Abstract
The present study examines the role of Pyk2 in acid regulation of sodium/hydrogen exchanger 3 (NHE3) activity in OKP cells, a kidney proximal tubule epithelial cell line. Incubation of OKP cells in acid media caused a transient increase in Pyk2 phosphorylation that peaked at 30 seconds and increased Pyk2/c-Src binding at 90 seconds. Pyk2 isolated by immunoprecipitation and studied in a cell-free system was activated and phosphorylated at acidic pH. Acid activation of Pyk2 (a) was specific for Pyk2 in that acid did not activate focal adhesion kinase, (b) required calcium, and (c) was associated with increased affinity for ATP. Transfection of OKP cells with dominant-negative pyk2K457A or small interfering pyk2 duplex RNA blocked acid activation of NHE3, while neither had an effect on glucocorticoid activation of NHE3. In addition, pyk2K457A blocked acid activation of c-Src kinase, which is also required for acid regulation of NHE3. The present results demonstrate that Pyk2 is directly activated by acidic pH and that Pyk2 activation is required for acid activation of c-Src kinase and NHE3. Given that partially purified Pyk2 can be activated by acid in a cell-free system, Pyk2 may serve as the pH sensor that initiates the acid-regulated signaling cascade involved in NHE3 regulation.
Introduction
Metabolic acidosis induces a series of homeostatic responses in various organs that serve in a concerted manner to return extracellular pH toward normal. These processes include increased acid secretion in the kidney, increased renal ammonia synthesis, increased osteoclast-mediated bone resorption, and muscle protein degradation (1). In the renal proximal tubule, metabolic acidosis induces increased rates of hydrogen secretion into the luminal fluid, citrate reabsorption from the luminal fluid, and ammonia synthesis. These processes involve regulation of multiple enzymes and transporters. Previous studies have demonstrated that this concerted response in the proximal tubule is mediated by an acid-regulated signaling pathway that includes activation of c-Src and ERK and increases in c-fos, c-jun, junB, and egr-1 expression (1–5).
Using maneuvers such as weak acid addition and NH3 prepulse that induce intracellular acidification in the absence of extracellular acidification, researchers have demonstrated that it is intracellular acidification that increases c-Src activity (5). However, the mechanism by which this occurs has not been elucidated. Presumably, the cell possesses a pH-sensitive protein, or pH sensor, that is activated by decreases in intracellular pH and then initiates a signaling cascade involving the above pathways. Because c-Src is activated by acid, we first examined p125FAK as a possible upstream activator. However, although p125FAK is activated in OKP cells by media acidification, activation occurs at 6 hours, which is later than c-Src activation (30–90 seconds) (5, 6).
In the present study, we examined the role of Pyk2, a member of the focal adhesion kinase (FAK) family of tyrosine kinases (7). Pyk2 is activated in OK cells by cholinergic agents, angiotensin II, and CO2 and plays a role in sodium bicarbonate cotransporter 1 (NBC-1) regulation (8). In multiple systems, Pyk2 activates c-Src and is activated by calcium. The results demonstrate that (a) media acidification activates Pyk2 in whole cells, (b) decreases in pH can activate Pyk2 in a cell-free system, and (c) Pyk2 activation is specific and required for acid activation of both c-Src kinase and sodium/hydrogen exchanger 3 (NHE3).
Results
Acid incubation induces Pyk2 phosphorylation in OKP cells.
To examine the role of Pyk2 in the cellular response to acidosis, we first examined the effect of media acidification on Pyk2 phosphorylation in OKP cells. Activated Pyk2 autophosphorylates at Tyr402 and activates c-Src, which then further phosphorylates Pyk2 on several additional sites (7). Pyk2 phosphorylation thus provides an accurate assay for Pyk2 activation (7).
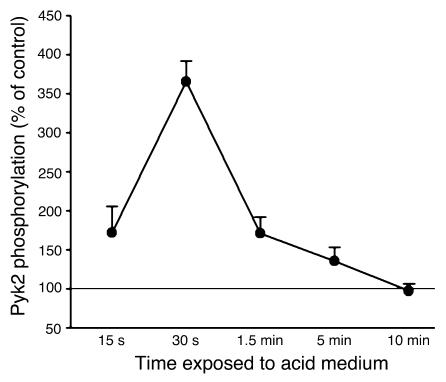
OKP cells were incubated at pH 7.4 or 6.8, Pyk2 was immunoprecipitated, and Pyk2 phosphorylation was assessed. As shown in Figure 1, incubation in acid media caused a transient increase in Pyk2 phosphorylation, which peaked at 30 seconds.
Figure 1.
Acid incubation induces Pyk2 phosphorylation in OKP cells. After growing to confluence, cells were rendered quiescent for 48 hours and then exposed to a medium of pH 7.4 (control) or 6.8 (acid) for the indicated time. Pyk2 was then immunoprecipitated and phosphorylation assayed, as described in Methods. Data are plotted as percentage of control; n = 6 for control and acid at each time point. Pyk2 phosphorylation was increased in 4 of 6 experiments (15 seconds), 6 of 6 experiments (30 seconds and 5 minutes), 5 of 6 experiments (90 seconds), and 2 of 6 experiments (10 minutes).
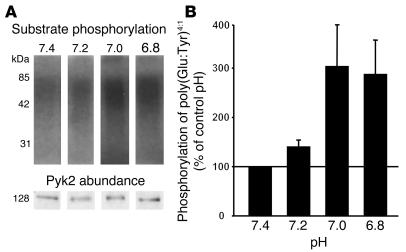
Pyk2 may be activated secondary to activation of another signaling molecule or could be activated directly by acid intracellular pH and thus would represent the pH sensor. To determine if Pyk2 can be directly activated by acid, we isolated Pyk2 from cell lysates by immunoprecipitation, incubated the Pyk2 at varying pH values, and assayed Pyk2 activity by its ability to phosphorylate a synthetic substrate [poly(Glu-Tyr)4:1]. Incubation at pH 7.0 or 6.8 for 5 minutes increases Pyk2 phosphorylation of poly(Glu-Tyr)4:1 to 304% and 288%, respectively, of control (Figure 2). These results demonstrate that acid pH can directly activate Pyk2 in the absence of cell contents and suggest that Pyk2 is directly regulated by proton concentration.
Figure 2.
Acid activates Pyk2 in a cell-free system. Wild-type OKP cells were grown to confluence and rendered quiescent for 48 hours. Pyk2 was immunoprecipitated as described in Methods and exposed to buffers at the indicated pH for 5 minutes; kinase activity was assayed as the ability of Pyk2 to phosphorylate poly(Glu-Tyr)4:1. (A) Typical autoradiograph (top) and Western blot (bottom). (B) Summary of the data, which are normalized for Pyk2 abundance and plotted as a percentage of control (pH 7.4); n = 9 for each pH. Pyk2 kinase activity was increased in 8 of 9 experiments at pH 7.2, 7.0, and 6.8.
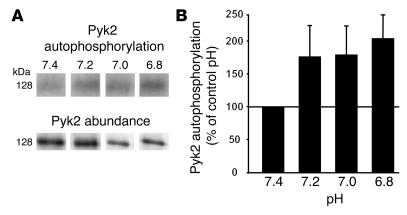
As noted above, activated Pyk2 autophosphorylates on Tyr402. Therefore, we measured Pyk2 autophosphorylation following acid incubation in vitro. Similar to acid stimulation of Pyk2 kinase activity, incubation in a cell-free system at pH 7.2, 7.0, or 6.8 for 5 minutes caused Pyk2 autophosphorylation to increase to 205% of control (Figure 3).
Figure 3.
Acid induces Pyk2 autophosphorylation in a cell-free system. Wild-type OKP cells were grown to confluence and rendered quiescent for 48 hours. Pyk2 was immunoprecipitated as described in Methods and exposed to buffers at the indicated pH for 5 minutes; Pyk2 autophosphorylation was assayed. (A) Typical autoradiograph (top) and Western blot (bottom). (B) Summary of the data, which are normalized for Pyk2 abundance and plotted as a percentage of control (pH 7.4); n = 9 for each pH. Pyk2 phosphorylation was increased in 5 of 9 experiments at pH 7.2 and 7.0 and in 8 of 9 experiments at pH 6.8.
Acid incubation does not activate FAK.
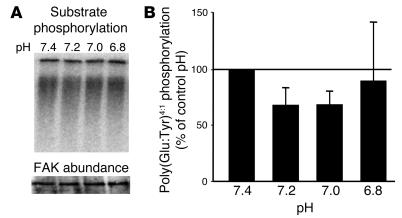
To determine if activation by acid is specific for Pyk2, studies were performed with FAK, a member of the same family of tyrosine kinases as Pyk2 (7). Although we have previously shown in whole cells that media acidification activates FAK, this effect required 6 hours of media acidification and thus it is unlikely that acid activation of FAK is a direct effect of acid. As shown in Figure 4, incubation in acid pH did not increase poly(Glu-Tyr)4:1 phosphorylation by FAK, demonstrating that the direct effect of acid on Pyk2 activity is specific.
Figure 4.
Acid does not activate FAK in a cell-free system. OKP cells were grown to confluence and rendered quiescent for 48 hours. FAK was immunoprecipitated as described in Methods and exposed to buffers at the indicated pH for 5 minutes; kinase activity was assayed as the ability of FAK to phosphorylate poly(Glu-Tyr)4:1. (A) Typical autoradiograph (top) and Western blot (bottom). (B) Summary of the data, which are normalized for FAK abundance and plotted as a percentage of control (pH 7.4); n = 3 for pH 7.4, 7.2, and 7.0, and n = 2 for pH 6.8.
Calcium is required for acid activation of Pyk2.
In many systems, Pyk2 activation is Ca2+ dependent. In the studies described above, Ca2+ was not added to the buffer solution; however, there is probably a small amount of Ca2+ in deionized water. To determine if Ca2+ is required for acid activation of Pyk2, activation was studied in the presence and absence of 1 mM EGTA. The presence of EGTA not only blocked acid activation of Pyk2 kinase but caused acid-induced inhibition of Pyk2 activity (ratio pH 6.8/pH 7.4 = 0.65 ± 0.05, n = 5). These data demonstrate a Ca2+ dependence of Pyk2 activation by acid and suggest that, in the absence of Ca2+, acid inhibits Pyk2 kinase activity.
Acid activation of Pyk2 kinase is associated with an increased affinity to ATP.
To begin to elucidate the mechanism of acid regulation of Pyk2 kinase activity, studies were performed in which kinase activity was assayed at pH 7.4 and 6.8 in the presence of varying concentrations of ATP (5, 20, 100, and 200 μM). Acid incubation did not affect maximal kinase activity, but rather the affinity for ATP increased. Using an Eadie-Hofstee analysis, the calculated KmATP was 129 ± 21 μM at pH 7.4 and 51 ± 6 μM at pH 6.8 (P < 0.03, n = 4). The calculated Vmax was 0.182 ± 0.111 at pH 7.4 and 0.246 ± 0.138 at pH 6.8 (NS, n = 4).
Dominant-negative Pyk2 prevents NHE3 activation by acid.
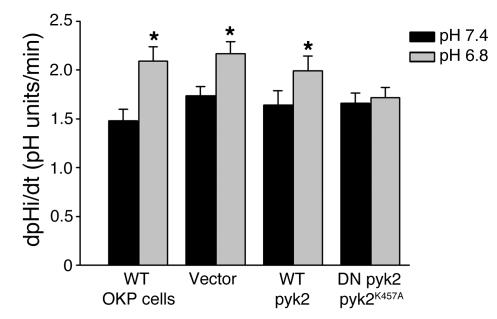
Subsequent studies used 2 approaches to determine the role of Pyk2 in acid activation of NHE3. First, OKP cells were transiently transfected with dominant-negative pyk2 (pyk2K457A) (7). Mutation of lysine 457 to alanine in the tyrosine-kinase domain of Pyk2 creates an inactive Pyk2 that inhibits wild-type Pyk2 activity (7, 9). Cells were incubated at pH 6.8 or 7.4 for 6 hours, and NHE3 activity was measured using 2′7′-bis (2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF). As shown in Figure 5, in wild-type OKP cells, incubation in acid media caused a 41% increase in NHE3 activity (P < 0.01). This response is similar to that previously reported (10). Similarly, in cells transiently transfected with vector alone or wild-type pyk2, acid incubation caused 25% and 21% increases, respectively, in NHE3 activity (P < 0.01, Figure 5). However, in cells transfected with dominant-negative pyk2K457A, acid incubation had no effect on NHE3 activity (3% increase, NS, Figure 5).
Figure 5.
Dominant-negative Pyk2 prevents NHE3 activation by acid. OKP cells were transiently transfected with 1 μg of the indicated DNA for 5 hours, grown to confluence over the next 19 hours, rendered quiescent for 24 hours, and exposed to control (pH 7.4) or acid (pH 6.8) media for 6 hours; Na/H antiporter activity was then assayed as the rate of Na-dependent increase in cell pH. Data are plotted as dpHi/dt, pH units/min; n = 6 (wild-type cells), n = 19 (vector transfected), n = 19 (wild-type Pyk2 transfected), and n = 17 (dominant-negative [DN] Pyk2K457A transfected). *P < 0.01 vs. pH 7.4.
To demonstrate the specificity of the dominant-negative Pyk2 effect, we examined its effect on glucocorticoid activation of NHE3. NHE3 activation by glucocorticoids does not involve tyrosine-kinase pathways and thus should not be blocked by inhibition of Pyk2. We have previously shown that inhibition of c-Src or mitogen-activated protein/ERK kinase (MEK) prevents acid activation of NHE3 but not activation by glucocorticoids (2, 4). Cells were incubated with 10–7 M dexamethasone or vehicle for 24 hours. In cells transfected with dominant-negative pyk2K457A, dexamethasone increased NHE3 activity by 48% (P < 0.01, n = 6), a degree of stimulation similar to that seen in wild-type OKP cells (11).
Small interfering pyk2 RNA prevents NHE3 activation by acid.
As a second approach, we decreased Pyk2 expression in OKP cells using small interfering pyk2 RNA (siRNApyk2) (12, 13). Because siRNAs require a perfect sequence match with their target mRNAs, we first cloned a partial sequence of the OKP pyk2. To accomplish this, PCR primers were generated as described in Methods based on pyk2 sequences conserved in human and rat. The PCR product generated was 405 bp, the predicted size, and when sequenced yielded a pyk2 sequence that was 88% identical to human and rat pyk2 and 85% identical to mouse pyk2 (GenBank accession numbers: opossum, AY195883; human, GI1245923; rat, GI1000679; and mouse, GI5905541). We then used an internal sequence to generate an siRNA that corresponded to a coding sequence, was distal to the first 100 bases of the coding sequence, and had a GC content of approximately 50%. The sequence used was 5′-(AA)UGCCCUUGAUAAGAAGUCC-3′, corresponding to nucleotides 325–343 of the cloned opossum pyk2 and nucleotides 872–890 (or 579–597 relative to the start codon) of the human pyk2 nucleotide sequence.
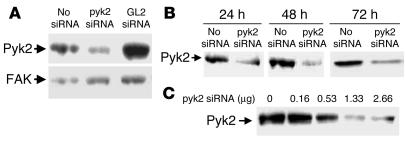
Transfection with siRNApyk2 caused a 90% decrease in Pyk2 protein abundance as assessed by immunoblot (Figure 6). As shown in Figure 6A, the expression of Pyk2 protein was specifically decreased by transfection with siRNApyk2 duplex but not by transfection with a nonspecific siRNA (siRNAGL2). Expression of a nontarget gene, FAK, which belongs to the same family as Pyk2, was not affected. Silencing was monitored at different time points after transfection and at different doses of siRNApyk2 duplex to find the greatest effect on Pyk2 protein abundance. As shown in Figure 6, B and C, Pyk2 was silenced within 24 hours of beginning transfection and remained silenced after 72 hours. The largest effect was seen at siRNApyk2 doses equal to or greater than 1.33 μg per well.
Figure 6.
siRNApyk2 decreases Pyk2 protein abundance in OKP cells. Typical blots. (A) Cells were transfected with 1.33 μg/well siRNApyk2 or siRNAGL2 (GL2 siRNA) or transfection reagent only (No siRNA) for 24 hours (at which time they were confluent), rendered quiescent for 24 hours, and harvested. FAK protein abundance was measured for comparison. (B) Cells were transfected with 1.33 μg/well siRNApyk2 for 24 hours (at which time they were confluent) and rendered quiescent. The length of quiescence varied from 0 to 48 hours, so that cells were harvested 24, 48, or 72 hours after the beginning of transfection. (C) Cells were transfected with the indicated amount of siRNApyk2 for 24 hours (at which time they were confluent), rendered quiescent for 24 hours, and harvested.
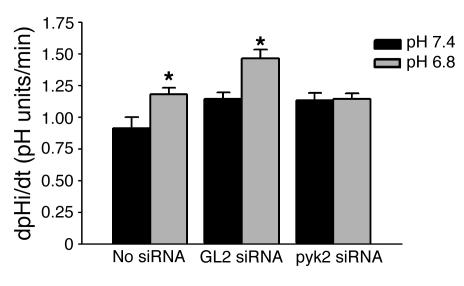
In control cells not treated with siRNA, acid incubation caused a 30% increase in NHE3 activity (P < 0.05, Figure 7). Similarly, in cells transfected with siRNAGL2, acid incubation caused a 28% increase in NHE3 activity (P < 0.05). However, in cells transfected with siRNApyk2, acid incubation had no effect on NHE3 activity (1% increase, NS), again demonstrating that Pyk2 is required for NHE3 activation (Figure 7).
Figure 7.
siRNApyk2 prevents NHE3 activation by acid. OKP cells were transiently transfected with 1.33 μg siRNApyk2 (pyk2 siRNA) per well for 24 hours (at which time they were confluent), rendered quiescent for 18 hours, and exposed to control (pH 7.4) or acid (pH 6.8) medium for 6 hours; then Na/H antiporter activity was assayed. Data are plotted as dpHi/dt, pH units/min. Controls were cells exposed to transfection reagent without siRNA (No siRNA) or cells transfected with a siRNA against a nonmammalian protein (GL2 siRNA); n = 7 (No siRNA), n = 6 (GL2 siRNA), and n = 10 (pyk2 siRNA). * P < 0.05 vs. pH 7.4.
To confirm the specificity of inhibition by siRNApyk2, we examined the effect of glucocorticoids on NHE3 activity. In cells transfected with siRNApyk2, 10–7 M dexamethasone caused a 33% increase in NHE3 activity (P < 0.05, n = 5). These results demonstrate that the effect of Pyk2 inhibition on NHE3 activation is specific for the acid-activated signaling pathway.
Acid incubation increases Pyk2 and c-Src binding, which is necessary for acid stimulation of NHE3 activity.
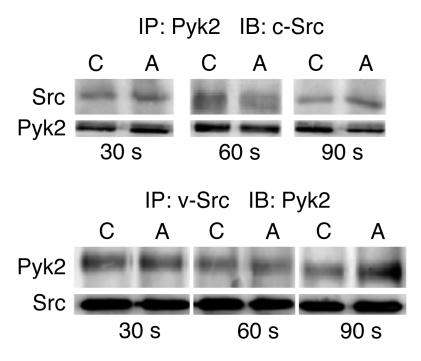
Following activation, Pyk2 autophosphorylates and then binds to and activates c-Src, which in turn further phosphorylates Pyk2 (14). We have previously shown that c-Src is necessary for acid regulation of NHE3 activity (2, 4, 5). To examine whether acid incubation increases binding of c-Src to Pyk2, coimmunoprecipitation studies were performed following incubation in a control (pH 7.4) or acid (pH 6.8) medium. By 90 seconds, the peak time of c-Src activation (5), there was a 107% increase in c-Src abundance in anti-Pyk2 immunoprecipitates and a 115% increase in Pyk2 abundance in anti-v-Src immunoprecipitates harvested from cells incubated in acid, compared to control media (Figure 8).
Figure 8.
Acid incubation increases Pyk2 and c-Src coimmunoprecipitation. Representative blots. OKP cells transiently transfected with pyk2 and c-Src were grown to confluence, rendered quiescent, and exposed to control (C) (pH 7.4) or acid (A) (pH 6.8) medium for the indicated time; Pyk2 or v-Src was immunoprecipated (IP) as indicated. The immunoprecipitates were divided and immunoblotting (IB) performed using anti-Pyk2 and anti-c-Src antibodies as indicated; n = 3 for each group.
Grb2 is an adaptor molecule with a putative binding site on Pyk2 (tyrosine 881) (14–17). Next we examined whether acid increased Grb2 binding to Pyk2. Results showed that Grb2 does coprecipitate with Pyk2 but that there was no effect of acid on coprecipitated Grb2 abundance at 30 and 90 seconds or at 2 minutes. At 60 seconds, acid significantly decreased the abundance of coprecipitated Grb2 (acid/control ratio = 0.69 ± 0.09, P < 0.04, n = 4).
To determine whether acid-induced c-Src binding to Pyk2 is necessary for acid regulation of NHE3 activity, we examined the effect of the Pyk2 mutant Pyk2Y402F on acid regulation of NHE3 activity. Mutation of tyrosine 402 to phenylalanine prevents binding of c-Src to activated Pyk2, and subsequent Src activation (9). In vector transfected cells, acid incubation increased NHE3 activity 24% ± 5% (n = 6, P < 0.03) whereas in pyk2Y402F transfected cells acid was without effect on NHE3 activity (4% ± 6% increase, NS, n = 6). Thus Pyk2 autophosphorylation and c-Src binding to Pyk2 are necessary for acid stimulation of NHE3 activity.
Pyk2 is required for acid activation of c-Src.
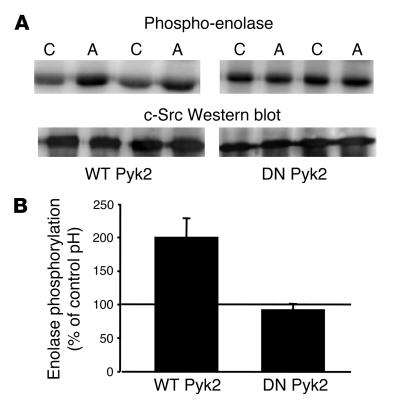
Having established that acid activates both Pyk2 and c-Src kinases and that both kinases and Pyk2/c-Src binding are required for acid regulation of NHE3, we next addressed the question of whether Pyk2 activation is required for acid activation of c-Src. For these studies, cells were transiently transfected with c-Src and either wild-type or dominant-negative Pyk2. Following incubation in control or acid media, c-Src kinase activity was assayed using acid-denatured enolase as the substrate. As shown in Figure 9, expression of the dominant-negative Pyk2 prevented acid activation of c-Src kinase.
Figure 9.
Pyk2 is required for acid activation of c-Src. OKP cells transiently transfected with wild-type or dominant-negative (DN) pyk2 and wild-type c-Src were grown to confluence, rendered quiescent, and exposed to control (pH 7.4) or acid (pH 6.8) medium for 90 seconds; c-Src was immunoprecipitated as described in Methods. c-Src kinase activity was assayed using acid-denatured enolase as the substrate. (A) Typical autoradiograph (top) and Western blot (bottom). (B) Summary of the data, which are normalized for c-Src abundance and plotted as percentage of control (pH 7.4); n = 6 for studies done with wild-type Pyk2 and n = 4 for studies done with the dominant-negative Pyk2. c-Src kinase activity was increased in 6 of 6 experiments in the presence of the wild-type Pyk2 and in 1 of 4 experiments in the presence of the dominant-negative Pyk2.
Discussion
Metabolic acidosis induces a diverse response in proximal tubule cells, including increases in the activities of NHE3, NBC-1, glutaminase, glutamate dehydrogenase, ATP citrate lyase, mitochondrial aconitase, sodium-dicarboxylate cotransporter 1 (NaDC-1) and phosphoenolpyruvate carboxykinase (PEPCK) (1). Increases in the activities of these enzymes and transporters results in increased rates of H+ secretion, citrate reabsorption, and ammonia synthesis, all of which participate in processes that return blood pH to normal. This coordinated response is due to activation of a signaling cascade that includes activation of c-Src and ERKs and increases in the expression of c-fos, c-jun, junB, and egr-1 (2–5). In addition, this response involves an increase in preproendothelin-1 gene expression, with secretion of endothelin 1 and activation of the endothelin B receptor (18, 19).
Although the above studies have defined components of a signaling cascade, they have not defined the protein that is directly activated by decreases in intracellular pH and that initiates the cascade. In an attempt to identify a possible pH sensor/activator of c-Src, we first examined p125FAK. While p125FAK was activated by acid in whole cells, its time course was delayed compared with c-Src activation, and thus it cannot be upstream (5, 6). Pyk2 is a nonreceptor tyrosine kinase that was first identified by Lev et al. (7). It is closely related to p125FAK and is also able to activate c-Src.
The present studies examined the role of Pyk2 in the acid signaling cascade that regulates NHE3 activity. We first found that exposing OKP cells to acid media transiently activated Pyk2. Peak Pyk2 activation occurred at 30 seconds, before peak c-Src activation at 30–90 seconds (5). This could be a direct effect of pH on Pyk2 or could be secondary to activation of another protein or intermediate. To address this, we isolated Pyk2 from cell lysates by immunoprecipitation, resuspended the precipitate, and exposed it to varying pHs for 5 minutes. Incubation at pH 6.8–7.2 consistently increased Pyk2 activity, assayed as the ability of Pyk2 to phosphorylate a synthetic substrate, and was associated with Pyk2 autophosphorylation. Activation by acid pH was specific for Pyk2 and was not seen with FAK. While all proteins have a pH optima, a pH sensor is a protein whose activity varies considerably over a smaller physiologically relevant range (e.g., pH 6.8–7.4 for Pyk2). Although these results suggest that Pyk2 is directly activated by decreases in pH, it is possible that the immunoprecipitate also contains a Pyk2 binding protein that is the pH sensor.
It should be noted that the degree of Pyk2 phosphorylation observed in the cell-free system (Figure 3) was increased to only 205% of control whereas Pyk2 phosphorylation increased to 365% of control in the intact cell upon acid activation (Figure 1). This difference was present even though the degree of acidification was likely greater in the cell-free system than would be achieved in the intact cell. This is likely due to the fact that in the intact cell Pyk2 is autophosphorylated and then further phosphorylated and activated by c-Src. When Pyk2 is activated in the cell-free system, c-Src is not present and thus only autophosphorylation occurs.
Our studies show that Pyk2 kinase activity can be regulated by pH in a cell-free system and that this involves an increase in the affinity of the kinase for ATP. Given that measured whole-cell ATP concentrations are in the millimolar range and the affinity for ATP is in the micromolar range, a change in the Michaelis-Menten constant for ATP would not seem to be physiologically relevant. However, Mandel and coworkers (20, 21) demonstrated that, although the isolated proximal tubule Na,K-ATPase has a Michaelis-Menten constant for ATP in the range of 300–500 μM and cellular ATP concentrations are in the millimolar range, maneuvers that decrease cellular ATP content by 30–45% in the intact tubule inhibit the Na,K-ATPase transport rate by 30–45%. These studies suggest that in the intact tubule, the Na,K-ATPase functions at the linear part of the ATP dependence curve. Thus it is possible that local ATP concentrations within microdomains of ATPases or kinases are lower than those measured in whole-cell lysates or that the affinity of these ATPases/kinases for ATP is lower than that measured on the isolated protein, possibly due to the presence of other regulatory molecules in the intact cell.
We have previously shown that inhibition of c-Src or MEK prevents acid activation of NHE3 (2, 4). In the present study, we used three approaches to demonstrate the role of Pyk2 in acid regulation of NHE3 and to determine if Pyk2 is necessary for acid signaling through c-Src. Pyk2 activation and Pyk2/c-Src binding are initiated by Pyk2 autophosphorylation on Tyr402, which leads to binding of the c-Src SH2 domain to a motif that includes the Tyr402 residue, with subsequent c-Src activation (9). Src activation leads to further Pyk2 phosphorylation in the kinase and C-terminal domains (14, 22). The 3 approaches used in our studies were to (a) decrease Pyk2 protein expression with siRNApyk2, (b) express a dominant-negative pyk2 construct (pyk2K457A) that removes a key lysine involved in ATP binding, and (c) express the pyk2Y402F mutant that prevents the SH2 domain in c-Src from binding to its consensus sequence (YAEI) on Pyk2. Our studies show that with all 3 approaches media acidification was unable to stimulate NHE3 activity, demonstrating that Pyk2 kinase activity is required for acid regulation of NHE3. In addition, these studies show that acid activation of c-Src is mediated by Pyk2.
Having demonstrated that (a) both Pyk2 (present studies) and c-Src (2, 4) are required for acid regulation of NHE3 activity, (b) acid incubation increases the abundance of c-Src that coprecipitates with Pyk2, (c) Pyk2/c-Src interaction is required for acid regulation of NHE3 activity, and (d) in the presence of the dominant-negative Pyk2K457A acid cannot activate c-Src kinase, we propose a signaling pathway that places Pyk2 as the direct upstream activator of c-Src. Pyk2 is generally activated as part of a signaling cascade in response to increases in cell Ca2+, activation of protein kinase C, or activation of G protein coupled receptors (7, 9). Based on its ability to respond to these multiple signals, Pyk2 is believed to play a role in the response to many extracellular agonists in several different cell types (14). In many systems, Pyk2 activation is mediated by increases in cell calcium (7). Acid activation of Pyk2 was inhibited in the presence of EGTA, suggesting calcium dependence. However, it should be noted that acid does not elicit a significant increase in cell calcium in OKP cells. Note that our unpublished observations indicate that lowering extracellular pH from 7.4 to 6.8 results in a small increase in cell Ca2+ from 44.5 ± 3.8 to 55.3 ± 2.6 nM. The small magnitude of the increase would be atypical for agonists that activate Pyk2 through changes in cell Ca2+. In addition, there was an unusual time course, with a sharp increase in the Fura-2 340/Fura-2 380 ratio and a constant plateau, suggesting that the small change may be due to a direct effect of pH on Fura-2 fluorescence. The present studies suggest that intracellular proton concentration also may serve as a regulator of Pyk2 activity.
The present results demonstrate an important role for Pyk2 in signaling the effect of decreased intracellular pH in the renal proximal tubule. Pyk2 is upstream of c-Src and may function as the pH sensor. Following Pyk2 activation and autophosphorylation, c-Src binds to Pyk2 and is activated, with subsequent activation of p125FAK and ERK and increased expression of c-fos, c-jun, junB, egr-1, and preproendothelin-1, resulting in NHE3 activation. Espiritu et al. (8) have similarly shown in OK cells that cholinergic agents, angiotensin II, and CO2 activate Pyk2 and that expression of dominant-negative Pyk2 inhibits activation of NBC-1 by these agonists. In inner medullary collecting duct cells, Pyk2 and p125FAK are both activated by hypertonicity, with Pyk2 activation occurring rapidly and p125FAK activation being more delayed (23). The temporal relationship between Pyk2 and p125FAK activation in inner medullary collecting duct cells is similar to that seen with acid incubation of OKP cells (6). Glucocorticoid activation of NHE3 is independent of Pyk2 and is likely mediated by glucocorticoid receptor–regulated gene expression. The role of Pyk2 in other acid-induced effects in the kidney and in other cells requires further investigation.
Methods
Materials.
All chemicals were obtained from Sigma-Aldrich with the following exceptions: penicillin and streptomycin (Whittaker MA Bioproducts); acetoxymethyl derivative of BCECF (Molecular Probes); TOPO TA Cloning kit, pcDNA3.1/HIS vector, ThermoScript RT-PCR System, Platinum Taq DNA Polymerase High Fidelity, and dNTP Mix (Invitrogen); pCI-neo, Tfx-50, BamHI, and EcoRI (Promega); DNA ligation kit (Takara); cell culture media, Lipofectamine Plus Reagent kits, and Superscript II kit (Gibco BRL); TransIT-TKO siRNA Transfection Reagent (Mirus Corp.); anti-v-Src (Ab-1) monoclonal antibody, NP-40, and protein G-Plus agarose (Calbiochem); goat anti-Pyk2 polyclonal IgG antibody, rabbit anti-c-Src (SRC2) polyclonal IgG antibody, goat and rabbit anti-FAK polyclonal IgG antibodies, rabbit anti-Grb2 polyclonal IgG antibody, and anti-phosphotyrosine antibody (PY99) (Santa Cruz Biotechnology Inc.); rabbit anti-Pyk2 polyclonal IgG antibody (Upstate Biotechnology, Inc.); HRP–labeled anti-rabbit IgG and anti-mouse IgG (Amersham); and ECL kit and 32P-γ-ATP (PerkinElmer).
Construction of expression vectors for wild-type Pyk2 (pcDNA3.1/HisA/Pyk2) and mutant Pyk2 (pcDNA3.1/HisA/Pyk2K457A and pcDNA3.1/HisA/Pyk2Y402F).
The pyk2 cDNA was amplified from Sprague-Dawley rat kidney RNA by PCR with the following composite primers encoding both ends of the rat pyk2 open reading frame flanked with BamHI and EcoRI adapter sequences: 5′-CGGGATCCCGATGTCCGGGGTGTCTGAGC-3′ and 5′-GGAATTCCTCACTCTGCAGGCGGGTGG-3′.
The pyk2 cDNA fragment was cloned into pCR2.1-TOPO vector with a TOPO TA Cloning kit and then subcloned into the pcDNA3.1/HIS vector using BamHI and EcoRI. The DNA sequence was confirmed to be identical to the full-length rat pyk2 sequence in GenBank, with an amino-terminal Xpress-tag.
The kinase-negative, dominant-negative mutants of pyk2, pyk2K457A, and pyk2Y402F were generated by replacing lysine 457 with alanine and tyrosine 402 with phenylalanine, respectively, using site-directed mutagenesis, following the manufacturer’s protocol (Stratagene). The mutations were confirmed by sequence analysis.
Preparation of OKP pyk2 siRNA.
To define the OKP pyk2 sequence, cDNA was prepared from OKP cell total RNA and amplified using the ThermoScript RT-PCR System, according to the manufacturer’s protocol. cDNA was amplified with 1 U of Platinum Taq DNA Polymerase High Fidelity for 30 cycles (denaturing at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 68°C for 90 seconds). PCR primers were designed from the human pyk2 sequence (GenBank accession number GI1245923) at sites of high homology between human and rat nucleotide sequences. Primers were 5′-GTTGGCTGAGTGCTATGGGCTGA-3′ (sense) and 5′-TCCTGCATCTGCTTTGGGAAAAA-3′ (antisense).
A 21-nucleotide siRNApyk2 duplex with dTdT overhangs, designed as AA followed by 19 nucleotides of the target OKP mRNA coding sequence (5′-AAUGCCCUUGAUAAGAAGUCC-3′) was synthesized, purified, and duplexed by Dharmacon Research.
Cell culture and transfection.
OKP cells were passaged in high-glucose (450 mg/dl) DMEM supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) (24, 25). When confluent, cells were rendered quiescent using a 1:1 mixture of low-glucose (100 mg/dl) DMEM and Ham’s F12 in the absence of serum. The length of the quiescent period is indicated in the figure legends.
For DNA transfection, OKP cells were grown to 80–90% confluence and then were transiently transfected for 5 hours with pcDNA3.1/HisB/LacZ (vector only), pcDNA3.1/HisA/Pyk2 (rat, wild-type pyk2), pcDNA3.1/HisA/Pyk2K457A (dominant-negative pyk2), or pcDNA3.1/HisA/Pyk2Y402F (c-Src binding deficient) plasmids or cotransfected for 5 hours with wild-type pyk2 (pcDNA3.1/HisA/Pyk2, rat) and wild-type c-Src (pcDNA3/c-Src, chicken), dominant-negative pyk2 (pcDNA3.1/HisA/Pyk2K457A, rat) and wild-type c-Src (pcDNA3/c-Src, chicken), or c-Src binding deficient pyk2 (pcDNA3.1/HisA/Pyk2Y402F, rat) and wild-type c-Src (pcDNA3/c-Src, chicken) plasmids using the Lipofectamine Plus kit per manufacturer’s instructions. When the transfection medium was removed, cells were incubated in passage medium for 19 hours. At 24 hours after the beginning of transfection, the cells were rendered quiescent.
For siRNA transfection, cells were grown in the same media but without antibiotics 24 hours before transfection. At 50–70% confluence, siRNApyk2 was transfected into OKP cells using TransIT-TKO siRNA Transfection Reagent, as described by the manufacturer. As a control, siRNAGL2 (corresponding to the firefly luciferase gene) was transfected under similar conditions (12). Other control cells were treated in the same manner but without the addition of siRNA to the transfection reagent. When confluent, cells were rendered quiescent.
To study the effects of media acidification on NHE3 activity, cells were grown on glass coverslips and, after being rendered quiescent, were incubated in control (pH 7.4) or acid (pH 6.8) medium for 6 hours (10). For studies examining the effect of medium acidification on c-Src kinase activity or the coprecipitation of Pyk2 and c-Src or Grb2, cells were grown in 60-mm culture dishes and, after being rendered quiescent, were incubated in control (pH 7.4) or acid (pH 6.8) medium for different times, as indicated in the figures. To study the effects of glucocorticoids on NHE3 activity, cells were grown on glass coverslips and, after being rendered quiescent, were incubated in 10–7 M dexamethasone or vehicle (ethanol) for 24 hours (11).
Na+/H+ antiporter activity.
As previously described, continuous measurement of cytoplasmic pH (pHi) was accomplished using the intracellularly trapped pH-sensitive dye BCECF (10, 25). To measure Na+/H+ antiporter activity, cells were acidified by addition of nigericin in a Na+-free solution in the absence of CO2/HCO3–. Na+/H+ antiporter activity was then measured as the initial rate of pHi increase (dpHi/dt) in response to Na+ addition. Chronic changes in media pH have no effect on buffer capacity (25). Results are therefore reported as dpHi/dt.
Immunoprecipitation.
Cells were grown to confluence, rendered quiescent, and then washed twice with ice cold PBS; they were lysed with 0.9 ml of ice-cold RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 50 mM β-glycerophosphate, 50 mM NaF, 1 mM EGTA, 1 mM Na3VO4, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 2 μg/ml pepstatin). All subsequent manipulations were performed on ice. Cells were scraped with a rubber policeman, rocked for 15 minutes at 4°C, and centrifuged at 14,000 g for 15 minutes at 4°C. The supernatant was diluted to 1 mg protein/ml, and then incubated with 5 μg/ml goat anti-Pyk2 or 1 μg/ml goat anti-FAK polyclonal IgG antibody overnight at 4°C with rotation, or with 4 μg/ml mouse v-Src (Ab-1) monocolonal Ab for 1.5 hours at 4°C with rotation. Protein G-Plus agarose (30 μl bead volume) was then added and the mixture incubated for 2 hours at 4°C and centrifuged; the pelleted beads were washed 3 times with RIPA buffer.
For coprecipitation studies, cells were grown in 60-mm culture dishes until confluent, rendered quiescent, and incubated in control (pH 7.4) or acid (pH 6.8) medium for the times indicated in the figures. Pyk2 or c-Src was immunoprecipitated as described above. After having been washed 3 times with RIPA buffer, the pelleted beads were suspended in 60 μl 2× SDS loading buffer and the mixture boiled for 5 minutes. After centrifugation, the supernatant was divided into 2 aliquots, one for immunoblotting with the precipitating antibody and the other for immunoblotting with the nonprecipitating antibody [c-Src (1:500 dilution), Pyk2 (1:1000 dilution), or Grb2 (1:200 dilution)]. The abundance of the coprecipitated protein was normalized to the abundance of the precipitated protein and expressed as a percentage of control (pH 7.4).
Immunoblot.
Pyk2 or FAK immunoprecipitated from cells grown in 100-mm culture dishes was subjected to 7.5% SDS-PAGE, and c-Src immunoprecipitated from cells grown in 60-mm culture dishes was subjected to 10% SDS-PAGE. The samples were then electrophoretically transferred to nitrocellulose. After blocking with blocking buffer (5% nonfat milk and 0.05% Tween 20 in PBS), blots were probed with a 1:1000 dilution of rabbit anti-Pyk2 IgG antibody, a 1:50 dilution of mouse antiphosphotyrosine antibody, a 1:500 dilution of rabbit anti-FAK antibody, or a 1:500 dilution of rabbit anti-c-Src antibody in blocking buffer overnight at 4°C. Blots were washed 3 times in PBS containing 0.1% Tween 20 for 10 minutes each and then incubated with a 1:5000 dilution of HRP-labeled anti-rabbit IgG or anti-mouse IgG in blocking buffer for 1 hour and washed as described above. Bands were visualized by ECL and quantified by densitometry.
In vivo phosphorylation and kinase assays.
To assay Pyk2 phosphorylation in vivo, confluent, quiescent cells were incubated in control (pH 7.4) or acid (pH 6.8) medium for the times indicated in the figures. Pyk2 was immunoprecipitated as described above. After washing 3 times with RIPA buffer, 60 μl 2× SDS loading buffer was added to the pelleted beads, and the mixture boiled for 5 minutes. The mixture was briefly centrifuged, and the supernatant was divided into 2 aliquots, one for Pyk2 immunoblot to determine Pyk2 abundance (rabbit polyclonal anti-Pyk2 IgG, 1:1000 dilution) and the second for immunoblot with PY99 (1:50 dilution) to measure phosphorylated Pyk2. Results were normalized for Pyk2 abundance and expressed as percentage of control (pH 7.4).
To measure c-Src kinase activity in vivo, c-Src was immunoprecipitated as described above from cells grown in 60-mm culture dishes that had been exposed to control (pH 7.4) or acid (pH 6.8) medium for the times indicated in the figures. The immunoprecipitate was washed 4 times with RIPA buffer and once with Buffer C (20 mM Na HEPES, 10 mM MgCl2, pH 7.4). The pelleted beads were then suspended in 500 μl of Buffer C, divided into 2 aliquots, and pelleted. One aliquot was used to determine c-Src abundance by immunoblot as described above, and the other aliquot was used to assay kinase activity. To assay c-Src kinase activity, the pellet was heated to 30°C for 1.5 minutes and then suspended in 20 μl of Buffer D (50 mM Na HEPES [pH 7.4], 10 mM MgCl2, 0.01 mM ATP, 0.3 mg/ml acid-denatured enolase, and 5 μCi [32P]-γ-ATP) at 30°C for 10 minutes with rotation. Reactions were stopped by the addition of 20 μl of 2× SDS loading buffer, and the mixture was boiled for 5 minutes. The mixture was then briefly centrifuged, and the supernatant resolved on a 10% SDS-PAGE gel. Phosphorylated enolase was analyzed by PhosphoImage and quantified by densitometry. Results were normalized for c-Src abundance and expressed as a percentage of control (pH 7.4).
In vitro Pyk2 autophosphorylation and Pyk2 and FAK kinase activities.
Pyk2 was immunoprecipitated as described above from cells grown in 100-mm culture dishes, and the beads were washed 3 times with RIPA buffer and once with Buffer A (10 mM MgCl2, 10 mM MnCl2, 150 mM NaCl and 0.2 M Mops) at pH 7.4, 7.2, 7.0, or 6.8. The pelleted beads were then incubated in 30 μl of Buffer A at the respective pH levels, with 1 μg/μl poly(Glu-Tyr)4:1, 20 μM ATP, and 5 μCi [32P]-γ-ATP for 5 minutes at 32°C with rotation. To examine the effect of acid media on the Michaelis-Menten constant for ATP and maximal kinase activity, cold ATP concentrations of 5, 20, 100, and 200 μM were used with a corresponding amount of hot ATP (1.25, 5, 25, and 50 μCi, respectively). To determine whether acid regulation of Pyk2 was calcium dependent, 1 mM EGTA was added to the reaction buffer. Pyk2 autophosphorylation was studied under the same conditions without the addition of poly(Glu-Tyr)4:1. Reactions were stopped by the addition of 30 μl of 2× SDS loading buffer, and the mixture was boiled for 5 minutes. The mixture was then briefly centrifuged and the supernatant divided into 2 aliquots. One aliquot was used for immunoblot of Pyk2 (rabbit polyclonal anti-Pyk2 IgG, 1:1000 dilution) to determine Pyk2 abundance. The other aliquot was resolved on 15% (kinase assay) or 7.5% (autophosphorylation) SDS-PAGE gels, analyzed by autoradiography or PhosphoImage, and quantified by densitometry. Results were normalized for Pyk2 abundance and expressed as a percentage of control (pH 7.4).
To assay FAK activity, FAK was immunoprecipitated as described above from cells grown in 100-mm culture dishes, and the beads were washed 3 times with RIPA buffer and once with Buffer B (5 mM MgCl2, 5 mM MnCl2, and 0.2 M MOPS) at pH 7.4, 7.2, 7.0, or 6.8. The pelleted beads were then incubated in 30 μl of Buffer B at the respective pH, with 1 μg/μl poly(Glu-Tyr)4:1, 5 μM ATP, and 5 μCi [32P]-γ-ATP for 10 minutes at 25°C with rotation. Reactions were stopped by the addition of 30 μl of 2× SDS loading buffer, and the mixture was boiled for 5 minutes. The mixture was then briefly centrifuged and the supernatant divided into two aliquots. One aliquot was used for immunoblot of FAK (rabbit, polyclonal anti-FAK IgG, 1:250 dilution) to determine FAK abundance. The other aliquot was resolved on a 15% SDS-PAGE gel, analyzed by PhosphoImage, and quantified by densitometry. Results were normalized for FAK abundance and expressed as a percentage of control (pH 7.4).
Statistics.
Densitometry was performed on a Molecular Dynamics densitometer using the Image Quant program. Statistical analysis was done using a paired or unpaired Student’s t test, as appropriate, with significance set at P < 0.05.
Acknowledgments
Technical assistance was provided by Ebtesam Abdel-Salam and Carol Wilde. These studies were supported by grants DK39298 and DK20543 from the NIH.
Footnotes
See the related Commentary beginning on page 1696.
Shaoying Li and Soichiro Sato contributed equally to this work.
Patricia Preisig and Robert J. Alpern contributed equally to this work.
Nonstandard abbreviations used: BCECF, 2′7′-bis (2-carboxyethyl)-5-(and-6)-carboxyfluorescein; FAK, focal adhesion kinase; MEK, mitogen-activated protein/ERK kinase; NaDC-1, sodium-dicarboxylate cotransporter 1; NBC-1, sodium bicarbonate cotransporter 1; NHE3, sodium/hydrogen exchanger 3; PEPCK, phosphoenolpyruvate carboxykinase; pHi, cytoplasmic pH; siRNApyk2, small interfering pyk2 RNA.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Alpern RJ, Sakhaee K. The clinical spectrum of chronic metabolic acidosis: homeostatic mechanisms produce significant morbidity. Am. J. Kid. Dis. 1997;29:291–302. doi: 10.1016/s0272-6386(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 2.Tsuganezawa H, et al. Role of c-Src and ERK in acid-induced activation of NHE3. Kidney Int. 2002;62:41–50. doi: 10.1046/j.1523-1755.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamaji Y, Moe OW, Miller RT, Alpern RJ. Acid activation of immediate early genes in renal epithelial cells. J. Clin. Invest. 1994;94:1297–1303. doi: 10.1172/JCI117448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaji Y, et al. Overexpression of csk inhibits acid-induced activation of NHE-3. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6274–6278. doi: 10.1073/pnas.92.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaji Y, Tsuganezawa H, Moe OW, Alpern RJ. Intracellular acidosis activates c-Src. Am. J. Physiol. 1997;272:C886–C893. doi: 10.1152/ajpcell.1997.272.3.C886. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Yin H, Preisig PA, Alpern RJ. Acid incubation stimulates p125FAK and paxillin expression and induces stress fiber and focal adhesion formation in OKP cells [abstract] J. Am. Soc. Nephrol. 2000;11:12A. [Google Scholar]
- 7.Lev S, et al. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 8.Espiritu DJD, Bernardo AA, Robey RB, Arruda JAL. A central role for Pyk2-Src interaction in coupling diverse stimuli to increase epithelial NBC activity. Am. J. Physiol. 2002;283:F663–F670. doi: 10.1152/ajprenal.00338.2001. [DOI] [PubMed] [Google Scholar]
- 9.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 10.Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am. J. Physiol. 1995;269:C126–C133. doi: 10.1152/ajpcell.1995.269.1.C126. [DOI] [PubMed] [Google Scholar]
- 11.Baum M, Cano A, Alpern RJ. Glucocorticoids stimulate Na/H antiporter in OKP cells. Am. J. Physiol. 1993;264:F1027–F1031. doi: 10.1152/ajprenal.1993.264.6.F1027. [DOI] [PubMed] [Google Scholar]
- 12.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein E, Denli AM, Hannon GJ. The rest is silence. RNA. 2001;10:1509–1521. [PMC free article] [PubMed] [Google Scholar]
- 14.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 15.Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Yano H, Schaefer E, Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 durng epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene. 2001;20:2626–2635. doi: 10.1038/sj.onc.1204359. [DOI] [PubMed] [Google Scholar]
- 17.Blaukat A, et al. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J. Biol. Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 18.Laghmani K, Preisig PA, Moe OW, Yanagisawa M, Alpern RJ. Endothelin-1/endothelin-B receptor–mediated increases in NHE3 activity in chronic metabolic acidosis. J. Clin. Invest. 2001;107:1563–1569. doi: 10.1172/JCI11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Licht C, Laghmani K, Yanagisawa M, Preisig PA, Alpern RJ. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004;65:1320–1326. doi: 10.1111/j.1523-1755.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 20.Gullans, S.R., and Mandel, L.J. 2000. Coupling of energy to transport in proximal and distal nephron. In The kidney: physiology and pathophysiology. D.W. Seldin and G. Giebisch, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 467–482.
- 21.Soltoff SP, Mandel LJ. Active ion transport in the renal proximal tubule, III: The ATP dependence of the Na pump. J. Gen. Physiol. 1984;84:643–662. doi: 10.1085/jgp.84.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SY, Avraham HK, Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a src-independent manner. J. Biol. Chem. 2004;279:33315–33322. doi: 10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Avraham H, Cohen DM. Urea and NaCl differentially regulate FAK and RAFTK/PYK2 in mIMCD3 renal medullary cells. Am. J. Physiol. 1998;275:F447–F451. doi: 10.1152/ajprenal.1998.275.3.F447. [DOI] [PubMed] [Google Scholar]
- 24.Cole JA, Forte LR, Krause WJ, Thorne PK. Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am. J. Physiol. 1989;256:F672–F679. doi: 10.1152/ajprenal.1989.256.4.F672. [DOI] [PubMed] [Google Scholar]
- 25.Cano A, Preisig P, Alpern RJ. Cyclic adenosine monophosphate acutely inhibits and chronically stimulates Na/H antiporter in OKP cells. J. Clin. Invest. 1993;92:1632–1638. doi: 10.1172/JCI116748. [DOI] [PMC free article] [PubMed] [Google Scholar]