Abstract
Steroid hormones of gonadal origin act on the neonatal brain, particularly the hypothalamus, to produce sex differences that underlie copulatory behavior. Neuroanatomical sex differences include regional volume, cell number, connectivity, morphology, physiology, neurotransmitter phenotype and molecular signaling, all of which are determined by the action of steroid hormones, particularly by estradiol in males, and are established by diverse downstream effects. Sex differences in distinct hypothalamic regions can be organized by the same steroid hormone, but the direction of a sex difference is often specific to one region or cell type, illustrating the wide range of effects that steroid hormones have on the developing brain. Substantial progress has been made in elucidating the downstream mechanisms through which gonadal hormones sexually differentiate the brain, but gaps remain in establishing the precise relationship between changes in neuronal morphology and behavior. A complete understanding of sexual differentiation will require integrating the diverse mechanisms across multiple brain regions into a functional network that regulates behavioral output.
Keywords: estradiol, hypothalamus, sex differences, sexual behavior, steroids
The brain is a major target of steroid hormones
Behavioral differences between males and females result from the potent ability of three families of steroid hormones to sexually differentiate the nervous system: estrogens, androgens and progestins. It has long been established that the brain is a target organ for steroid hormones, as estrogens, androgens, progestins (as well as adrenal steroids) all bind in the brain with high affinity (Stumpf & Sar, 1976). Gonadal steroids each bind to their own cognate receptors, which are members of a nuclear receptor superfamily and which act in their classical capacity as transcription factors after dimerizing, translocating to the nucleus and binding to appropriate hormone response elements on DNA (King & Greene, 1984; O’Malley & Tsai, 1992). In addition to this classical mode of steroid hormone action, steroid hormone receptors can regulate gene transcription independently of hormone response elements by associating with co-factors such as c-fos, which themselves bind to specific sequences of DNA on gene promoters (Paech et al., 1997; Uht et al., 1997). Steroid hormones also rapidly activate kinases, proteases and other molecules that initiate signaling cascades and lead to gene transcription without hormone receptors ever acting directly on DNA (Gu et al., 1996; Zhou et al., 1996; Watters et al., 1997; Bi et al., 2001; Abraham et al., 2004; Zadran et al., 2009). Lastly, hormones can act within seconds to change cell physiology (Kelly et al., 1976) and these rapid effects are now largely attributed to their action on transmembrane receptors (Towle & Sze, 1983; Mermelstein & Micevych, 2008), although some of the receptors mediating these effects are still being characterized (Toran-Allerand, 2005). These multiple modes of steroid hormone action converge on the developing nervous system to produce sex differences in the brain and in behavior.
Steroids exert organizational and activational effects on the brain
As so elegantly demonstrated in the seminal study by Phoenix et al. (1959), sex differences in reproductive behavior are achieved via a combination of organizational and activational effects of hormones. Hormones first act on the developing brain during a perinatal sensitive period to shape subsequent responsivity to the adult hormonal profiles that mediate reproductive physiology and behavior. Organizational effects are such that the developmental hormonal milieu both potentiates as well as limits the brain’s subsequent response to adult hormone exposure. In this way a brain organized as male during development has a greatly reduced ability to produce female sexual behavior in adulthood, even when receiving female-typical hormone replacement. Thus, the organizational effects of hormones ensure that the brain of an animal is appropriately matched to the hormonal signals it will receive in adulthood. Additionally, sensory circuits in the nervous system are differentiated such that they become limited in their ability to respond to sexually relevant stimuli, such as olfactory signals.
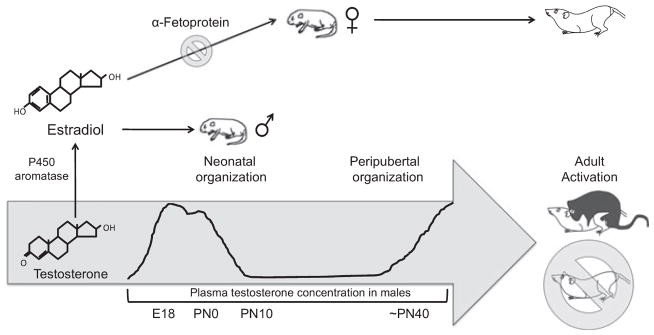
In the rodent, the organizing hormonal signal is the perinatal androgen surge from the testes, which begins prenatally (at approximately embryonic day 18), peaks on the day of birth and rapidly declines within hours (Konkle & McCarthy, in press; Fig. 1). Emerging evidence from hamster studies also implicates an additional peripubertal organizing period in further priming the brain to respond to activational steroid hormones in adulthood (reviewed in Schulz et al., 2009), although much of this work remains to be replicated in other rodent species.
Fig. 1.
The sensitive period for the organizational effects of steroids on the brain. In males, plasma testosterone levels rapidly increase late during the embryonic period and peak on postnatal day (PN) 0. Once in the brain, testosterone is converted by the aromatase enzyme into estradiol, which acts to masculinize and defeminize the hypothalamus / preoptic area. A second organizational period occurs peripubertally, when steroid hormones begin to increase. The developing female brain is protected from the masculniizing effects of the maternal dam’s estradiol by α-fetoprotein, a circulating steroid binding globulin that sequesters estradiol in the bloodstream of the fetus. In adulthood, the differentiated hypothalamus / preoptic area are subject to the activational effects of gonadal hormones, resulting in sex-specific copulatory behavior.
Masculinization, defeminization and feminization are distinct processes of sexual differentiation
Sexual differentiation of the brain involves three processes: masculinization, defeminization and feminization. Masculinization and defeminization are active but distinct processes in the male brain, both of which rely on gonadal hormone action during the perinatal period. Masculinization is the process through which the brain becomes capable of producing male sexual behavior, which includes sexual motivation as well as the copulatory behavior itself, consisting of mounting, erection, intromitting and ejaculating. Defeminization is the process through which the brain loses the ability to produce female-typical sex behavior. Feminization is a default program that proceeds in the absence of organizing steroid hormone action, but is nonetheless an active process. Feminization results in a brain that mediates female sexual responding, which in the rodent consists of a combination of proceptive solicitous behavior and the receptive posture called lordosis. Although the process is poorly understood, feminization, too, is probably dependent upon estrogens of ovarian origin, possibly acting outside the critical period for masculinization (Bakker & Baum, 2008). As the female brain is never exposed to the masculinizing organizational effects of androgens, it is inherenlty kept incapable of producing behavioral responsivity to the activational effects of androgens in adulthood.
Masculinization and defeminization of the hypothalamus are both driven by gonadal hormones, but are distinguishable from one another, in that they are differentially sensitive to neonatal hormone levels (Debold & Whalen, 1975), occur across slightly different developmental time courses (Wallen & Baum, 2002) and involve different brain regions. Masculinization and defeminization of a single brain region can also be controlled by different underlying mechanisms, as our laboratory has shown to be the case in the preoptic area (Todd et al., 2005). Therefore, it is possible for a single adult male to display either male or female copulatory behaviors given the appropriate activational hormones if the mechanisms underlying brain defeminization are interfered with developmentally while those underlying masculinization are left undisturbed. The organizational role of each class of gonadal hormones on these different processes of sex differentiation will be explored subsequently in this review, with a focus on the mechanisms through which gonadal hormones shape the morphology of the developing brain and adult sexual behavior.
Androgens in the brain are converted to estrogens
The organizing effects of steroid hormones are derived from the testicular androgen surge, yet androgens acting on androgen receptors do not alone organize the rodent brain to produce sex-specific copulatory behavior. Rather, in the late 1970s, it became apparent that neonatal estradiol could masculinize the rodent brain as easily as testosterone. The Aromatization Hypothesis reconciled the potent ability of estrogens to masculinize the brain with the established fact that the perinatal androgen surge from the testes was necessary for masculinization to occur. Testosterone is enzymatically converted by cytochrome p450 aromatase into estradiol in the brain, which then acts to produce a complex mosaic of sexually differentiated brain regions. The aromatase enzyme is highly expressed in the brains of both males and females, especially in sexually differentiated areas such as the hypothalamus, and especially during the perinatal androgen surge (George & Ojeda, 1982; Roselli & Resko, 1993; Lauber & Lichtensteiger, 1994). Preventing aromatase activity in the neonate blocks the masculinizing effect of androgens on partner preference (Bakker et al., 1993a, 2002) and impairs several aspects of copulatory behavior, including intromission latency and ejaculation (Bakker et al., 2004).
Once aromatized from testosterone, estradiol acts in the brain on several different estrogen receptors (ERs): ERα, once thought to be the only estrogen receptor, the more recently characterized ERβ, and the G-protein-coupled receptors ER-X and GPR30. The effects of ERα and ERβ can occur either in the nucleus in the ‘classical’ fashion or at the membrane (Mermelstein & Micevych, 2008), ER-X appears to act also largely at the membrane and to activate intracellular signaling cascades (Toran-Allerand et al., 2002; Qiu et al., 2003), whereas GPR30 is most highly expressed on the endoplasmic reticulum and acts to alter intracellular calcium signaling (Revankar et al., 2005). Interestingly, recent knockout mouse models show that ERα is more closely coupled with masculinization and ERβ with defeminization (Kudwa et al., 2006).
The developing female brain is protected from the masculinizing effects of maternal estrogens by α-fetoprotein, a plasma protein which has a high affinity for estradiol and sequesters the hormone in the bloodstream (McEwen et al., 1975). α-Fetoprotein does not bind androgens, and therefore in males testosterone synthesized in the testes gains access to the brain and is locally aromatized to estradiol. As a result, males experience significantly higher estradiol levels in sexually differentiating brain regions than do females (Amateau et al., 2004). Although there is debate over whether α-fetoprotein acts solely to sequester estradiol or also to selectively transport it into the female brain to produce behavioral feminization (Toran-Allerand, 1980, 1987; Bakker & Baum, 2008), female mice with a mutated and therefore dysfunctional form of α-fetoprotein have masculinized sexual behavior and masculine neurochemistry, confirming that α-fetoprotein protects the female brain from masculinization and defeminization (Bakker et al., 2006). Interestingly, α-fetoprotein levels drop significantly in the early postnatal period (Raynaud, 1973; Meijs-Roelofs & Kramer, 1979; Andrews et al., 1982), so α-fetoprotein may first protect the female brain from masculinization and defeminization during the sensitive period for these processes, and then its absence subsequently permits estrogens of ovarian origin to participate in brain feminization.
Sex differences in the brain take many forms
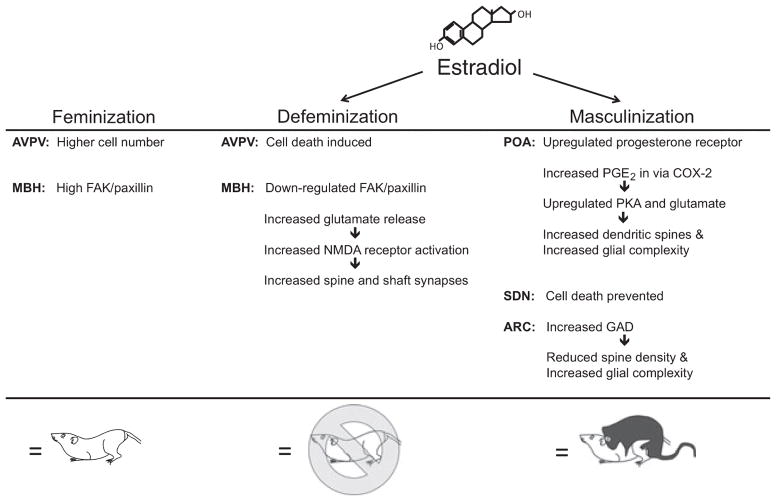
Sex differences in the brain can be broadly categorized as structural, neurochemical or molecular. Structural sex differences range from macroscopic (e.g. differences in brain region area, volume, cell number or projection density) to the microscopic (e.g. differences in cell size, neurite complexity or morphology, dendritic length and spine number). Neurochemical sex differences have been established in neurotransmitter, enzyme or local hormone levels. Molecular sex differences occur in signaling cascade activation, gene expression and epigenetic modifications. Each of these types of sex differences can be found in the hypothalamus and all are regulated by the organizing effects of estradiol (McCarthy et al., 2009; Fig. 2).
Fig. 2.
Estradiol is responsible for both masculinizing and defeminizing the hypothalamus / preoptic area. Estradiol induces a host of molecular and morphological changes in a regionally specific manner. Feminization of the brain is the default program, and prepares the brain for female sexual behavior in adulthood. For abbreviations, see text.
At the broadest level, neonatal estradiol exposure produces sex differences in cell number and in the size or volume of certain regions of the hypothalamus. The two best characterized are the sexually dimorphic nucleus of the preoptic area (SDN-POA), which is larger in males, and the anteroventral periventricular nucleus (AVPV), which is larger in females. These two are a perfect point–counterpoint in that they demonstrate the specificity of estradiol’s actions, either promoting or preventing cell death depending on the brain region (Arai et al., 1996).
The SDN-POA is over five times larger in males than females because it contains many more cells (Gorski et al., 1978). Although we still lack a precise understanding of the role of the SDN in sexual behavior, the profound sex difference in cell number in the SDN-POA is regulated by neonatal estrogen exposure (Rhees et al., 1990). Estradiol prevents cell death in the SDN of males (Arai et al., 1996; Davis et al., 1996) through NMDA receptor activation and a classic downstream expression of the anti-apoptotic protein bcl-2 (Hsu et al., 2001, 2005). In the case of the AVPV, which is involved in producing the luteinizing hormone (LH) surge necessary for ovulation, a single neonatal dose of aromatizable androgen to a female is sufficient to reduce the number of dopaminergic cells to that of a male (Simerly et al., 1985a) and to prevent the ability to produce a preovulatory LH surge (Simerly et al., 1985b). Correspondingly, treating males with an ER antagonist produces cell death in dopaminergic neurons, via downstream upregulation of caspases (Waters & Simerly, 2009). In addition to a sex difference in the number of dopaminergic cells, there are also twice as many estrogen-sensitive GABAergic / glutamatergic cells in the female AVPV (Ottem et al., 2004), and death of these cells is mediated by tumor necrosis factor alpha, which is higher in males (Krishnan et al., 2009). The pro-apoptotic protein Bax also contributes to cell death in GABAergic cells, but does not regulate the sex difference in dopaminergic cell number (Forger et al., 2004). There is yet another sex difference in the AVPV – females have significantly more kisspeptin-positive cells, which are a distinct population from the dopaminergic AVPV neurons (Kauffman et al., 2007). Estradiol also regulates this sex difference, decreasing the number of kisspeptin-positive cells in the AVPV, which are involved in producing the LH surge that is critical for ovulation (Homma et al., 2009). Therefore, estradiol in the AVPV regulates cell death of multiple cell types in the male and appears to do so through different signaling cascades in each instance (Krishnan et al., 2009). Surprisingly, while the sex differences in both of these nuclei is established via cell death during perinatal development, the dimorphism is further reinforced by hormonally regulated neurogenesis in the peripubertal organizational period (Ahmed et al., 2008).
Sex differences in cell morphology and synaptic patterning are regulated by a variety of molecular mechanisms
Many subregions of the hypothalamus contain neurons with sex differences in cell morphology and synaptic connectivity, including differences in neurite or dendritic morphology, dendritic spine number or density, and synapse number or type. Each of these microscopic morphological sex differences is shaped by early estradiol exposure and regulated by distinct molecular underpinnings, sometimes even in the same brain region. The specific mechanisms of cellular sex differentiation have been best characterized in three hypothalamic areas: the arcuate nucleus (ARC), the preoptic area (POA) and the medial basal hypothalamus (MBH) (Fig. 2).
Estradiol in the ARC regulates sex differences in physiology
The ARC of the hypothalamus is located around the third ventricle, near the median eminence. Neurons in the ARC regulate the anterior pituitary release of hormones, including GnRH and prolactin, and control the LH surge (Micevych et al., 2009), lactation (Smith & Grove, 2002) and stress-induced suppression of reproduction (Dobson et al., 2003), as well as controlling non-reproductive functions such as appetite regulation and growth hormone release (reviewed in Bluet-Pajot et al., 1998; Bouret & Simerly, 2006). There are robust sex differences in cell morphology in the ARC. At the ultrastructural level, males have more axosomatic synapses, which are probably GABA-ergic, and females have a higher density of axodendritic spine synapses, which are glutamatergic (Matsumoto & Arai, 1980; Mong et al., 1999). Glia in the ARC are also dimorphic, with males having more complex, stellate astrocytes than females (Mong et al., 1996, 2001; Mong & McCarthy, 1999). These sex differences in cell morphology and synaptic patterning in the ARC are all regulated by estradiol – neonatal castration of males feminizes synapse type (Matsumoto & Arai, 1980; Mong et al., 2001) and spine density (Mong et al., 1999), while treating females neonatally with steroid hormones masculinizes each of these morphological parameters (Mong & McCarthy, 1999; Mong et al., 1999).
The mechanisms through which estradiol inhibits spine formation in the male ARC have yet to be described, but the sex differences in glial morphology in the ARC are dependent upon GABA signaling. Activating GABAA receptors with muscimol induces stellate astrocytic morphology, and reducing the synthesis of the major GABA synthesizing enzyme, GAD, prevents this morphological change (Mong et al., 2001). As estrogen receptors have not been detected in the astrocyte population in the ARC and GAD is expressed only by neurons, it is concluded that estradiol acts first on GABAergic neurons, which then release GABA onto the astrocytes and induce morphological changes. It remains unclear whether hormone-induced glial differentiation then leads to down-regulated spine density in neighboring neurons, or whether estradiol regulates dendritic morphology via entirely independent mechanisms.
Glial morphology and neuron–glia interactions play an integral role in neuroendocrine processes in adulthood (Garcia-Segura et al., 2008). Rapid, hormonally regulated morphological changes in specialized glia of the median eminence are necessary to allow GnRH neuron terminals physical access to the hypophyseal portal capillaries to produce the release of GnRH (Prevot et al., 1999). Similarly, astrocytes in the rostral POA vary in process number and surface area over the estrous cycle of adult females (Cashion et al., 2003), as do both neurons and glia in the ARC (Witkin et al., 1991; Garcia-Segura et al., 1994). This morphological change regulates afferent synaptic connectivity and subsequent hormone release. It is crucial, then, that the effects of gonadal hormones on glia are well characterized in order to gain a comprehensive understanding of how hormones organize sex-specific reproductive behaviors.
Estradiol acting on the POA contributes to behavioral masculinization
The medial preoptic area is critical for the expression of male sexual behavior (Larsson & Heimer, 1964) and receives input from most major hypothalamic regions and limbic areas, such as the amygdala, bed nucleus of the stria terminalis and septum, as well as sensory areas conveying information about sexually relevant stimuli (Simerly & Swanson, 1986), and sends reciprocal projections to other hypothalamic and extrahypothalamic regions involved in motivated behavior and motor regions involved in the expression of sexual behavior (Simerly & Swanson, 1988). Robust sex differences in neuronal morphology and connectivity as well as glial morphology in the POA are programmed by steroid hormone exposure during the critical period. Given its central role in the control of reproductive behavior, its not surprising there is a sex difference in synaptic connectivity in the POA (Raisman & Field, 1973). In males, POA neurons have over twice the number of dendritic spines as females, with neonatal estradiol exposure being sufficient to masculinize spine number in females (Amateau & McCarthy, 2002a, 2004). Astrocytes of the POA are also sexually dimorphic, with males having longer and more numerous processes as a result of estradiol action during the neonatal period (Amateau & McCarthy, 2002b).
Downstream of estradiol in the POA is prostaglandin E2 (PGE2), a lipid molecule which is synthesized following the estradiol-mediated upregulation of cyclooxygenase-2 (COX-2), the major enzyme responsible for prostanoid synthesis in the brain. PGE2 is necessary and sufficient to masculinize morphology in the POA (Amateau & McCarthy, 2002a). This PGE2-induced masculinization of the POA is behaviorally relevant, as administration of PGE2 to neonatal females allows for adult masculine copulatory behavior, whereas treating males with a COX-2 inhibitor significantly reduces these behaviors, even when estradiol is co-administered (Amateau & McCarthy, 2004; Wright & McCarthy, 2009). Glutamate and protein kinase A (PKA) are the downstream effectors of PGE2 in the POA, with antagonism of the AMPA-kainate receptor, the metabotropic glutamate receptors and PKA all preventing PGE2-induced increases in spine number as well as masculine sexual behavior (Amateau & McCarthy, 2002a; Wright & McCarthy, 2009).
One fascinating aspect of the PGE2-induced sexual differentiation of the POA is that it specifically influences behavioral masculinization, while having no detectable effect on defeminization. This is evidenced by the fact that females masculinized with PGE2 neonatally are nonetheless capable of female sexual behavior in adulthood when treated with estrogens and progestins (Todd et al., 2005); in other words, they are masculinized, but not defeminized. An additional behavioral assay for PGE2-induced defeminization in the POA is maternal behavior, a female-typical behavior that is also mediated by the POA (Numan et al., 1985; Numan, 1986). Indeed, neonatal PGE2 administration to females does not impair maternal behavior (Todd et al., 2005), indicating that even in the same brain region masculinized by estradiol-driven prostaglandin signaling, there is no effect on defeminization of reproductive behavior.
Estradiol acting on the MBH contributes to behavioral defeminization
Immediately adjacent to the POA is the medial basal hypothalamus, which includes the ventromedial nucleus (VMN). The VMN is the critical node in the female sexual behavior circuit; stimulation of the VMN facilitates receptivity and lordosis (Pfaff & Sakuma, 1979b), while lesions of the VMN abolish lordosis responding (Pfaff & Sakuma, 1979a). Synaptic patterning and neuronal morphology in the VMN are sexually dimorphic, with the male VMN having more synapses on dendritic shafts and spines (Matsumoto & Arai, 1986), and a more highly branched dendritic morphology (Mong et al., 1999). As with the POA, the sex difference in synaptic patterning is hormonally regulated; testosterone masculinizes this synaptic patterning in females (Matsumoto & Arai, 1986) and increases branch number (Mong et al., 1999), whereas aromatase inhibitors prevent the masculine pattern (Lewis et al., 1995). However, in contrast to the POA, the sex differences in spine number and dendritic branching in the MBH are not mediated by PGE2 (Todd et al., 2005), nor are they accompanied by changes in glial morphology, as is the case in both the POA and the ARC (Mong et al., 1999). Steroid-induced differentiation of the MBH does have one commonality with that of the POA, however – glutamate. Antagonism of the AMPA- and NMDA-type glutamate receptors prevents estradiol-induced spinogenesis (Todd et al., 2007). Interestingly, estradiol in this brain region acts presynaptically, by increasing glutamate release which binds to post-synaptic receptors activating mitogen-activated protein kinase and leading to spine formation (Schwarz et al., 2008). Moreover, glutamate receptor activation is critical to behavioral defeminization – NMDA receptor antagonism during the period of sexual differentiation prevents the steroid hormone-induced defeminization of adult sexual behavior (Schwarz & McCarthy, 2008).
An additional component of estradiol-induced defeminization may involve the suppression of genes important to normal female brain development. In the MBH there are at least two closely related molecules expressed at two-fold or higher magnitude in females compared with males – focal adhesion kinase (FAK) and paxillin (Speert et al., 2007). Both of these molecules are important components of the focal adhesion complex, which negatively regulates cell motility and neurite outgrowth (Brown & Turner, 2004; Rico et al., 2004; Robles & Gomez, 2006). In the developing MBH, estradiol down-regulates FAK within 6 h and paxillin within 48 h while also increasing neurite outgrowth in hypothalamic cultures (Speert et al., 2007). As males have longer dendrites with more spines in the MBH, it follows that FAK and paxillin may negatively regulate neurite outgrowth in females, and that estradiol down-regulates this negative regulator of neurite extension in males. It is an intriguing possibility that FAK and paxillin are two molecular components of active feminization, and that estradiol acts to ‘override’ this signal and result in a defeminized neuronal phenotype.
Androgens contribute to behavioral masculinization via extrahypothalamic targets
Androgens also contribute to the sexual differentiation of brain and behavior, largely via effects on extrahypothalamic targets (Fig. 3). Behaviorally, androgens alone cannot support masculinization, with aromatase knockout males or males given aromatase inhibitors showing disrupted adult partner preference (Bakker et al., 1993a,b), and increased intromission and ejaculation latencies (Bakker et al., 2004). Males receiving aromatase inhibitors prenatally or just after birth also show enhanced lordosis and proceptive behavior (Whalen & Olsen, 1981; Dominguez-Salazar et al., 2002), implying androgens do not support behavioral feminization. These studies are confounded in that androgen receptor expression is regulated by estradiol (McAbee & Doncarlos, 1999; Shah et al., 2004; Juntti et al., 2010), and thus aromatase inhibition may also be altering androgen receptor levels. As normal behavioral masculinization and defeminization are estrogen-dependent, blocking estrogen synthesis may lead to compensatory mechanisms that interfere with normal developmental actions of androgens.
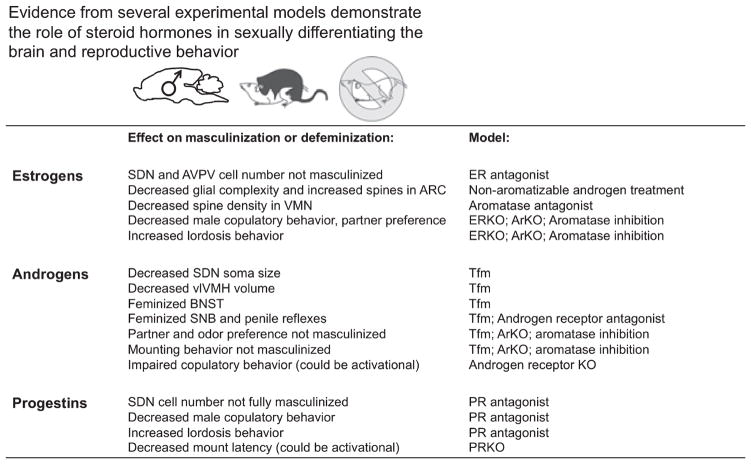
Fig. 3.
Estrogens, androgens and progestins all contribute to sexual differentiation. Evidence from multiple pharmacological and transgenic experimental models implicate each type of gonadal hormone in sexual differentiation of the brain and reproductive behavior. For abbreviations, see text.
Both rats and mice with the testicular feminization mutation (Tfm), which have a non-functioning androgen receptor, show normal partner and odor preference, mounting and thrusting (Olsen, 1979; Bodo & Rissman, 2007), and castrated Tfm male rats show more female sex behavior than intact Tfm males (Olsen, 1979). Tfm studies thus also implicate estrogens, and not androgens, as the driving force behind behavioral masculinization and defeminization. Tfm males do, however, show subtle changes in hypothalamic morphology, including decreased soma size in the SDN-POA (Morris et al., 2005), and decreased volume of the ventromedial hypothalamus (Dugger et al., 2007). These brain regions are implicated in the expression of masculine and feminine sexual behavior, respectively, although the specific consequences of the morphological changes in Tfm rats for behavior have not been determined. Further complicating the matter is that aromatase activity is lower in Tfm males (Roselli et al., 1987), such that decreased estrogen levels may be responsible for the observed feminization. In fact, normal males treated with non-aromatizable androgens show corresponding decreases in aromatase mRNA expression in the hypothalamus, implicating androgens in the regulation of local estrogen synthesis (Abdelgadir et al., 1994).
Androgen receptor knockout mice, unlike Tfm males, show copulatory deficits, including reductions in mounting, intromission and ejaculation even when administered androgens or estrogens in adulthood (Sato et al., 2004; Raskin et al., 2009; Juntti et al., 2010). The fact that knockout animals do not have androgen receptors either developmentally or in adulthood precludes the ability to determine whether developmental and / or adult androgen receptor function is necessary to produce masculinized and defeminized behavior. Detailed anatomical study of androgen receptor knockouts has yet to be done, and will be helpful in determining whether androgens play a role in masculinizing the hypothalamus.
Androgens have been more firmly established in the regulation of sex differentiation of extrahypothalamic brain regions involved in copulatory behavior. The spinal nucleus of the bulbocavernosus (SNB), a motor nucleus involved in the production of penile reflexes in males, is highly androgen-dependent (Breedlove & Arnold, 1983a,b). Androgens save SNB motoneurons from cell death (Nordeen et al., 1985) and support masculine patterns of dendritic arborization (Goldstein et al., 1990), and anti-androgen-treated or Tfm male rats have a feminized SNB (Breedlove & Arnold, 1981, 1983a). Additionally, the bed nucleus of the stria terminalis, which is involved in the production of copulatory behavior (Emery & Sachs, 1976), shows regional sex differences in volume (Guillamon et al., 1988), and is partially feminized in Tfm males (Durazzo et al., 2007). Overall, evidence from multiple experimental models and various brain regions converges to suggest that androgens play a role in the sexual differentiation of copulatory behavior, largely via effects on extrahypothalamic targets.
Progestins support behavioral masculinization and defeminization
Progestins also contribute to the sexual differentiation of the hypothalamus and sexual behavior, although they have received considerably less attention than the other gonadal hormones. Progesterone levels in the neonate are not different in males and females (Weisz & Ward, 1980), but there are significant sex differences in progesterone receptor (PR) expression in the developing hypothalamus. Males have higher PR in the medial preoptic nucleus, periventricular nucleus, AVPV and arcuate nucleus (Quadros et al., 2002a,c, 2007). Sex differences in PR are age-dependent, with males having higher preoptic PR expression from embryonic day 18 until postnatal day 10, and females having a later surge in PR expression around postnatal day 10 which attenuates the sex difference (Quadros et al., 2002a). This sex difference in PR is regulated by estrogens, with the delayed production of estradiol in the female resulting in delayed PR up-regulation relative to the earlier androgen surge in males (Quadros et al., 2002a).
Progesterone signaling during the neonatal period regulates hypothalamic development as well as adult male sexual behavior (Fig. 3). Antagonizing progesterone action prevents the androgen-induced masculinization of the SDN-POA (Quadros et al., 2002b), implicating progesterone as a mediator of this masculinization. Progesterone receptor antagonism during development also negatively impacts masculine sexual behavior (Lonstein et al., 2001) and attenuates normal defeminization (Weinstein et al., 1992). PR knockouts, in contrast, have decreased mount latencies, although this effect may also be activational in nature (Schneider et al., 2005). Despite this suggestive data, research has yet to show the means through which progesterone acts to sexually differentiate the developing brain. Moreover, PR antagonism studies used RU-486, a compound which also antagonizes the glucocorticoid receptor, and thus these experiments are far from conclusive. The mechanisms through which progesterone contributes to sexual differentiation is an area ripe for discovery; such research could greatly enhance our understanding of whether the different gonadal hormones act independently or cross talk to produce and maintain behavioral sex differences.
Gonadal hormones and hypothalamic sex differences –concepts and conclusions
This review has focused on the mechanisms through which gonadal hormones organize the rodent hypothalamus to produce masculine or feminine sexual behavior. We as a field have an understanding of several well-characterized sex differences in the hypothalamus, how these sex differences are achieved mechanistically, as well a broad understanding of whether these sex differences are necessary for the production of adult sexual behavior. Although advances are being made in elucidating several unique mechanisms of gonadal hormone action on the brain, there remains a gap between directly associating changes in the neuronal substrate with changes in behavior. Moreover, an integrated conceptualization of hormonally induced sexual differentiation has not been realized. It is unknown whether each cellular mechanism is independent of others or whether there is cross talk between signaling cascades and brain regions that alters the outcome in subtle ways not yet detected? The influence of estradiol on the developing rodent brain is pervasive, having many more effects than those reviewed here and often involving modulation of the disparate molecular signaling cascades that govern normal brain development. Yet reconciling the potent ability of estradiol to regulate the development of the male rodent brain (and the avian brain) with the lack of estradiol-driven masculinization in primates remains a challenge. Generating a cohesive picture of gonadal hormone action on the developing brain and then causally connecting that to adult physiology and behavior as well as generalizing these mechanisms across mammalian and even vertebrate phylogeny is a fundamental future goal. Recent data suggest that rapid and / or membrane effects of gonadal hormones contribute to the sexual differentiation of the hypothalamus (Speert et al., 2007; Schwarz et al., 2008), but future research will surely continue to characterize these non-classical actions. Future research will also continue to explore the extent to which the classical effects of gonadal hormones on gene transcription can be dampened, enhanced or maintained long-term via both classic protein-synthesis-mediated permanent changes and enduring imprints imposed via epigenetic changes to the genome. Recent work has also implicated direct effects of sex chromosome complement on sexual differentiation of the brain, which are independent of hormonally mediated organization (Arnold & Chen, 2009). Future research will thus better characterize how chromosomal sex and gonadal sex interact to produce sexually differentiated brain and behavior.
Abbreviations
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- COX-2
cyclooxygenase-2
- ER
estrogen receptor
- FAK
focal adhesion kinase
- LH
luteinizing hormone
- MBH
medial basal hypothalamus
- PGE2
prostaglandin E2
- PKA
protein kinase A
- POA
preoptic area
- PR
progesterone receptor
- SDN-POA
sexually dimorphic nucleus of the preoptic area
- SNB
spinal nucleus of the bulbocavernosus
- Tfm
testicular feminization mutation
- VMN
ventromedial nucleus
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002a;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002b;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Andrews GK, Dziadek M, Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J Biol Chem. 1982;257:5148–5153. [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the ‘‘four core genotypes’’ mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci. 1993a;107:480–487. doi: 10.1037//0735-7044.107.3.480. [DOI] [PubMed] [Google Scholar]
- Bakker J, van Ophemert J, Slob AK. Organization of partner preference and sexual behavior and its nocturnal rhythmicity in male rats. Behav Neurosci. 1993b;107:1049–1058. doi: 10.1037//0735-7044.107.6.1049. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluet-Pajot MT, Epelbaum J, Gourdji D, Hammond C, Kordon C. Hypothalamic and hypophyseal regulation of growth hormone secretion. Cell Mol Neurobiol. 1998;18:101–123. doi: 10.1023/a:1022579327647. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70:295–301. doi: 10.1111/j.1399-0004.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983a;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983b;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM. The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology. 2003;144:274–280. doi: 10.1210/en.2002-220711. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Debold JF, Whalen RE. Differential sensitivity of mounting and lordosis control systems to early androgen treatment in male and female hamsters. Horm Behav. 1975;6:197–209. doi: 10.1016/0018-506x(75)90036-7. [DOI] [PubMed] [Google Scholar]
- Dobson H, Ghuman S, Prabhakar S, Smith R. A conceptual model of the influence of stress on female reproduction. Reproduction. 2003;125:151–163. doi: 10.1530/rep.0.1250151. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75:337–346. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A, Morris JA, Breedlove SM, Jordan CL. Effects of the testicular feminization mutation (tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats. Neurosci Lett. 2007;419:168–171. doi: 10.1016/j.neulet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Emery DE, Sachs BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav. 1976;17:803–806. doi: 10.1016/0031-9384(76)90044-5. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Duenas M, Torres-Aleman I, Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994;19:445–453. doi: 10.1016/0306-4530(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Lorenz B, DonCarlos LL. The role of glia in the hypothalamus: implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction. 2008;135:419–429. doi: 10.1530/REP-07-0540. [DOI] [PubMed] [Google Scholar]
- George FW, Ojeda SR. Changes in aromatase activity in the rat brain during embryonic, neonatal, and infantile development. Endocrinology. 1982;111:522–529. doi: 10.1210/endo-111-2-522. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988;44:281–290. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH / LH surge system in male rats. Biol Reprod. 2009;81:1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Yang RC, Shih HC, Hsieh YL, Chen UY, Hsu C. Prenatal exposure of testosterone prevents SDN-POA neurons of postnatal male rats from apoptosis through NMDA receptor. J Neurophysiol. 2001;86:2374–2380. doi: 10.1152/jn.2001.86.5.2374. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Shao PL, Tsai KL, Shih HC, Lee TY, Hsu C. Gene regulation by NMDA receptor activation in the SDN-POA neurons of male rats during sexual development. J Mol Endocrinol. 2005;34:433–445. doi: 10.1677/jme.1.01601. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time-course of estradiol, testosterone and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. doi: 10.1210/en.2010-0607. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Larsson K, Heimer L. Mating behaviour of male rats after lesions in the preoptic area. Nature. 1964;202:413–414. doi: 10.1038/202413a0. [DOI] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult GDX. Brain Res Dev Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Quadros PS, Wagner CK. Effects of neonatal RU486 on adult sexual, parental, and fearful behaviors in rats. Behav Neurosci. 2001;115:58–70. doi: 10.1037/0735-7044.115.1.58. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sexual dimorphism in ‘wiring pattern’ in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res. 1980;190:238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Male–female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140:3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Plapinger L, Chaptal C, Gerlach J, Wallach G. Role of fetoneonatal estrogen binding proteins in the associations of estrogen with neonatal brain cell nuclear receptors. Brain Res. 1975;96:400–406. doi: 10.1016/0006-8993(75)90755-6. [DOI] [PubMed] [Google Scholar]
- Meijs-Roelofs HM, Kramer P. Maturation of the inhibitory feedback action of oestrogen on follicle-stimulating hormone secretion in the immature female rat: a role for alpha-foetoprotein. J Endocrinol. 1979;81:199–208. doi: 10.1677/joe.0.0810199. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol. 2001;432:259–267. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487:217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Numan M. The role of the medial preoptic area in the regulation of maternal behavior in the rat. Ann N Y Acad Sci. 1986;474:226–233. doi: 10.1111/j.1749-6632.1986.tb28014.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Morrell JI, Pfaff DW. Anatomical identification of neurons in selected brain regions associated with maternal behavior deficits induced by knife cuts of the lateral hypothalamus in rats. J Comp Neurol. 1985;237:552–564. doi: 10.1002/cne.902370411. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Induction of male mating behavior in androgen-insensitive (tfm) and Normal (King-Holtzman) male rats: effect of testosterone propionate, estradiol benzoate, and dihydrotestosterone. Horm Behav. 1979;13:66–84. doi: 10.1016/0018-506x(79)90035-7. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46:163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA / glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979a;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979b;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, Beauvillain JC. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94:809–819. doi: 10.1016/s0306-4522(99)00383-8. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Goldstein AY, De Vries GJ, Wagner CK. Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol. 2002a;14:761–767. doi: 10.1046/j.1365-2826.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol. 2002b;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002c;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol. 2007;504:42–56. doi: 10.1002/cne.21427. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud JP. Influence of rat estradiol binding plasma protein (EBP) on uterotrophic activity. Steroids. 1973;21:249–258. doi: 10.1016/s0039-128x(73)80009-1. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O’Malley B, Levine JE. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm Behav. 2008;54:662–668. doi: 10.1016/j.yhbeh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985a;330:55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985b;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol. 2002;23:225–256. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- Speert DB, Konkle AT, Zup SL, Schwarz JM, Shiroor C, Taylor ME, McCarthy MM. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148:3391–3401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7:1163–1170. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA / kainate receptors, not GABA(A) receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev Neurobiol. 2007;67:304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Coexistence of alpha-fetoprotein, albumin and transferrin immunoreactivity in neurones of the developing mouse brain. Nature. 1980;286:733–735. doi: 10.1038/286733a0. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Neuronal uptake of alpha-fetoprotein (AFP) synthesized and secreted by hepatocytes in liver / brain co-cultures. Neurosci Lett. 1987;83:35–40. doi: 10.1016/0304-3940(87)90212-6. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. J Steroid Biochem. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- Uht RM, Anderson CM, Webb P, Kushner PJ. Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology. 1997;138:2900–2908. doi: 10.1210/endo.138.7.5244. [DOI] [PubMed] [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff D, editor. Hormones, Brain, and Behavior. Academic Press; New York: 2002. pp. 385–424. [Google Scholar]
- Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Weinstein MA, Pleim ET, Barfield RJ. Effects of neonatal exposure to the antiprogestin mifepristone, RU 486, on the sexual development of the rat. Pharmacol Biochem Behav. 1992;41:69–74. doi: 10.1016/0091-3057(92)90061-j. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Olsen KL. Role of aromatization in sexual differentiation: effects of prenatal ATD treatment and neonatal castration. Horm Behav. 1981;15:107–122. doi: 10.1016/0018-506x(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA / kainate, and metabotropic glutamate receptor signaling. J Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci USA. 2009;106:21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]