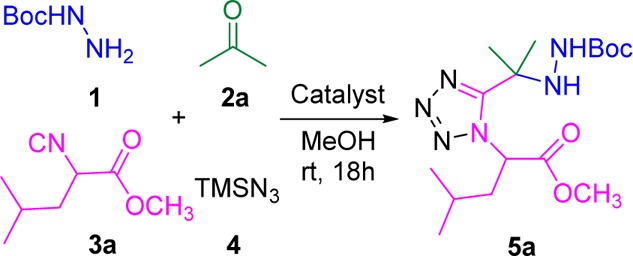
Table 1. Screening of the Lewis Acid Catalysts for Ugi-Tetrazole Adduct.
| entry | catalyst | conc. (equiv) | yield (%)a |
|---|---|---|---|
| 1 | - | - | 45 |
| 2 | ZnCl2 | 0.1 | 72 |
| 3 | SnCl2 | 0.1 | 60 |
| 4 | (CF3SO3)2Zn | 0.1 | 62 |
| 5 | CF3SO3Ag | 0.1 | 43 |
| 6 | CF3SO3K | 0.1 | 47 |
| 7 | (CF3SO3)2Mg | 0.1 | 75 |
| 8 | CF3SO3Li | 0.1 | 53 |
| 9 | (CF3SO3)3In | 0.1 | 38 |
| 10 | (CF3SO3)3Al | 0.1 | 47 |
Isolated yield.
We found that divalent Lewis acid catalysts afforded high yields, (CF3SO3)2Mg was found to be the best catalysts affording 75% yield of 5a. ZnCl2 gave a similar good yield of 72%. In addition, (CF3SO3)2Zn and SnCl2 resulted in slightly lower yields (62% and 60%). In contrast, poorer yields were observed in the reactions catalyzed by other commonly used Lewis acids. Hence, (CF3SO3)2Mg was the Lewis acid catalyst of choice for the subsequent explorations.
With the optimal catalyst for Ugi-4CR in hand, the conditions for postcyclization were further optimized. At first, we tried to cyclize the Ugi-adduct under acidic conditions in one pot (Scheme 2). We speculated that it is possible to form both 6-membered (6) and 7-membered (7) cyclized products as the Boc-protecting group will be removed in acidic condition. Although the 7-membered product suffered from more strain during the ring formation than the 6-membered product, the exposed primary amine would be also highly interesting for further transformations.
Scheme 2. One-Pot Cyclization of Ugi-Adduct under Acidic Condition.
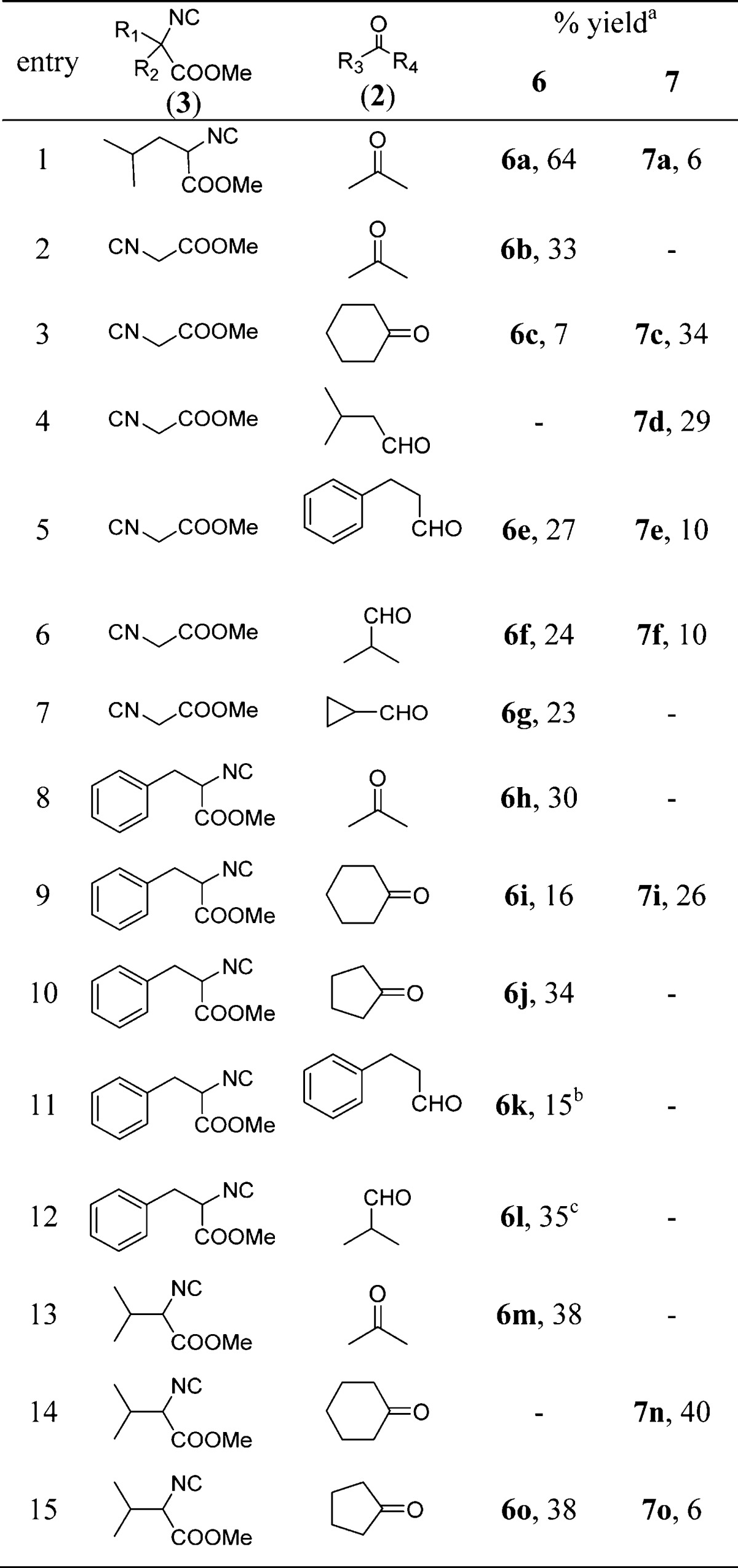
We commenced our one pot postcyclization under acidic conditions using Ugi-adduct 5a. Four different acids: trifluoroacetic acid, acetyl chloride, 6 M aqueous hydrochloric acid, and 2 M methanolic hydrochloric acid were screened (Table 2). Results showed that two equivalents of trifluoroacetic acid afforded poor yields of 6a (10%) and traces of 7a. When 6 M aqueous hydrochloric acid solutions was used as the solvent, a 33% yield of the 6-membered product (6a) was afforded. Interestingly, two equivalent of acetyl chloride in methanol as solvent gave 36% yield of the 6-membered product (6a), as well as little amount of 7-membered product (7a, 5% yield). Surprisingly, when we used 2 M methanolic hydrochloric acid as solvent, the yield of 6-membered (6a) product significantly increased to 64% and minor 7-membered (7a) product (6% yield) was formed. Hence, 2 M methanolic hydrochloric acid was chosen as the acid for postcyclization. Furthermore, we explored the possibility to accomplish the overall 2-step sequence in a one-pot fashion. The unpurified Ugi-adduct obtained was directly treated with 2 M methanolic hydrochloric acid. We were pleased to observe that 43% of yield 6-membered product 6a and 6% yield of 7-membered product 7a could be obtained in the one-pot procedure. After these interesting results, the generality of this one-pot procedure was then explored by varying the isocyanide and oxo-components (Table 3).
Table 2. Screening of Acidic Conditions for Post-cyclization.
| yield
(%)a |
||||
|---|---|---|---|---|
| entry | acid, solvent | amount, time | 6a | 7a |
| 1 | TFA, MeOH | 2 equiv., 18 h | 10 | traces |
| 2 | 6 M aq. HCl | 5 mL, 18 h | 33 | traces |
| 3 | acetyl chloride, MeOH | 2 equiv., 18 h | 36 | 5 |
| 4 | 2 M HCl in MeOH | 5 mL, 4 h | 64 | 6 |
Isolated yield.
Table 3. Typical Structures and Yields of Post-Cyclization Product under Acid Condition.
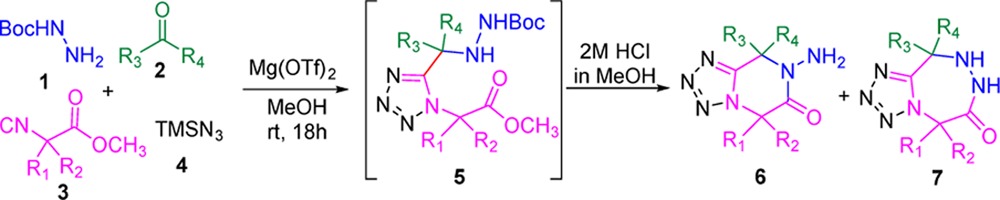
One-pot isolated yields.
dr ratio = 25:1.
dr ratio = 1:1.
Various derivatives of methyl glycine isocyanides along with ketones and aldehydes were synthesized. When we used methyl glycine isocyanides with acetone, 6-memberd ring 6b was obtained in 33% yield. Surprisingly, with cyclohexanone we observed only a minor amount of 6-membered ring 6c (7% yield) along with spiro-7-membered ring 7c (34% yield). A similar trend was observed with isovaleraldehyde, that gave 7-membered ring 7d in 29% yield accompanied by 6d in only trace amount. With hydrocinnamaldehyde (entry 5), we obtained 6e in 27% yield along with 10% yield of 7-memberd ring (7e). Similar results were obtained with isobutyraldehyde (entry 6), in this case we obtained 24% yield of 6f and 10% yield of 7f. In addition, the isocyanide derived from methyl phenylalaninate was used in combination with various oxo components. With cyclopropanecarboxaldehyde (entry 7) and acetone (entry 8) we obtained only 6-membered ring 6g and 6h in 23% and 30% yield, respectively. Cyclopentanone (entry 10), hydrocinnamaldehyde (entry 11) and isobutyraldehyde (entry 12) gave only 6-memberd ring 6j (34% yield), 6k (15% yield) and 6l (35% yield). In case of 6k and 6l, we observed the diastereomeric mixtures in 25:1 and 1:1 ratio, respectively. Cyclohexanone gave 6i (16% yield) and 7i (26% yield). Interestingly, in the final 7-membered product 7i we observed a mixture of two ring-flip conformers in 1:1 ratio in the NMR spectra. A similar phenomenon is often observed in the related 7-membered benzodiazepines based on ring flipping and axial and equatorial orientation of bulky substituents. Furthermore, we tested the isocyanide derived from valine methyl ester with various oxo-components like acetone and cyclohexanone. Surprisingly, we were able to isolate only the 6-membered ring 6m in 38% yield with acetone and exclusively the 7-membered ring 7n in 40% yield with cyclohexanone. With cyclopentanone (entry 15) we obtained 6-membered and 7-membered ring 6o and 7o in 38% and 6% yield, respectively. Although the overall yield of the one-pot reaction under acidic condition is relatively low, we were surprised to find that both 6-membered and 7-membered products were obtained. As we can see from cyclization products 6e and 7e, 6f and 7f, and 6o and 7o, 6-membered product was formed as the major product and the 7-membered product was formed in less than 10% yield. Notably, when cyclohexanone was used as the oxo-component, the 7-membered ring was observed as the major product (7c, 7i, and 7n). In general the yields and selectivity of the reaction under acidic conditions was unsatisfactory. Therefore, we tried to cyclize the Ugi-adduct under basic conditions (Scheme 3, Table 4).
Scheme 3. Cyclization under Basic Conditions.
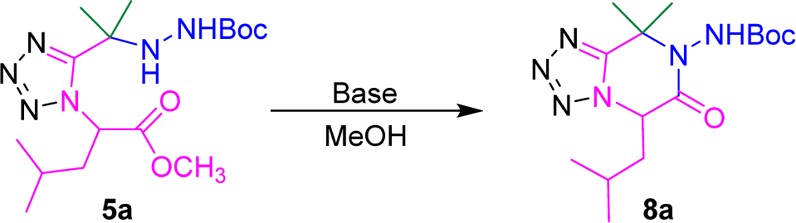
Table 4. Screening of Basic Conditions for Post-cyclization.
| entry | catalyst | conc. (equiv) | time (h) | yield (%)a |
|---|---|---|---|---|
| 1 | DIPEA | 0.1 | 1 | 20 |
| 2 | DIPEA | 0.1 | 12 | 25 |
| 3 | NaOCH3 | 0.1 | 1 | 33 |
| 4 | NaOCH3 | 0.1 | 12 | 40 |
| 5 | NaOCH3 | 1 | 1 | 86 |
| 6 | NaOCH3 | 2 | 1 | 80 |
Isolated yield.
As the Boc-protecting group is generally stable under basic conditions, only the 6-membered products with the Boc-group on are expected to be formed. Initially, two bases (DIPEA and NaOCH3) which are often used in cyclization chemistry were evaluated. It was found that when they were used in catalytic amounts, very poor yield was observed in both cases after 1h. In addition, prolonging the reaction time from 1 to 12 h had little effect either. As NaOCH3 has given higher yield than DIPEA, we further increased the amount of NaOCH3 to one equivalent, which significantly improved the reaction yield to 86%. However, increasing the base amount to two equivalents failed to further improve the reaction yield.
The results of the cyclization under basic condition are summarized in Table 5. We tested different methyl glycine isocyanide derivatives obtained from various amino acids in Ugi-tetrazole reaction with the various oxo-components. To avoid the formations of diastereomeric mixture after the post modification on Ugi-adducts, we selected symmetric ketones as oxo-components. Interestingly, with magnesium triflate as Lewis acid catalyst in the Ugi-tetrazole reaction, good to excellent yields of adducts (5a–5l, 56–90%) was obtained. These pure isolated Ugi-tetrazole adducts were subjected to cyclization with sodium methoxide (1 equiv) in methanol (0.5 M) at room temperature. All Ugi-tetrazole adducts (5a–5l) gave 6-membered cyclized product (8a–8l) in moderate to excellent yields (38–87%). Furthermore, we wanted to develop this reaction sequence in one pot. To test this strategy, we selected the methyl leucine isocyanide with acetone as an oxo-component, mono-Boc hydrazine and TMSN3. We observed the Ugi tetrazole adduct formation was good by SFC-MS and TLC analysis. After the addition of sodium methoxide in the reaction mixture for basic cyclization, we observed multiple product formation. The major side product was the hydrolyzed methyl ester of the Ugi-tetrazole adducts to the corresponding acids; however, 8a was isolated only in 41% yield.
Table 5. Typical Structures and Yields of Post-cyclization Product under Basic Condition.
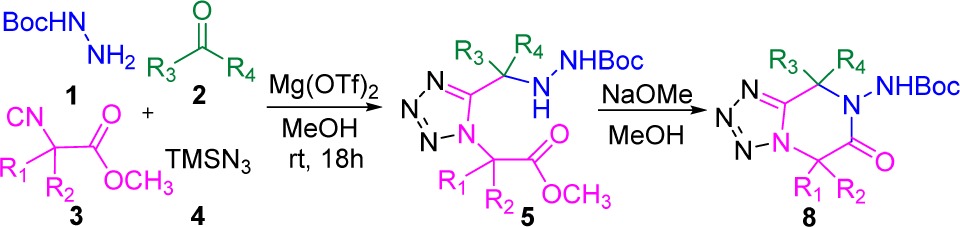
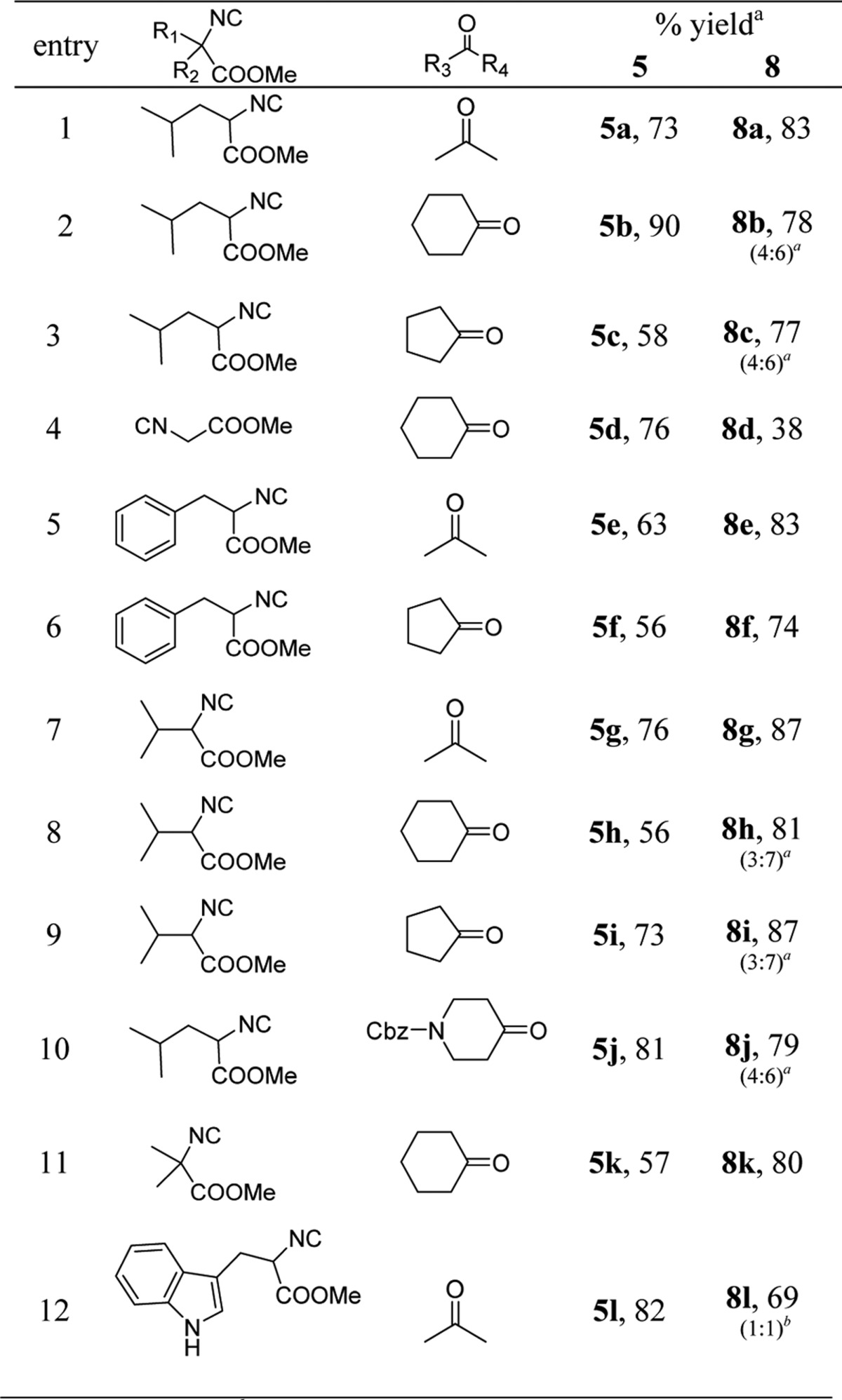
Isolated yields.
N-Boc rotamers.
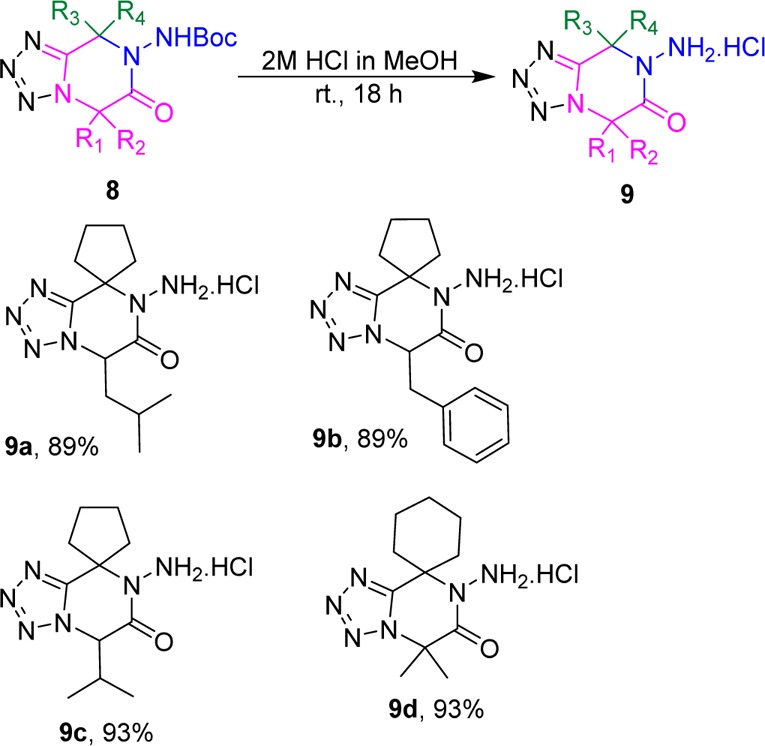
As we observed the one pot postcyclization under acidic condition using Ugi-adduct 5 was poor yielding to compounds 6. We further deprotected the compounds 8 under acidic condition using 2 M methanolic hydrochloric acid at room temperature for 18h (Table 6). We obtained exo-NHBoc deprotected compounds as hydrochloric salts (9) in excellent yields after evaporation of solvents.
Table 6. Deprotection of Compound 8.
To further support structural proof, we were also able to grow crystals of some Ugi-adducts, 6-membered products and 7-membered products (Figure 1).
Figure 1.
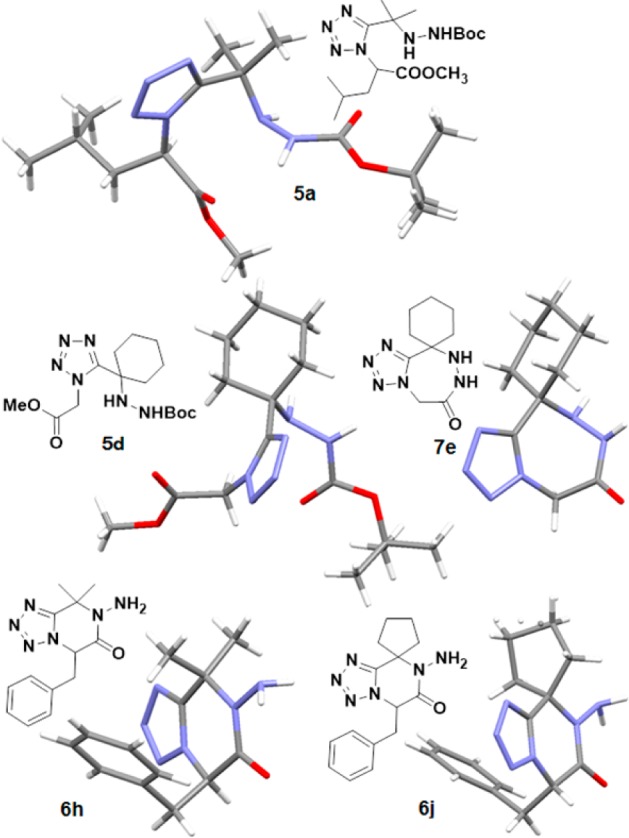
Crystal structures of 5a, 5d, 7e, 6h and 6j.
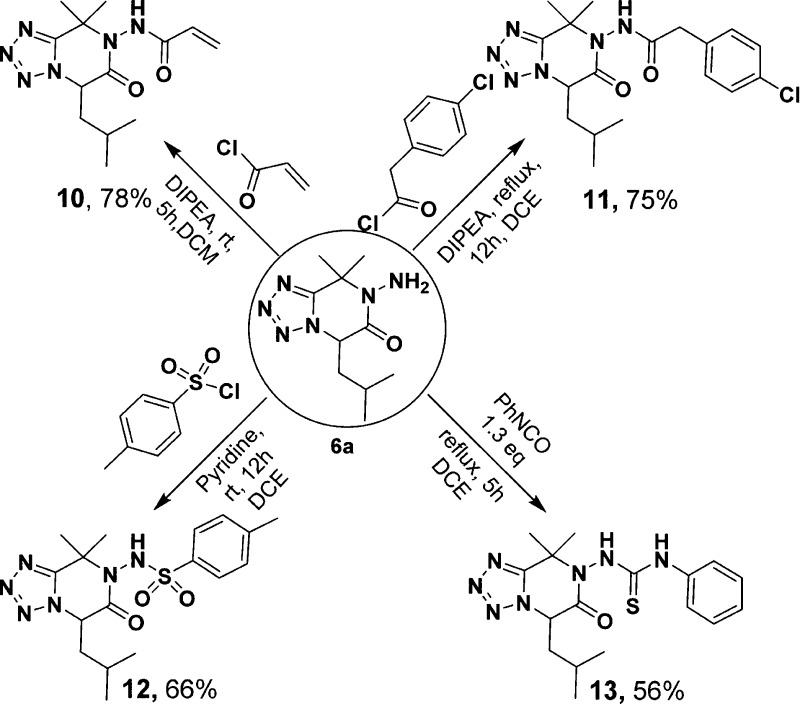
Lastly, the diversity application of the free exo-NH2 group in compounds 6a for biological studies was accomplished by synthesizing some derivatives through acylation, sulfonylation and thiourea formation (Scheme 4). Compounds 6a reacted with 1.2 equiv of acryloyl chloride to give compound 10 in 78% yields. Upon treatment with 4-chlorobenzoyl chloride in DCE under reflux, compound 11 (75% yield) was afforded. The reaction of 6a with 4-methylbenzenesulfonyl chloride led to 12 in 66% yield. In addition, 6a was also subjected to phenyl thioisocyanate with pyridine in DCE to give 13 in 56% yield.
Scheme 4. Application of the Primary Amine in Ugi-Adduct 6a.
Conclusions
In summary, we described here the application of mono-Boc-protected hydrazine in the Ugi tetrazole synthesis and its postcyclization. Both 7-aminotetrazolopyrazinone (6) and tetrazolotriazepinone (7) products were afforded in one-pot under acidic conditions. Under basic condition, Boc-protected 7-aminotetrazolopyrazinone (8) products were obtained in good to excellent yield. The exposed free primary amine could be further used as the building block to synthesize more complex compounds.
Acknowledgments
This work was financially supported (A.D.) by the NIH (2R01GM097082-05), the Innovative Medicines Initiative (Grant Agreement No. 115489), and received funding from the European Union’s Horizon 2020 research and innovation program under MSC ITN “Accelerated Early stage drug discovery” (AEGIS), Grant Agreement No. 675555. K. K, K-T. J supported by the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08). We acknowledge the China Scholarship Council for supporting Y.W.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscombsci.7b00009.
Experimental procedures, compound data, NMR spectra, HRMS, and crystal structure determinations (PDF)
Crystallographic information file for compound 5a (CIF)
Crystallographic information file for compound 5d (CIF)
Crystallographic information file for compound 6h (CIF)
Crystallographic information file for compound 6j (CIF)
Crystallographic information file for compound 7e (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Adams J. L.; Smothers J.; Srinivasan R.; Hoos A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discovery 2015, 14, 603–622. 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- Aeluri M.; Chamakuri S.; Dasari B.; Guduru S. K.; Jimmidi R.; Jogula S.; Arya P. Small molecule modulators of protein–protein interactions: selected case studies. Chem. Rev. 2014, 114, 4640–4694. 10.1021/cr4004049. [DOI] [PubMed] [Google Scholar]
- Doyle S. K.; Pop M. S.; Evans H. L.; Koehler A. N. Advances in discovering small molecules to probe protein function in a systems context. Curr. Opin. Chem. Biol. 2016, 30, 28–36. 10.1016/j.cbpa.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkin M. R.; Tang Y.; Wells J. A. Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chem. Biol. 2014, 21, 1102–1114. 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Besnard J.; Jones P. S.; Hopkins A. L.; Pannifer A. D. The joint european compound library: Boosting precompetitive research. Drug Discovery Today 2015, 20, 181–186. 10.1016/j.drudis.2014.08.014. [DOI] [PubMed] [Google Scholar]; b Hamza D.; Kalliokoski T.; Pouwer K.; Morgentin R.; Nelson A.; Müller G.; Piechot D.; Tzalis D.; et al. Expansion of chemical space for collaborative lead generation and drug discovery: the European Lead Factory Perspective. Drug Discovery Today 2015, 20, 1310–1316. 10.1016/j.drudis.2015.09.009. [DOI] [PubMed] [Google Scholar]; c Mullard A. European lead factory opens for business. Nat. Rev. Drug Discovery 2013, 12, 173–175. 10.1038/nrd3956. [DOI] [PubMed] [Google Scholar]; d Nelson A.; Roche D. Innovative approaches to the design and synthesis of small molecule libraries. Bioorg. Med. Chem. 2015, 23, 2613. 10.1016/j.bmc.2015.02.046. [DOI] [PubMed] [Google Scholar]; e Paillard G.; Cochrane P.; Jones P. S.; Caracoti A.; van Vlijmen H.; Pannifer A. D. The ELF Honest Data Broker: informatics enabling public–private collaboration in a precompetitive arena. Drug Discovery Today 2016, 21, 97–102. 10.1016/j.drudis.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Domling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- Dömling A.; Wang W.; Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Bienayme H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Sinha M. K.; Khoury K.; Herdtweck E.; Dömling A. Various cyclization scaffolds by a truly Ugi 4-CR. Org. Biomol. Chem. 2013, 11, 4792–4796. 10.1039/c3ob40523k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; Khoury K.; Herdtweck E.; Dömling A. A Universal Isocyanide for Diverse Heterocycle Syntheses. Org. Lett. 2014, 16, 5736–5739. 10.1021/ol5024882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; Khoury K.; Herdtweck E.; Dömling A. MCR synthesis of a tetracyclic tetrazole scaffold. Bioorg. Med. Chem. 2015, 23, 2699–2715. 10.1016/j.bmc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarganes-Tzitzikas T.; Patil P.; Khoury K.; Herdtweck E.; Dömling A. Concise Synthesis of Tetrazole–Ketopiperazines by Two Consecutive Ugi Reactions. Eur. J. Org. Chem. 2015, 2015, 51–55. 10.1002/ejoc.201403401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb W.; Neuville L.; Zhu J. Ugi-post functionalization, from a single set of Ugi-adducts to two distinct heterocycles by microwave-assisted palladium-catalyzed cyclizations: tuning the reaction pathways by ligand switch. J. Org. Chem. 2009, 74, 3109–3115. 10.1021/jo900210x. [DOI] [PubMed] [Google Scholar]
- a Tempest P.; Ma L.; Thomas S.; Hua Z.; Kelly M. G.; Hulme C. Two-step solution-phase synthesis of novel benzimidazoles utilizing a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett. 2001, 42, 4959–4962. 10.1016/S0040-4039(01)00919-4. [DOI] [Google Scholar]; b Nixey T.; Kelly M.; Semin D.; Hulme C. Short solution phase preparation of fused azepine-tetrazoles via a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett. 2002, 43, 3681–3684. 10.1016/S0040-4039(02)00636-6. [DOI] [Google Scholar]; c Gunawan S.; Ayaz M.; De Moliner F.; Frett B.; Kaiser C.; Patrick N.; Xu Z.; Hulme C. Synthesis of tetrazolo-fused benzodiazepines and benzodiazepinones by a two-step protocol using an Ugi-azide reaction for initial diversity generation. Tetrahedron 2012, 68, 5606–5611. 10.1016/j.tet.2012.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xu Z.; Ayaz M.; Cappelli A. A.; Hulme C. General one-pot, two-step protocol accessing a range of novel polycyclic heterocycles with high skeletal diversity. ACS Comb. Sci. 2012, 14, 460–464. 10.1021/co300046r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; Zhang J.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Hydrazine in the Ugi Tetrazole Reaction. Synthesis 2016, 48, 1122–1130. 10.1055/s-0035-1561353. [DOI] [Google Scholar]
- a Godet T.; Bonvin Y.; Vincent G.; Merle D.; Thozet A.; Ciufolini M. A. Titanium catalysis in the Ugi reaction of α-amino acids with aromatic aldehydes. Org. Lett. 2004, 6, 3281–3284. 10.1021/ol048850x. [DOI] [PubMed] [Google Scholar]; b Dai W. M.; Li H. Lewis acid-catalyzed formation of Ugi four-component reaction product from Passerini three-component reaction system without an added amine. Tetrahedron 2007, 63, 12866–12876. 10.1016/j.tet.2007.10.050. [DOI] [Google Scholar]; c Bonger K. M.; Wennekes T.; Filippov D. V.; Lodder G.; van der Marel G. A.; Overkleeft H. S. The Effect of Lewis Acids on the Stereochemistry in the Ugi Three-Component Reaction with D-lyxo-Pyrroline. Eur. J. Org. Chem. 2008, 2008, 3678–3688. 10.1002/ejoc.200800340. [DOI] [Google Scholar]; d Li Z.; Sharma U. K.; Liu Z.; Sharma N.; Harvey J. N.; Van der Eycken E. V. Diversity-Oriented Synthesis of β-Lactams and γ-Lactams by Post-Ugi Nucleophilic Cyclization: Lewis Acids as Regioselective Switch. Eur. J. Org. Chem. 2015, 2015, 3957–3962. 10.1002/ejoc.201500270. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.