Abstract
Observational data indicate that behaviors that shift energetic homeostasis, such as exercise, may decrease the risk of developing breast cancer by reducing the amount of energy-dense, metabolically active adipose tissue. Between December 2008 and April 2013, we conducted a single-blind, 5-month, clinical trial that randomized premenopausal women at high risk of developing breast cancer to one of three groups: 150 min/wk of aerobic exercise (low dose), 300 min/wk of aerobic exercise (high dose), or control. Body composition was assessed using dual-energy x-ray absorptiometry. Background parenchymal enhancement (BPE) was quantified using computerized algorithms on breast dynamic contrast-enhanced MRI. Over 5 months, compared with the control group: the low-dose and high-dose groups lost −1.5 ± 0.5 and −1.3 ± 0.5 kg of body mass (linear Ptrend = 0.032); −1.5 ± 0.4 and −1.4 ± 0.3 kg of fat mass (linear Ptrend = 0.003); −1.3 ± 0.3 and −1.4 ± 0.3% of body fat (linear Ptrend < 0.001); −15.9 ± 5.4 and −26.6 ± 5.0 cm2 of subcutaneous adipose tissue (linear Ptrend < 0.001); and −6.6 ± 1.9 and −5.0 ± 1.9 cm2 visceral adipose tissue (nonlinear Ptrend = 0.037). For each −1 cm2 reduction in visceral adipose tissue, BPE decreased by −3.43 ± 1.34 cm2 (P = 0.010) and explained 9.7% of the variability in BPE. Changes in other aforementioned body composition outcomes did not significantly correlate with changes in BPE. These mechanistic data support observational evidence that shifting energetic homeostasis through exercise may alter the risk of developing breast cancer. Additional adequately powered studies are needed to confirm and expand upon our findings that changes in body composition are associated with changes in BPE.
Introduction
Women with BRCA1/2 gene mutations have an increased risk of developing breast cancer. Estimates for lifetime risk of developing breast cancer among BRCA1/2 mutation carriers vary from 30% to 80% (1). Factors most strongly related to the risk of developing breast cancer among BRCA1/2 mutation carriers include sex hormone concentrations and reproductive characteristics (2). Additional variability in lifetime risk among BRCA1/2 mutation carriers may be explained by factors related to energetic homeostasis.
Observational evidence suggests that energy expenditure through physical activity may be associated with a lower risk of developing breast cancer among BRCA1/2 mutation carriers (3) and premenopausal women (4). Conversely, the storage of excess energy through weight gain and unfavorable alterations in body composition, particularly the accumulation of abdominal fat (5), may be associated with a higher risk of developing breast cancer among BRCA1/2 mutation carriers (6, 7) and premenopausal women (8, 9). Weight loss in early adulthood may also be associated with a lower risk of developing breast cancer among BRCA1/2 mutation carriers (10). These observational data are consistent with the hypothesis that behaviors which shift energetic homeostasis may reduce the risk of developing breast cancer by increasing energy expenditure and reducing the amount of energy-dense, metabolically active adipose tissue. However, the biologic mechanisms that mediate the relationship between energy balance–related factors and breast cancer risk have not been elucidated.
Background parenchymal enhancement (BPE) is the volume and intensity that normal fibroglandular breast tissue enhances when measured using MRI with contrast (11). Studies suggest that increased BPE may be predictive of a 3-to 10-fold elevation in breast cancer risk (11, 12). Quantifying changes in BPE may be a useful outcome to predict response to risk-reducing interventions (11, 13). Women with increased BPE have a higher body mass index (BMI) than women with lower BPE, a known risk factor for breast cancer (14). However, it is unknown if improving body composition correlates with reductions in BPE. If exercise-induced changes in body composition are correlated with reductions in BPE, it would provide mechanistic data to corroborate the findings from previous observational studies that have identified associations between physical activity or body composition and breast cancer risk (15).
The Women In Steady Exercise Research (WISER) Sister study was a three-armed randomized controlled trial with the primary aim to test the dose–response effects of 150 and 300 minutes per week (min/wk) of aerobic exercise versus usual care control over 5 months on endogenous sex hormones among 139 healthy premenopausal women at elevated risk of developing breast cancer (16). The primary and key secondary outcomes of the WISER Sister study including estrogen, progesterone, and BPE have been reported (17). Here, we report additional secondary outcomes including body composition measures and characterize the relationship between changes in body composition and changes in BPE. Our primary hypothesis was that exercise would favorably alter body composition outcomes in dose–response fashion and that improvements in body composition would correlate with reductions in BPE.
Materials and Methods
Participants
Eligibility requirements included: female sex, no personal history of cancer, BMI ≤ 50 kg/m2, age of 18 to 50 years, eumenorrheic (menstrual cycles, 23–35 days in length), time since starting menstruation ≥4 years, intact ovaries and uterus, no hormonal contraceptive use (past 3 months for oral and vaginal methods, past 12 months for medroxyprogesterone), prior tubal ligation or willingness to use nonhormonal birth control during study, no eating disorder as assessed using the Eating Disorder Diagnostic Scale (18), not currently in a weight loss program, not pregnant in past 6 months, not currently breastfeeding, not planning to become pregnant, no more than seven alcoholic beverages per week, self-reported aerobic exercise of <75 min/wk over past 6 months, not planning to move during study, no medical conditions that would preclude safe participation in exercise, and a predicted lifetime breast cancer risk ≥18% evidenced by a documented BRCA1/2 mutation for participant or first-degree relative, and/or Claus model risk >18% (19), and/or Gail model risk >18% (20). Participants were recruited from across the continental United States using national organizations that provide resources to women at elevated risk of breast cancer, such as Facing Our Risk of Cancer Empowered (FORCE) and the Cancer Genetics Network (21). Costs for travel (airfare, hotel) to complete in-person measurement visits were paid for by the study. Additional details about recruitment have been published previously (16).
Women were stratified on years since starting menstruation (<10 vs. ≥10 years) and BMI (<30 kg/m2 vs. ≥30 kg/m2) then randomized in equal ratio to one of three groups: low-dose aerobic exercise (150 min/wk), high-dose aerobic exercise (300 min/wk), or control (usual activities). The study was approved by the University of Pennsylvania Institutional Review Board. Women provided written informed consent and written approval from their physician prior to participation.
Intervention
Aerobic exercise was performed over 5 months using study-provided in-home treadmills (Smooth Fitness, Model 5.65). A 5-month intervention was selected to be long enough to induce changes in urinary hormones (primary outcome), while balancing the feasibility of recruitment, complexity of delivering a distance-based intervention, and completing the study within the funding period. Participants were provided with a heart rate monitor to objectively record heart rate during each exercise session. The heart rate monitor had a long-term memory to record up to 99 bouts of exercise using a 1-minute epoch for each exercise bout. Every month, a new heart rate monitor was provided to each participant, and monitors from the previous month with recorded exercise data were returned to study staff via postal mail for data download and analysis. Participants also used exercise logs to record the date, time, average heart rate, and exercise duration. Participants were contacted by a certified exercise professional each week to promote compliance and facilitate progression of exercise volume (i.e., frequency, duration, and intensity). Exercise intensity in the first month was 65% to 70% of the age-predicted maximum, and in months two to five, heart rate was maintained at 70% to 80% of the age-predicted maximum (i.e., moderate intensity). The low-dose and high-dose groups progressed toward the goal of 150 or 300 min/wk of exercise over 4 and 10 weeks, respectively. The control group was asked to maintain their prestudy levels of exercise and not to engage in new activities during the study. Control group participants were provided with an in-home treadmill after completing the study. Additional details of the exercise intervention have been provided elsewhere (16).
Measurements
Baseline and follow-up measures were obtained by trained staff that were blinded to treatment assignment and followed standardized protocols. Demographic characteristics were self-reported. Predicted lifetime risk of breast cancer was quantified using genetic testing results and the Claus and Gail models (19, 20). Cardiorespiratory fitness was quantified using the Bruce protocol (22). Physical activity was quantified using the modifiable physical activity questionnaire (23). The modi-fiable physical activity questionnaire has satisfactory test–retest reliability (ρ = 0.62–0.96), reproducibility (ρ = 0.88–0.92), and is correlated with objectively measured physical activity (ρ = 0.62; ref. 23). Caloric intake was quantified using 3-day food records that were analyzed by a registered dietitian using the Nutrition Data System for Research software (v.2009). The collection and quantification of urinary hormones including estrogen and progesterone have been described in detail elsewhere (16).
Body composition outcomes
Anthropometric measures included height (m) and body mass (kg), which were used to calculate BMI (kg/m2). All participants underwent whole-body dual-energy x-ray absorptiometry (DXA; Hologic Discovery A). All DXA scans were reviewed for accuracy by a bio-nutritionist who was blinded to study group (24). The DXA scanner was calibrated daily using a soft-tissue phantom. DXA was used to quantify fat mass (kg), body fat (%), visceral adipose tissue (VAT; cm2), subcutaneous adipose tissue (SAT; cm2), and lean mass (kg) using Hologic APEX v.13.4 software. DXA-derived VAT has been validated against CT-derived VAT (r = 0.93; P < 0.001; ref. 25) and has been used among premenopausal women across a large weight spectrum (26).
Background parenchymal enhancement
Bilateral dynamic contrast-enhanced MRI (DCE-MRI) examinations were conducted between days six and ten of the menstrual cycle at baseline and follow-up using a 1.5-Telsa Siemens scanner using published methods (27). At follow-up, images were obtained using the same field of view and slab dimensions used in the initial exam to ensure consistency for within-participant comparison. We used validated fully automated computerized methods to quantify absolute volume of BPE (cm2; refs. 13, 28, 29). Budget cuts prevented breast MRI for all participants. The first 68 participants underwent breast MRI (22, 22, and 24 in the control, low-dose, and high-dose groups, respectively). There were fewer nonwhite women than white women (P = 0.001), but no other differences were observed between participants who underwent breast MRI versus not.
Statistical analysis
All statistical analyses were completed using Stata MP Version 14.1 (StataCorp). Descriptive statistics presented for baseline variables include counts and proportions for categorical variables and mean ± SDs for continuous variables. Categorical baseline characteristics were compared between the three groups using the Fisher exact test, and continuous baseline characteristics were compared between the three study groups using the Kruskal–Wallis test. The sample size for this trial was determined for the primary outcome, urinary estrogen. For the secondary outcomes reported herein, we had 80% power to detect effect sizes of 0.23 and larger (16). All inferential analyses were conducted on an intention-to-treat basis.
Changes in body composition outcomes were evaluated from baseline to follow-up between the three groups using repeated-measures mixed-effects regression models. This statistical approach includes all available data and accounts for the correlation between repeated measures. The baseline value of the dependent variable was included as a covariate in the regression models (30). Group-by-time interaction terms were included as fixed effects in the regression model. Results from the repeated-measures mixed-effects regression models are presented as least-square mean ± SE. To evaluate the presence of a dose–response relationship across randomized groups, a test of trend was conducted by examining linear and nonlinear (quadratic) contrasts. Sensitivity analysis was performed to assess the robustness of the primary analyses using a repeated-measures analysis of covariance (RM-ANCOVA) with last-observation-carried-forward (LOCF) imputation. Results from the sensitivity analysis did not differ from those presented herein. The longitudinal relationship between body composition outcomes and BPE was examined using a mixed model that consolidated the three randomized groups and included participants who completed breast MRI measures. The proportion of variance in BPE explained by body composition was calculated using the R2 method as described by Bryk and Raudenbush for mixed-effects regression models (31).
Results
Between December 2008 and March 2012, 139 women residing in over 100 cities in 33 states within the United States were recruited and randomized with data collection ending for all study participants in April 2013. Characteristics of study participants are presented in Table 1. Age ranged from 18 to 49 years. The lifetime-predicted breast cancer risk from the Claus model ranged from 8.3% to 46.0%, and the Gail model ranged from 9.6% to 51.4%. All women had a lifetime risk of developing breast cancer ≥18% using at least one method of prediction.
Table 1.
Description of demographic characteristics
| Characteristic | Overall (N = 139) | Control (N = 46) | Low dose (N = 45) | High dose (N = 48) |
|---|---|---|---|---|
| Age, y | 34.3 ± 6.9 | 34.6 ± 7.5 | 35.2 ± 6.4 | 33.4 ± 6.8 |
| Race | ||||
| White | 118 (85%) | 39 (85%) | 40 (89%) | 39 (81%) |
| Other | 21 (15%) | 7 (15%) | 5 (11%) | 9 (19%) |
| Education | ||||
| ≤High school | 4 (3%) | 3 (7%) | 1 (2%) | 0 (0%) |
| Some college | 40 (29%) | 12 (26%) | 13 (29%) | 15 (31%) |
| ≥College | 95 (68%) | 31 (67%) | 31 (69%) | 33 (69%) |
| Employed full time (% yes) | 80 (58%) | 24 (52%) | 28 (62%) | 28 (58%) |
| Marital status | ||||
| Single/divorced/separated | 55 (40%) | 23 (50%) | 11 (24%) | 21 (44%) |
| Married/partnered | 84 (60%) | 23 (50%) | 34 (76%) | 27 (56%) |
| Children (% yes) | 83 (60%) | 24 (52%) | 34 (76%) | 52 (52%) |
| BRCA gene mutation status | ||||
| Positive | 49 (35%) | 14 (30%) | 18 (40%) | 17 (35%) |
| Negative | 12 (9%) | 7 (15%) | 2 (4%) | 3 (6%) |
| Not tested | 78 (56%) | 25 (55%) | 25 (56%) | 28 (59%) |
| Predicted breast cancer risk (%) | ||||
| Claus modela | 24.5 ± 10.3 | 24.1 ± 10.1 | 24.3 ± 10.0 | 25.1 ± 11.0 |
| Gail modelb | 22.6 ± 8.6 | 25.0 ± 10.2 | 21.4 ± 8.1 | 20.7 ± 5.9 |
NOTE: Values are mean ± SD or N (%).
N = 135, Claus score is not calculated for women who did not have a female first/second-degree relative with breast cancer.
N = 69, Gail score is not calculated for women ≤ 35 years.
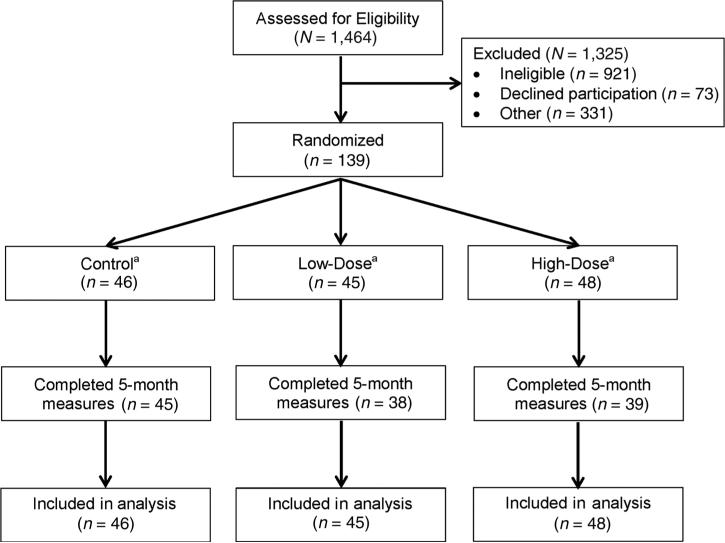
Figure 1 shows the 139 randomized participants, including 17 women (12.2%) who did not complete 5-month measures. The most commonly cited reason for attrition was attributed to personal life events unrelated to exercise. Attrition was monitored throughout the study, and the importance of participant retention was reinforced by the study coordinator and principal investigator. As an alternative to terminating participation in the study entirely, all participants were offered the option to cease exercise but agree to complete end of study measures. Participants who did not complete 5-month measures were more likely to be nonwhite (33% vs. 9%; P = 0.005), have less than a college degree (55% vs. 4%; P < 0.001), be single or divorced (22% vs. 6%; P = 0.008), with a lower predicted breast cancer risk (Gail model only; 17.1 ± 7.3 vs. 23.2 ± 8.6%; P = 0.04) at baseline compared with participants who completed 5-month measures. Participants who did not complete 5-month measures had shorter maximal treadmill time (6.8 ± 2.1 vs. 8.3 ± 1.6 minutes; P = 0.007), higher body mass (88.1 ± 14.4 vs. 72.1 ± 16.4 kg; P < 0.001), BMI (31.5 ± 5.4 vs. 26.1 ± 6.0 kg/m2; P < 0.001), fat mass (37.4 ± 10.2 vs. 27.7 kg; P < 0.001), body fat percentage (42.0 ± 4.9 vs. 37.1 ± 6.7%; P = 0.004), VAT area (104.8 ± 52.8 vs. 77.9 ± 50.8 cm2; P = 0.02), SAT area (496.7 ± 140 vs. 338.6 ± 145.1 cm2; P < 0.001), at baseline compared with participants who completed 5-month measures. No other reported study variables differed between participants who did, versus did not, complete the study.
Figure 1.
Flow of participants through the study: a22, 22, and 24 participants in the control, low-dose, and high-dose exercise groups had breast MRI data, respectively (N = 68 subsample).
Energy balance variables are presented in Table 2. Over 5 months, mean adherence to the low-dose exercise prescription was 127 ± 51 min/wk (85% of prescribed dose) and to the high-dose exercise prescription was 214 ± 73 min/wk (81% of prescribed dose). In both groups, 96% of all exercise bouts were confirmed with the objective heart rate monitor data (i.e., 4% failure rate on the heart rate monitors). After 5 months, linear dose–response increases were observed for maximal treadmill time and self-reported physical activity (P < 0.001). No change in caloric consumption was observed. There were no unexpected or serious adverse events related to the intervention.
Table 2.
Exercise capacity and energy balance variables at baseline and change during 5 months
| Characteristic | Baseline (mean ± SD) | Δ Baseline to month 5 (LS mean ± SE) | Δ from control (LS mean ± SE) |
|---|---|---|---|
| Maximal treadmill time, minutes | |||
| Control | 8.1 ± 1.6 | −0.14 ± 0.09 | – |
| Low dose | 7.9 ± 1.6 | 0.80 ± 0.10a | +0.95 ± 0.10b |
| High dose | 8.3 ± 1.9 | 1.10 ± 0.11a | +1.23 ± 0.10b,c |
| Test for trend | Linear, P < 0.001; Quadratic, P = 0.008 | ||
| Self-reported physical activity, MET-Hr/Wk | |||
| Control | 8.3 ± 6.5 | −6.15 ± 0.65a | – |
| Low dose | 9.0 ± 9.1 | −0.01 ± 0.94 | +6.40 ± 0.83b |
| High dose | 7.3 ± 6.7 | 2.32 ± 1.06a | +8.13 ± 0.91b |
| Test for trend | Linear, P < 0.001; Quadratic, P = 0.091 | ||
| Self-reported caloric consumption, calories | |||
| Control | 1,840 ± 554 | −18.90 ± 69.55 | – |
| Low dose | 1,804 ± 556 | −12.59 ± 89.83 | −4.53 ± 81.59 |
| High dose | 1,867 ± 500 | −34.21 ± 66.47 | −7.33 ± 69.77 |
| Test for trend | Linear, P = 0.874; Quadratic, P = 0.978 |
Abbreviation: LS mean, least squares mean.
Significantly different from baseline (within-group), P < 0.05.
Significantly different from control, P < 0.05.
Significantly different from low dose, P < 0.05.
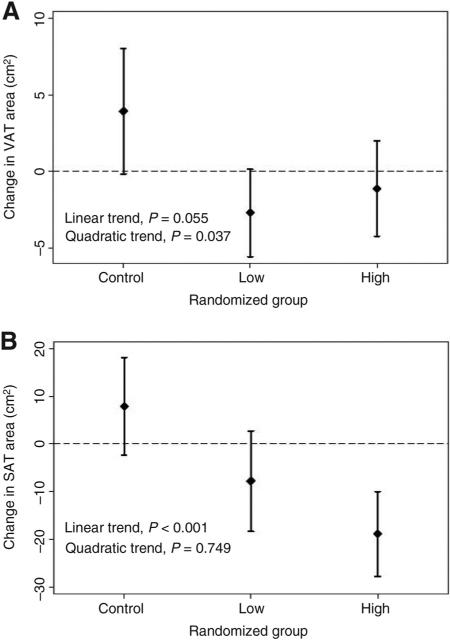
Body composition outcomes are presented in Table 3. Compared with the control group, the low-dose and high-dose groups lost −1.5 ± 0.5 and −1.3 ± 0.5 kg of body mass (linear Ptrend = 0.032); −1.8 ± 0.6% and −1.8 ± 0.5% (linear Ptrend = 0.001). Similar linear dose–response patterns were observed for fat mass and body fat percentage. Compared with the control group, the low-dose and high-dose groups lost −15.9 ± 5.4 and −26.6 ± 5.0 cm2 of SAT (linear Ptrend < 0.001; Fig. 2); −4.5 ± 1.6% and −7.8 ± 1.5% (linear Ptrend < 0.001). Compared with the control group, the low-dose and high-dose groups lost −6.6 ± 1.9 and −5.0 ± 1.9 cm2 VAT (nonlinear Ptrend = 0.037; Fig. 2); −8.3 ± 2.3% and −6.2 ± 2.3% (nonlinear Ptrend = 0.031). Change in body mass significantly (P < 0.001) correlated with changes in fat mass (r = 0.90), body fat percentage (r = 0.63), VAT (r = 0.69), and SAT (r = 0.79). Adjustment for change in body mass did not substantively alter the effect estimates for the aforementioned outcomes (results not shown). No statistically significant dose–response effects were observed for BMI (P = 0.080) or lean mass (P = 0.900). Sensitivity analyses using RM-ANCOVA did not differ from the reported mixed-model analyses.
Table 3.
Body composition outcomes at baseline and change during 5 months
| Characteristic | Baseline (mean ± SD) | Δ Baseline to month 5 (LS mean ± SE) | Δ from control (LS mean ± SE) |
|---|---|---|---|
| Body mass, kg | |||
| Control | 74.2 ± 16.3 | 1.04 ± 0.52a | – |
| Low dose | 74.5 ± 17.4 | −0.49 ± 0.40 | −1.53 ± 0.47b |
| High dose | 73.4 ± 17.9 | −0.31 ± 0.35 | −1.35 ± 0.46b |
| Test for trend | Linear, P = 0.032; Quadratic, P = 0.093 | ||
| BMI, kg/m2 | |||
| Control | 26.8 ± 6.2 | 0.15 ± 0.10 | – |
| Low dose | 26.8 ± 6.0 | −0.18 ± 0.15 | −0.33 ± 0.13b |
| High dose | 26.7 ± 6.5 | −0.13 ± 0.13 | −0.28 ± 0.12b |
| Test for trend | Linear, P = 0.080; Quadratic, P = 0.279 | ||
| Fat mass, kg | |||
| Control | 28.9 ± 11.3 | 0.65 ± 0.37 | – |
| Low dose | 29.6 ± 11.1 | −0.86 ± 0.34a | −1.52 ± 0.36b |
| High dose | 28.2 ± 11.9 | −0.74 ± 0.29a | −1.38 ± 0.34b |
| Test for trend | Linear, P = 0.003; Quadratic, P = 0.047 | ||
| Body fat, % | |||
| Control | 37.7 ± 7.0 | 0.25 ± 0.25 | – |
| Low dose | 38.6 ± 6.1 | −1.07 ± 0.28a | −1.33 ± 0.27b |
| High dose | 37.0 ± 7.1 | −1.17 ± 0.26a | −1.41 ± 0.26b |
| Test for trend | Linear, P < 0.001; Quadratic, P = 0.067 | ||
| VAT, cm2 | |||
| Control | 82.6 ± 54.7 | 3.94 ± 2.08 | – |
| Low dose | 89.4 ± 51.4 | −2.68 ± 1.45 | −6.62 ± 1.85b |
| High dose | 72.0 ± 48.3 | −1.10 ± 1.58 | −5.05 ± 1.93b |
| Test for trend | Linear, P = 0.055; Quadratic, P = 0.037 | ||
| SAT, cm2 | |||
| Control | 353.3 ± 145.5 | 7.92 ± 5.24 | – |
| Low dose | 375.0 ± 141.7 | −7.75 ± 5.36 | −15.86 ± 5.43b |
| High dose | 346.4 ± 171.3 | −18.86 ± 4.55a | −26.65 ± 5.06b,c |
| Test for trend | Linear, P < 0.001; Quadratic, P = 0.749 | ||
| Lean mass, kg | |||
| Control | 45.3 ± 5.8 | 0.40 ± 0.23 | – |
| Low dose | 44.9 ± 7.0 | 0.37 ± 0.19 | −0.03 ± 0.22 |
| High dose | 45.2 ± 7.2 | 0.43 ± 0.16a | +0.03 ± 0.20 |
| Test for trend | Linear, P = 0.900; Quadratic, P = 0.862 |
Abbreviation: LS mean, least squares mean.
Significantly different from baseline (within-group), P < 0.05.
Significantly different from control, P < 0.05.
Significantly different from low dose, P < 0.05.
Figure 2.
Change in (A) VAT and (B) SAT from baseline to 5 months between randomized groups.
At baseline, multiple body composition outcomes were significantly (P < 0.001) correlated with BPE, including body mass (r = 0.70), BMI (r = 0.73), fat mass (r = 0.71), body fat percentage (r = 0.68), VAT (r = 0.64), SAT (r = 0.64), and lean mass (r = 0.57). We have previously reported that exercise reduced BPE in dose–response fashion (P = 0.009), such that after 5 months compared with the control group, BPE was reduced by −82.2 ± 37.6 cm2 and −155.1 ± 35.2 cm2 in the low-dose and high-dose exercise groups, respectively (17). After 5 months, change in VAT was the only body composition outcome to correlate with change in BPE. For each −1 cm2 reduction in VAT, BPE decreased by −3.43 ± 1.34 cm2 (P = 0.010) and explained 9.7% of the variability in BPE (Table 4). Change in body composition outcomes did not correlate with changes in urinary estrogen and progesterone (results not shown; ref. 17).
Table 4.
Relationship between change in body composition variables and change in BPE during 5 months
| Characteristic | r | Unit of change | Δ BPE (cm2) per unit of change in body composition (LS mean ± SE) | R2 | P |
|---|---|---|---|---|---|
| Body mass, kg | 0.28 | −1kg | −8.10 ± 5.02 | 7.0% | 0.107 |
| BMI, per kg/m2 | 0.22 | −1kg/m2 | −23.01 ± 19.14 | 4.6% | 0.229 |
| Fat mass, kg | 0.28 | −1kg | −13.26 ± 9.14 | 1.3% | 0.147 |
| Body fat, % | 0.20 | −1% | −13.26 ± 9.14 | 1.3% | 0.147 |
| VAT, cm2 | 0.30 | −1cm2 | −3.43 ± 1.34 | 9.7% | 0.010 |
| SAT, cm2 | 0.12 | −1cm2 | −0.57 ± 0.47 | 2.6% | 0.231 |
| Lean mass, kg | 0.17 | +1kg | +20.25 ± 13.33 | 4.3% | 0.129 |
Abbreviations: LS mean, least squares mean; r, Pearson correlation coefficient between change in body composition and change in BPE; R2, proportion of variability of change in BPE explained by change in body composition variable (N = 68).
Discussion
Moderate-intensity aerobic exercise among premenopausal women at high risk of breast cancer resulted in dose–response reductions in body composition outcomes, including total body mass, fat mass, body fat percentage, VAT, and SAT. Change in VAT correlated with change in BPE; other body composition outcomes were not statistically significant correlates of change in BPE. The findings from this randomized trial provide preliminary mechanistic data to support observational evidence that suggests shifting energetic homeostasis may alter the risk of developing breast cancer (3, 6, 7, 10, 32).
We observed only modest changes in body mass. Over 5 months, the control group gained 1 kg of body mass (P = 0.046), whereas the exercise groups maintained body mass. In the absence of caloric restriction, exercise is useful for weight maintenance (33). VAT is the primary fat tissue compartment associated with chronic disease risk (34, 35). A significant nonlinear (quadratic) dose–response reduction in VAT was observed. Compared with the control group, the low- and high-dose exercise groups lost −6.6 cm2 [95% confidence interval (CI), −10.2, −3.0] and −5.0 cm2 (95% CI, −8.8, −1.3) of VAT, respectively. The magnitude of VAT reduction observed was modestly smaller than prior studies (36, 37). Among 173 sedentary postmenopausal women, 12 months of moderate-intensity aerobic exercise (225 min/wk) reduced VAT by −8.6 cm2 compared with the control group (36). Among 400 physically inactive postmenopausal women, 150 min/wk of aerobic exercise was not significantly different from 300 min/wk of aerobic exercise in reducing VAT (between group Δ: −1.5 cm2; P = 0.50; ref. 37), which is consistent with our nonlinear observation. The average BMI (26.8 kg/m2) in our study was lower than other studies [29.2 kg/m2 (37) and 30.5 kg/m2 (36)] that have examined the efficacy of exercise to improve body composition. Consequently, study participants in prior studies had 64% (37) to 83% (36) higher VAT at baseline, relative to participants in our study. Differences in baseline VAT area may explain, in part, the proportionally attenuated reductions in VAT observed in our study. Alternative explanations may include differences intervention length, exercise adherence, and study completion rates.
BPE is an imaging biomarker that measures blood flow by differentiating MRI contrast uptake in fibroglandular tissue versus that of the adipose breast tissue (11, 14). BPE is strongly predictive of breast cancer risk (12), and breast MRI is more sensitive than mammography in detecting early breast tumors (11). BPE has been proposed as an endpoint to quantify response to risk-reducing interventions (11, 13). Our study confirms a prior report that baseline BMI correlates with BPE (14) and extends this observation to other body composition outcomes. To our knowledge, our study is the first to report that longitudinal changes in body composition correlate with reductions in BPE. Change in VAT accounted for a significant proportion of variance in BPE (9.7%). Each −1 cm2 reduction in VAT correlated with a −3.4 cm2 (95% CI, −6.1, −0.8) reduction in BPE. These observations should be interpreted as exploratory, as our statistical power was limited to demonstrate significant correlations with other important body composition parameters, such as body mass and fat mass. Additional investigation using adequately powered studies is needed to examine the relationship between BPE and these additional body composition parameters. The observed relationships are consistent with the hypothesis that the mechanism through which physical activity reduces premenopausal breast cancer risk may be due, in part, to reductions in body fat, particularly in the abdominal cavity.
There are several limitations to this trial. Breast MRI was only completed in a subset of participants. With exception of race, participants who completed MRI measures were similar to those who did not undergo MRI. The small sample size of participants with MRI data limited our statistical power to detect correlations between changes in other body composition outcomes with change in BPE. We were unable to examine specific changes within the VAT compartment, such as mesenteric, omental, and retroperitoneal fat (38). Participants who did not complete 5-month measures differed from those who did complete 5-month measures with respect to several demographic, clinical, and anthropometric variables, which indicates follow-up data were not missing completely at random and therefore our effect estimates may be influenced by a selection bias (plausibly away from the null because participants who did not complete the trial had poorer body composition than those who did complete the trial). Sensitivity analyses suggested that our findings were not different using an alternative method of analysis (RM-ANCOVA) with LOCF imputation. The duration of the exercise intervention was modest, lasting 5 months, whereas other studies have lasted 12 months (36, 37). The absolute risk change associated with these improvements in body composition and BPE is not known and therefore the clinical importance of these findings warrants additional investigation. Furthermore, it is not known if BPE and body composition convey similar prognostic information regarding the absolute risk of developing breast cancer. It is unknown if reducing VAT among women at average risk of breast cancer and with higher VAT volumes would yield reductions in BPE that are of similar magnitude to the current study.
There are several strengths to this trial. To our knowledge, this is the first randomized trial to leverage DXA as an imaging modality to quantify longitudinal changes in VAT. BPE quantification was fully automated, which attenuates measurement error (13, 28, 29). Data collection was completed by staff blinded to study group. A large proportion of study participants had complete body composition outcome data at 5 months. Adherence to the exercise prescriptions in both groups was high. National recruitment increases the generalizability to high-risk premenopausal women across the United States.
In conclusion, the findings from this study demonstrate the feasibility and dose–response effects of moderate-intensity aerobic exercise to favorably alter numerous body composition outcomes among premenopausal women at high risk of developing breast cancer. Change in VAT correlated with change in BPE, and future adequately powered studies are needed to confirm and expand upon this finding. The findings from this randomized trial provide mechanistic data to support observational evidence that shifting energetic homeostasis through exercise may alter the risk of developing breast cancer.
Acknowledgments
Grant Support
This study was supported by R01-CA131333 from the NCI (to K.H. Schmitz).
Footnotes
Authors' Contributions
Conception and design: K.H. Schmitz
Development of methodology: J.C. Brown, D. Kontos, M.D. Schnall, S. Wu, K.H. Schmitz
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J.C. Brown, M.D. Schnall, K.H. Schmitz
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J.C. Brown, D. Kontos, M.D. Schnall, S. Wu, K.H. Schmitz
Writing, review, and/or revision of the manuscript: J.C. Brown, D. Kontos, M.D. Schnall, S. Wu, K.H. Schmitz
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.C. Brown, K.H. Schmitz
Study supervision: J.C. Brown, K.H. Schmitz
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Antoniou A, Pharoah P, Narod S, Risch HA, Eyfjord JE, Hopper J, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narod S. Modifiers of risk of hereditary breast cancer. Oncogene. 2006;25:5832–6. doi: 10.1038/sj.onc.1209870. [DOI] [PubMed] [Google Scholar]
- 3.King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 4.Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst. 2008;100:728–37. doi: 10.1093/jnci/djn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the sister study. Cancer. 2015;121:3700–8. doi: 10.1002/cncr.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nkondjock A, Robidoux A, Paredes Y, Narod S, Ghadirian P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res Treat. 2006;98:285–94. doi: 10.1007/s10549-006-9161-8. [DOI] [PubMed] [Google Scholar]
- 7.Manders P, Pijpe A, Hooning MJ, Kluijt I, Vasen HF, Hoogerbrugge N, et al. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2011;126:193–202. doi: 10.1007/s10549-010-1120-8. [DOI] [PubMed] [Google Scholar]
- 8.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: A systematic review and doseresponse metaanalysis. Obes Rev. 2013;14:665–78. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 9.Harvie M, Hooper L, Howell A. Central obesity and breast cancer risk: A systematic review. Obes Rev. 2003;4:157–73. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 10.Kotsopoulos J, Olopado OI, Ghadirian P, Lubinski J, Lynch HT, Isaacs C, et al. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005;7:R833–43. doi: 10.1186/bcr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pike MC, Pearce CL. Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol. 2013;24(Suppl 8):viii37–41. doi: 10.1093/annonc/mdt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260:50–60. doi: 10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Weinstein SP, DeLeo MJ, 3rd, Conant EF, Chen J, Domchek SM, et al. Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: Preliminary evaluation in a cohort of BRCA1/2 mutation carriers. Breast Cancer Res. 2015;17:67. doi: 10.1186/s13058-015-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hruska CB, Rhodes DJ, Conners AL, Jones KN, Carter RE, Lingineni RK, et al. Background parenchymal uptake during molecular breast imaging and associated clinical factors. AJR Am J Roentgenol. 2015;204:W363–70. doi: 10.2214/AJR.14.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettapiece-Phillips R, Narod SA, Kotsopoulos J. The role of body size and physical activity on the risk of breast cancer in BRCA mutation carriers. Cancer Causes Control. 2015;26:333–44. doi: 10.1007/s10552-014-0521-0. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz KH, Williams NI, Kontos D, Kurzer MS, Schnall M, Domchek S, et al. Women in steady exercise research (WISER) sister: Study design and methods. Contemp Clin Trials. 2015;41:17–30. doi: 10.1016/j.cct.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KH, Williams NI, Kontos D, Domchek S, Morales KH, Hwang W, et al. Dose response effects of aerobic exercise on estrogen among women at high risk for breast cancer: A randomized controlled trial. Breast Cancer Res Treat. 2015;154:309–18. doi: 10.1007/s10549-015-3604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stice E, Telch CF, Rizvi SL. Development and validation of the eating disorder diagnostic scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2000;12:123. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- 19.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of earlyonset breast cancer. Implications for risk prediction. Cancer. 1994;73:643–51. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 21.Anton-Culver H, Ziogas A, Bowen D, Finkelstein D, Griffin C, Hanson J, et al. The cancer genetics network: Recruitment results and pilot studies. Community Genet. 2003;6:171–7. doi: 10.1159/000078165. [DOI] [PubMed] [Google Scholar]
- 22.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–62. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 23.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 24.Powers C, Fan B, Shepherd J. Importance of image review for accurate reporting of hologic DXA visceral adipose tissue. J Clin Densitom. 2014;3:399. [Google Scholar]
- 25.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-Energy X-Ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20:1109–14. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredella MA, Gill CM, Keating LK, Torriani M, Anderson EJ, Punyanitya M, et al. Assessment of abdominal fat compartments using DXA in premen opausal women from anorexia nervosa to morbid obesity. Obesity. 2013;21:2458–64. doi: 10.1002/oby.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boston RC, Schnall MD, Englander SA, Landis JR, Moate PJ. Estimation of the content of fat and parenchyma in breast tissue using MRI T 1 histograms and phantoms. Magn Reson Imaging. 2005;23:591–9. doi: 10.1016/j.mri.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Weinstein SP, Conant EF, Kontos D. Automated fibroglandular tissue segmentation and volumetric density estimation in breast MRI using an atlas-aided fuzzy C-means method. Med Phys. 2013;40:122302. doi: 10.1118/1.4829496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Weinstein SP, Conant EF, Schnall MD, Kontos D. Automated chest wall line detection for whole-breast segmentation in sagittal breast MR images. Med Phys. 2013;40:042301. doi: 10.1118/1.4793255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzmaurice G, Laird N, Ware J. Applied longitudinal analysis . Wiley; Hoboken, New Jersey: 2004. [Google Scholar]
- 31.Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Sage Publications, Inc; Thousand Oaks, California: 1992. [Google Scholar]
- 32.Pijpe A, Manders P, Brohet RM, Coll[notdef]ee JM, Verhoef S, Vasen HF, et al. Physical activity and the risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2010;120:235–44. doi: 10.1007/s10549-009-0476-0. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. American college of sports medicine position stand. appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 34.Despres JP, Lemieux I, Prud'homme D. Treatment of obesity: Need to focus on high risk abdominally obese patients. BMJ. 2001;322:716–20. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 36.Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, et al. Effect of exercise on total and intra-abdominal body fat in post-menopausal women. JAMA. 2003;289:323–30. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 37.Friedenreich CM, Neilson HK, O'Reilly R, Duha A, Yasui Y, Morielli AR, et al. Effects of a high vs. moderate volume of aerobic exercise on adiposity outcomes in postmenopausal women: A randomized clinical trial. JAMA Oncol. 2015;1:766–76. doi: 10.1001/jamaoncol.2015.2239. [DOI] [PubMed] [Google Scholar]
- 38.Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]