Acute disseminated encephalomyelitis (ADEM) mostly occurs in children and can be triggered by infections and vaccinations. Recently, 40% of patients with ADEM were found to be seropositive for myelin oligodendrocyte glycoprotein antibodies (MOG-abs).1 Furthermore, a subgroup of adult patients negative for aquaporin-4 antibody fulfilling diagnostic clinical and radiologic criteria for neuromyelitis optica spectrum disorder (NMOSD) harbor high-titer serum MOG-abs.2 We present clinical, serologic, and histopathologic features of 2 adult patients with a clinical diagnosis of ADEM according to the diagnostic criteria3 associated with intrathecal MOG-abs synthesis. MOG-abs were determined by live-cell immunofluorescence on HEK293T cells expressing full-length human MOG-enhanced green fluorescent protein at a starting dilution of 1:20 in serum and 1:2 in CSF using an epifluorescence microscope and end-point titration as previously described.2
Case reports.
Case report 1.
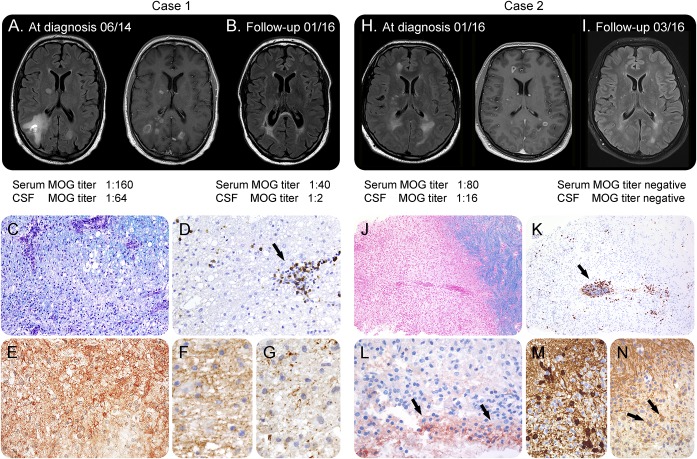
A 49-year-old man developed subacute encephalopathy with bradyphrenia, dysphoria, and anhedonia together with progressive central paresis of the right leg, a sensory level below TH10, myalgia, and deteriorated to tetraparesis despite treatment with aciclovir, ceftriaxone, and methylprednisolone. No preceding infections, vaccinations, or prior neurologic symptoms were reported. MRI showed extensive spinal cord and cerebral lesions (figure 1, A and B, and figure e-1 at Neurology.org/nn). CSF analysis demonstrated pleocytosis (133 white blood cells [WBCs]/μL) and elevated protein (1,572 mg/L) without CSF-restricted oligoclonal IgG. No infectious agents were detected. Brain biopsy performed 4 weeks after the onset of symptoms showed active, confluent demyelination with IgG and complement deposition, perivascular and parenchymal B- and T-cell accumulation, parenchymal macrophage infiltration, and oligodendrocyte apoptosis associated with selective loss of minor myelin proteins consistent with overlapping features of MS patterns II and III (figure 1, C–G). Plasma exchange and intravenous cyclophosphamide led to improvement. No new clinical or radiologic activity was observed on follow-up (17 months).
Figure 1. Neuropathology of MOG-antibody–associated demyelination.
Case 1 (A–G): MRI showed large bilateral hazy, partly Gd-enhancing lesions in the deep white matter and periventricular zone (A, left axial-fluid-attenuated inversion recovery (FLAIR), right Gd-enhanced axial T1) that regressed partially during follow-up (B, axial-FLAIR). Histopathology showed a demyelinating lesion (C, Luxol fast blue) and inflammatory infiltrates mainly composed of CD8-positive T cells (D, CD8; arrow). The lesion showed deposits of activated complement complex C9neo on degenerating fibers and in macrophages (E). In early active demyelinating lesion zones, MOG was still present (F; MOG), while myelin-associated glycoprotein (MAG) was lost (G; MAG). Case 2 (H–N): MRI showed large bilateral hazy lesions in the deep white matter and periventricular zone as well as gadolinium rim-enhancing, well-demarcated lesions in the deep white matter (H, left axial-FLAIR, right Gd-enhanced axial T1) that regressed on follow-up (I, axial-FLAIR). Biopsy of a ring-enhancing lesion revealed a well-demarcated demyelinating lesion (J, Luxol fast blue), T-cell dominated inflammation (K, CD8), and mild perivascular complement deposition (L, C9neo; arrows) reminiscent of MS pattern II. Numerous remyelinating oligodendrocytes were encountered (M, tubulin polymerization promoting protein/p25) that were partly MOG positive (N; MOG; arrows). Magnification: C–G; L–N: ×400; J, K: ×100 (MRIs [A, B] from the Institute of Neuroradiology, Magdeburg, Germany). Further panels are provided in figure e-1. MOG = myelin oligodendrocyte glycoprotein.
Retrospective analysis of the initial CSF and serum confirmed MOG-abs (IgG1, IgG3, and IgM) in the CSF (IgG 1:64, follow-up 17 months later 1:2) and serum (IgG 1:160, follow-up 17 months later 1:40). Initial intrathecal MOG-abs-IgG index was 22.3 (<4, total IgG-CSF 0.143 g/L, serum 8.0 g/L).
Case report 2.
A 34-year-old man presented with aphasia and somnolence followed by hypesthesia and paresis of lower extremities, bladder dysfunction, and ataxia. MRI showed multiple cerebral and spinal lesions (figures 1, H and I and e-1). CSF studies revealed pleocytosis (151 WBCs/μL), elevated protein (1,260 mg/L), and CSF-restricted oligoclonal bands. Biopsy 6 weeks after symptom onset showed a confluent, well-demarcated demyelinating lesion with a rim of parenchymal macrophages, T-cell–dominated inflammation, and mild complement deposition, reminiscent of MS pattern II (figure 1, J–N). The patient responded well to treatment with methylprednisolone, IV immunoglobulin (IVIG), and plasma exchange. Three-month follow-up showed regression of MRI lesions. CSF-restricted oligoclonal bands were still present. Last follow-up 9 months after the onset did not reveal any new clinical or radiologic activity.
Retrospective serum and CSF analyses confirmed IgG and IgM MOG-abs (initial serum 1:80, CSF 1:16; 3-month follow-up serum <1:40, CSF <1:2). Initial intrathecal MOG-abs-IgG index was 32.4 (<4, total IgG-CSF 0.073 g/L, serum 1.2 g/L).
Discussion.
We report 2 adult patients with a subacute, multifocal clinical presentation with encephalopathy and MRI features fulfilling clinical diagnostic criteria of ADEM,3 who both had (1) an MS-like histopathology on brain biopsy and (2) intrathecal MOG-abs synthesis.
Pathologically, ADEM is distinct from MS with minor perivascular demyelination in ADEM vs confluent plaque–like demyelination in MS while both conditions share perivascular inflammation. Neuropathologic reports of MOG-abs–associated demyelination are scarce and show MS typical confluent demyelination with astrocyte preservation in a patient with a clinical syndrome of NMOSD,4 MS pattern II–like pathology in a patient with a clinically isolated syndrome,5 a patient fulfilling criteria for relapsing MS,6 and an overlap of pathologic MS and NMOSD features in a patient clinically classified as ADEM, who also had anti–aquaporin-4 antibodies.7
Our cases imply that, although clinically and radiologically presenting as ADEM-like syndrome, MOG-abs–associated demyelinating disorders could pathologically resemble MS.
MOG-abs are increasingly recognized in adult patients with inflammatory CNS demyelination with a yet-to-be-defined spectrum encompassing NMOSD, ADEM, and uni- or bilateral isolated optic neuritis (ON).1 They are considered as highly sensitive and specific when tested with appropriate methods using live (unfixed) cell-based assays. MOG-abs are more consistently detected in the serum than in the CSF, and intrathecal MOG-abs synthesis is unusual.2 While CSF MOG-abs in children with ADEM are unusual, future systematic examination of adults with multifocal demyelinating CNS syndromes with MOG-abs will hopefully elucidate, whether our observation of intrathecal MOG-abs synthesis is a coincidental or causal association. Our observations (1) indicate that MOG-abs–associated inflammatory demyelination independent of clinical presentation histopathologically resembles MS and (2) should encourage clinicians to test for MOG-abs in inflammatory CNS diseases suggestive of ADEM, ON, or NMOSD in the serum and CSF using appropriate test methods.
Supplementary Material
Footnotes
Supplemental data at Neurology.org/nn
Author contributions: Péter Körtvélyessy and Markus Breu have access to all the data and take responsibility for the data, accuracy of the data analysis, the conduct of the research design and conceptualization of the study and analysis and interpretation of the data, and drafting of the manuscript for intellectual content. Marc Pawlitzki: design and conceptualization of the study and revising the manuscript for intellectual content. Imke Metz: accuracy of the data analysis, the conduct of the research design, analysis and interpretation of the data, and drafting of the manuscript for intellectual content. Mike Matzke, Hans-Jochen Heinze, and Christian Mawrin: design and conceptualization of the study and revising the manuscript for intellectual content. Paulus Rommer, Gabor G. Kovacs, and Christian Mitter: accuracy of the data analysis and revising the manuscript for intellectual content. Hans Lassmann. Wolfgang Brück, Klaus-Peter Wandinger, and Markus Reindl: accuracy of the data analysis, the conduct of the research design analysis and interpretation of the data, and drafting of the manuscript for intellectual content. Romana Höftberger and Frank Leypoldt have access to all the data and takes responsibility for the data, accuracy of the data analysis, the conduct of the research design and conceptualization of the study and analysis and interpretation of the data, and drafting of the manuscript for intellectual content.
Study funding: This study was supported by a research grant from the “Medizinisch-Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien, Project 15022,” the “Jubiläumsfonds der Österreichischen Nationalbank, Project 16919,” and the Austrian Federal Ministry of Science and Economy (grant BIG WIG MS).
Disclosure: P. Körtvelyessy received travel funding from Eisai gGmbH. M. Breu reports no disclosures. M. Pawlitzki served on the scientific advisory board for Novartis and received travel funding from Novartis. I. Metz served on the advisory board for Roche; received travel funding and/or speaker honoraria from BiogenIdec, Bayer Healthcare, TEVA, Serono, Novartis, and Genzyme; and received research support from BiogenIdec and German Ministry for Education and Research. H.-J. Heinze served on the scientific advisory board for UKE Hamburg Eppendorf, Hertie Institute for Clinical Brain Research, and Neuro-Nielsen; served on the editorial board for Frontiers in Human Neuroscience; is vice-speaker for German Centre for Neurodegenerative Diseases; and received research support from Deutsche Forschungs Gemeinschaft, DFG, BMBF, Bundesministerium für Bildung und Forschung, SFB 779, and Excellence initiative of the state Saxony-Anhalt. M. Matzke received travel funding and speaker honoraria from Bayer-Schering, Merck Serono, Novartis, Sanofi-Aventis, Biogen, and TEVA; received research support from Novartis and Merck Serono. C. Mawrin reports no disclosures. P. Rommer consulted for Teva and received speaker honoraria from Merck and Roche. G.G. Kovacs served on the scientific advisory board for Gedeon Richter Plc. Pharmaceutical Company; is on the editorial board for Acta Neuropathologica, Neuropathology and Applied Neurobiology and Clinical Neuropathology; holds a patent for Anti-alpha-synuclein; receives publishing royalties from Cambridge University Press; received research support from Federal Ministry of Health, FP7 EU Project Develage. C. Mitter reports no disclosures. M. Reindl is an academic editor for PLoS One, The University Hospital, and Medical University of Innsbruck (Austria, Markus Reindl); receives payments for antibody assays (NMDAR, AQP4, and other autoantibodies) and for MOG and AQP4 antibody validation experiments organized by Euroimmun (Luebeck, Germany). W. Brück served on the advisory board for Genzyme, Novartis, Biogen, and Teva; received speaker honoraria from Teva, Sanofi, Genzyme, Novartis, Merck Serono, Biogen, Roche, and Bayer; is on the editorial board for Acta Neuropathologica, Therapeutic Advances in Neurological Disorders, Multiple Sclerosis International and Neuropathology, and Applied Neurobiology; received research support from Teva Pharma, Novartis, Biogen, Genzyme, German Research Foundation, German Ministry for Education and Research, Tschira Foundation, and German Multiple Sclerosis Foundation; and was an expert witness for Teva. K.-P. Wandinger reports no disclosures. H. Lassmann received travel funding and speaker honoraria from BiogenIdec, Novartis, Roche, and Teva; is on the editorial board for journals in the fields of Neurology and Neuroscience; has consulted for BiogenIdec and AMGEN; and received research support from Austrian Science Fund and European Union PITN. R. Höftberger reports no disclosures. F. Leypoldt served on the scientific advisory board for Roche; received travel funding and/or speaker honoraria from Grifols, Teva, Biogen, Fresenius, and Bayer; and does commercial antibody testing service for 20% of his time. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 2013;9:455–461. [DOI] [PubMed] [Google Scholar]
- 2.Höftberger R, Sepulveda M, Armangue T, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 2015;21:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JJ, Jaunmuktane Z, Mummery C, Brandner S, Leary S, Trip SA. Inflammatory demyelination without astrocyte loss in MOG antibody-positive NMOSD. Neurology 2016;87:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarius S, Metz I, König FB, et al. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in 'pattern II multiple sclerosis' and brain biopsy findings in a MOG-IgG-positive case. Mult Scler 2016;22:1541–1549. [DOI] [PubMed] [Google Scholar]
- 6.Spadaro M, Gerdes LA, Mayer MC, et al. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol 2015;2:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Pauli F, Höftberger R, Reindl M, et al. Fulminant demyelinating encephalomyelitis: insights from antibody studies and neuropathology. Neurol Neuroimmunol Neuroinflamm 2015;2:e175 doi: 10.1212/NXI.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.