Abstract
Background
The connection between Alzheimer’s disease (AD) and olfactory deficits is well documented and further, alterations in olfactory functioning may signal declines in functions associated with dementia. The aim of the present comprehensive meta-analysis was to investigate the nature of olfactory deficits in mild cognitive impairment (MCI).
Methods
Articles were identified through computerised literature search from inception to 30 June 2016 using PubMed, MEDLINE and PsychInfo databases. In order to control for differences in sample size during effect size computation, studies were weighted according to their inverse variance estimates.
Results
31 articles (62 effects) were identified, which included 1993 MCI patients and 2861 healthy older adults (HOA). Included studies contrasted odour identification, discrimination, detection threshold and/or memory between cases and controls. Moderate to large and heterogeneous effects were seen for olfactory deficits in MCI relative to HOA (d=−0.76, 95% CI −0.87<δ<−0.64). Moderator analysis revealed that tests of odour identification yielded larger effect sizes than those of odour detection threshold or memory. In addition, a potential interaction between age and sex was observed, with male patients carrying a larger burden of olfactory deficit and older female patients performing better on olfactory tests.
Conclusions and relevance
Olfactory deficits are present and robust in MCI. Odour identification is most impaired in MCI, which parallels the most prominent sensory deficit seen in AD. As such, a simple-to-administer test of odour identification warrants inclusion in the screening of individuals at risk for developing AD.
INTRODUCTION
Earlier identification and diagnosis of individuals likely to develop Alzheimer’s disease (AD) is critical for potential intervention and treatment early in the course of the disease. Hence, there has been intense focus on individuals at risk for developing dementia, in particular those with mild cognitive impairment (MCI). To this effect, recent studies of neuropsychological function in MCI are aimed at early detection and prevention strategies. Recent work1 verifies prior findings on the diagnostic utility of detailed neuropsychological and cognitive screening inventories in AD and MCI. However, challenges remain in differentiating incipient dementia from healthy ageing, thus additional methods that provide brief, accurate and cost-effective approaches to predicting the onset of AD are desirable.
Several lines of evidence implicate alterations of the olfactory system in the pathogenesis of Alzheimer’s type dementia.2–4 Deficits in odour identification are large2,5 and likely denote fundamental neuroanatomic and neurophysiologic abnormalities that are specific to the peripheral olfactory system3,6 and primary olfactory cortex.7 Poorer olfactory ability is associated with structural brain changes in the hippocampus and entorhinal cortex, two regions affected in dementia.8–10 Most importantly, olfactory dysfunction is correlated with AD pathology on postmortem examination,11 biopsies of the olfactory epithelium note the presence of AD pathology (eg, Aβ) in AD patients12 and the presence of tau protein is measurable in nasal secretions of AD individuals.13 On a practical note, olfactory screening is routine in otorhinolaryngology,14,15 reliable,16 and quick and easy to administer17,18 making it ideal for clinical screening in dementia.
Over the past 2 decades, several studies have measured olfactory performance in MCI. Deficits are observed in multiple olfactory domains, including odour detection threshold, identification, discrimination and memory. Several of these studies find that olfactory deficits precede the onset of illness,19,20 distinguish patients with prodromal symptoms (eg, MCI) from healthy older adults (HOA)21,22 and may predict which vulnerable individuals are most likely to develop AD.19,21,23–25 However, these findings are not consistent across studies and discrepancies may relate to heterogeneity of the MCI diagnosis, illness stage or severity, age or olfactory methodology. Improvement in the specificity of these olfactory deficits may arise through the investigation in MCI subtypes. For example, impaired odour identification and detection has been found in MCI amnestic subtype (aMCI) with more severe deficits in aMCI individuals with deficits in multiple domains.22 Also, a recent, prospective population-based study found odour identification deficits were associated with incident aMCI and with progression from aMCI to AD.23 Yet, to date, there is neither thorough, quantitative investigation of the nature of olfactory psychophysical performance deficits in MCI, nor within MCI subtypes.
In the current study, we conduct a comprehensive meta-analysis of existing studies examining psychophysical olfactory function in patients with MCI. A meta-analytic approach allowed for the combination of results across studies to provide a more powerful estimate of true population differences. We examined olfactory functioning in adults with MCI, including MCI subtype, as compared with healthy age-matched comparison groups. Further, we sought to identify the impact of various potential moderators, such as demographic and clinical variables that have been previously identified as different between patient and healthy comparison groups. We hypothesise that olfactory performance will be lower in MCI as compared with controls, even after assessing the known contributions of age and sex.
MATERIALS AND METHODS
Literature search strategy
Articles were identified through computerised literature search using PubMed, MEDLINE and PsychInfo databases to find relevant studies with the search terms “mild cognitive impairment” or “MCI” AND each of the following: “olfactory, olfaction, smell”. The search was limited to English language articles that enrolled human subjects. Additionally, a thorough manual review of articles was performed using cross-references from identified original articles and reviews. Studies eligible for inclusion used performance-based measures of olfactory functioning which provided statistical information that permitted meta-analytical methods to be used. This search procedure yielded 39 articles that addressed olfactory function in MCI patients.
Data extraction
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) standard26 was followed in the extraction of relevant studies and data (see online supplementary materials). Studies that were included in the meta-analysis followed these criteria: (1) a focus on standard or experimental tasks of olfactory function in patients with MCI, (2) had an age-matched comparison group of healthy unrelated participants with no history of MCI and (3) provided data or statistical information that allowed for the calculation of effect size. Based on criteria 1–3, three authors (MJM, LB, SK) initially reviewed each potential study. Initially, recommendations from the National Institute on Ageing-Alzheimer’s (NIA-A) Association workgroup was used to ensure adequate diagnostic criteria were used for MCI.27 Previous studies of MCI have revealed there is significant variability in cognitive profiles associated with early stages of AD beyond the typical ‘amnestic’ profile28,29 and that this heterogeneity may suggest different neurobiological routes to AD. In turn, we sought to further subtype the MCI diagnosis when possible, defining the following MCI categorisations: (1) Amnestic (aMCI), single domain impairment in memory; (2) Amnestic plus (aMCI+), impairment in memory with additional cognitive deficit(s); (3) Single domain (sdMCI), impairment in a single domain of cognition excluding memory; (4) Multiple domain (mdMCI), impairment in multiple cognitive domains excluding memory; (5) Unspecified (MCI Mixed), no identified subtypes perhaps reflecting an amalgam of subtypes. Subsequently, two principal investigators (DRR and PJM) independently reviewed the diagnostic criteria in each manuscript to ensure study subjects met criteria for the diagnosis of MCI. All disagreements were resolved via discussion and a consensus decision was reached. After passing this stage, relevant data were extracted for meta-analytic analysis, including data on tests of olfactory function, clinical criteria, demographic information and mini-mental state examination (MMSE) scores, if provided.
After review, eight articles of the original 39 were excluded, resulting in 31 publications that reported comparative results of psychophysical olfactory testing (see online supplementary materials for article inclusion list), totalling 62 effects for analysis (table 1). Reasons for exclusion were: (1) absence of healthy comparison groups (N=5); (2) insufficient characterisation of MCI sample (N=2) and (3) limited olfactory methodology (N=1). A complete list of included and excluded articles is presented in the online supplementary materials.
Table 1.
Olfactory studies of mild cognitive impairment
| Author | Year | Olfactory test type |
MCI (n) |
HOA (n) |
Odour identification test |
|---|---|---|---|---|---|
| Bahar-Fuchs | 2010S1 | I | 24 | 19 | UPSIT* |
| Bahar-Fuchs | 2010S3 | I, M | 13 | 11 | UPSIT* |
| Bahar-Fuchs | 2010S2 | I | 13 | 10 | UPSIT* |
| Bahar-Fuchs | 2011 | I | 25 | 22 | UPSIT* |
| Conti | 2013 | Di, I, M | 88 | 46 | CA-SIT |
| DeArment | 2008 | I | 8 | 8 | UPSIT |
| Devanand | 2000 | I | 90 | 45 | UPSIT |
| Devanand | 2008 | I | 148 | 63 | UPSIT |
| Devanand | 2010S9 | I | 290 | 802 | UPSIT |
| Devanand | 2010S10 | I | 127 | 59 | UPSIT |
| Djordjevic | 2008 | De, I | 51 | 33 | UPSIT |
| Eibenstein | 2005 | I | 29 | 29 | SS-OIT |
| Huart | 2015 | De, Di | 13 | 13 | – |
| Laakso | 2009 | I, M | 72 | 486 | Homemade |
| Lehrner | 2009 | I | 64 | 40 | UPSIT |
| Lojkowska | 2011 | De, I | 49 | 33 | SS-OIT |
| Magerova | 2011 | I | 30 | 11 | SS-OIT |
| McKinnon | 2010 | I | 33 | 207 | UPSIT |
| Peters | 2003 | De, Di, I | 8 | 8 | UPSIT |
| Seligman | 2013 | I | 112 | 132 | SS-OIT |
| Servello | 2015 | De, Di, I | 25 | 28 | SS-OIT |
| Sparks | 2005 | I | 21 | 42 | UPSIT |
| Steinbach | 2010 | I, De, Di | 29 | 29 | SS-OIT |
| Tabert | 2005 | I | 147 | 63 | UPSIT |
| Turana | 2014 | I | 51 | 58 | Homemade |
| Vasavada | 2015 | I | 12 | 27 | UPSIT |
| Vyhnalek | 2015 | I | 107 | 27 | MHST |
| Wang | 2002 | I | 28 | 30 | B-SIT |
| Westervelt | 2008 | I | 88 | 21 | B-SIT |
| Williams | 2009 | Di, I | 21 | 47 | SS-OIT |
| Wilson | 2007 | I | 177 | 412 | B-SIT |
This table lists only those studies (k=31) included in the current meta-analysis. Studies are presented in alphabetical order by first author. Publication year, olfactory test type (I, identification; De, detection; Di, discrimination; M, memory) and sample sizes of the mild cognitive impairment (MCI) and healthy older adult (HOA) groups are included. Superscripted numbers are used to disambiguate studies published in the same calendar year (Refer to the online supplemental material Reference List).
Modified version of the UPSIT.
B-SIT; Brief Smell Identification Test; CA-SIT Culturally Adapted Smell Identification Test; MHST Motol Hospital Smell Test; SS-OIT Sniffin' Sticks-Odor Identication Test; UPSIT University of Pennsylvania Smell Identification Test.
Methodological variables
We sought to define olfactory function broadly, looking at effect size across four basic domains including psychophysical tests of: (1) odour identification, (2) odour discrimination, (3) odour detection threshold and (4) odour memory. Assignment of olfactory tests to selected domains was guided by the classifications made in source articles and consensus of the authors.
Moderator variables
In the event of significant effect of heterogeneity in effect sizes across studies, categorical moderator analysis was undertaken for: (1) Olfactory domain (ie, identification, discrimination, detection threshold sensitivity, memory) (2) MCI subtypes (ie, aMCI, aMCI+, sdMCI, mdMCI and samples where subtypes were not specified (MCI Mixed)) and (3) Odour identification test type (ie, University of Pennsylvania Smell Identification Test (UPSIT), Brief-Smell Identification Test (B-SIT), Sniffin’ Sticks Identification, or other). Within the patient population, the following demographic and clinical moderator variables were coded for meta-regression: (1) mean age at the time of testing, (2) sex (ie, % male), (3) years of education and (4) MMSE test scores. The included articles were searched for additional demographic characteristics, including smoking status/burden and laterality of presentation; however, these data were not sufficiently reported to be included in formal analyses.
Statistical analyses
All analyses were carried out using Comprehensive Meta-Analysis V.2.0 (Biostat, 2005) using standard random-effects models. Differences in olfactory function were analysed across all eligible studies. The mean difference in olfactory scores between MCI patients and healthy comparison subjects were standardised using Cohen’s d, the difference between the two raw means divided by the pooled SD. When means and SDs were not available, d was calculated from reported univariate F tests, t-statistics or p values. CIs for each effect were reported. In order to control for differences in sample size during effect size computation, studies were weighted according to their inverse variance estimates (see online supplementary materials for details). Prior convention has classified effect sizes as small (d=0.2), medium (d=0.5) or large (d≥0.8) based on these methods.30 Random-effect models were used to compute the significance level of the mean effect sizes for each study and selected demographic and clinical variables. Effect size homogeneity across studies was assessed using the Cochran Q-statistic.31 In the case of overall effect size heterogeneity, potential moderators were analysed using the Q-statistic and meta-regression techniques. Publication bias was evaluated graphically through the use of a funnel plot as well as an adjusted rank-correlation test, according to the methods of Begg and Mazumdar,32 Egger et al,33 and Duval and Tweedy.34
RESULTS
Overall meta-analysis results
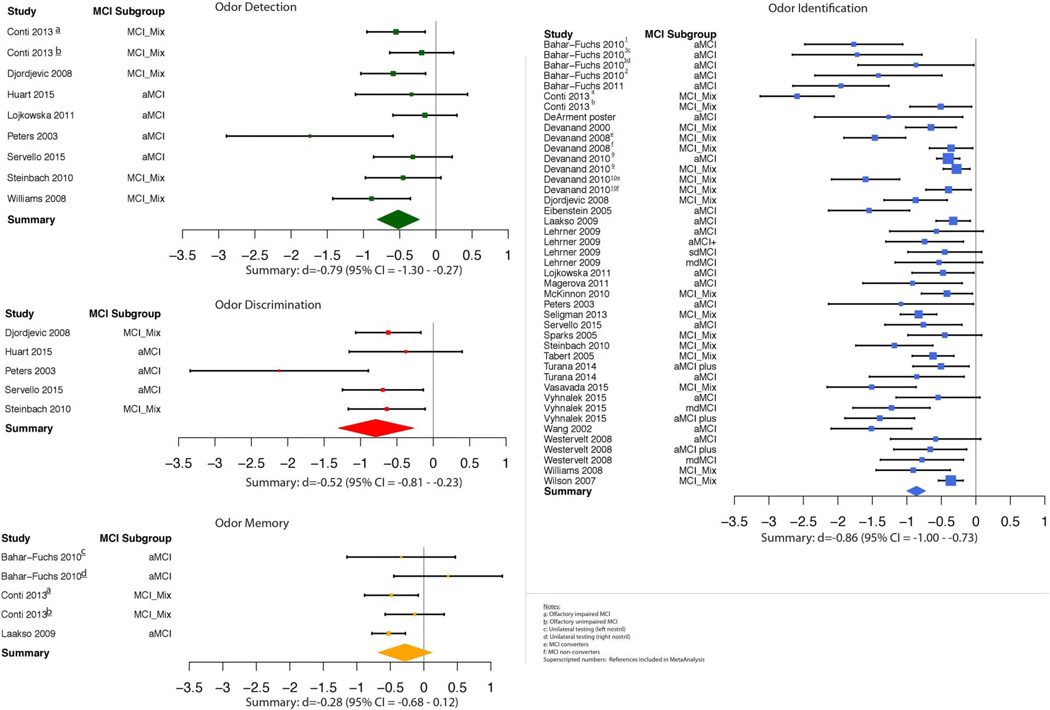
Analysis of effect sizes across olfactory domains for the MCI sample revealed effect sizes in the medium to large range of magnitude (k=62, d=−0.76, 95% CI −0.87<δ<−0.64) that were significantly heterogeneous (QB(61)=237.63, p<1.0×10−5). Individual study effect sizes by olfactory domain are displayed in figure 1. Given that the variability in effect sizes between MCI and healthy comparison groups differed more than would be expected from sampling error alone, analysis of potential moderator variables was undertaken.
Figure 1.
Individual study effect sizes by olfactory domain. Error bars represent 95% CIs.
Moderator analysis
Olfactory domain
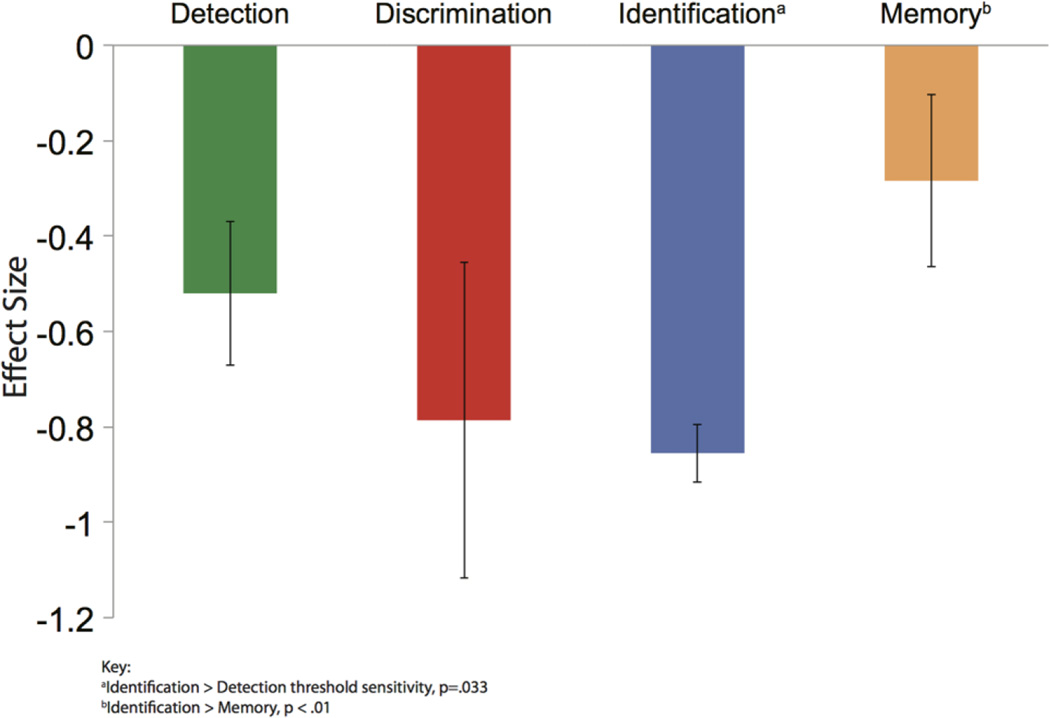
Analysis revealed significant heterogeneity among effect sizes (QB(3)=10.25, p=0.017). The effect size for odour identification (d=−0.86, 95% CI −1.00<δ<−0.73) did not differ from odour discrimination (d=−0.79, 95% CI −1.30<δ<−0.27; p=0.63), but was larger than odour memory (d=−0.28, 95% CI −0.68<δ<0.12; p<0.01) and odour detection threshold (d=−0.52, 95% CI=−0.81<δ<−0.23; p=0.04). The effect size for odour discrimination was nominally, but not statistically, larger than odour memory (p=0.09) and odour detection threshold (p=0.25). Odour memory did not differ from odour detection threshold (p=0.44; see figure 2).
Figure 2.
Average effect sizes (Cohen’s d) in mild cognitive impairment by olfactory domain. Error bars represent 95% CIs.
Odour identification test type
Within the odour identification domain, analysis of the four different odour identification tests (ie, UPSIT, B-SIT, Sniffin’ Sticks or other), did not reveal significant heterogeneity among effect sizes (QB (3)=6.06, p=0.10).
Mini-mental state examination
The average MMSE score for MCI patients was 26.93 (range 24.30–28.80). Results of meta-regression did not reveal a significant relationship between MMSE scores and olfactory deficit (k=44, Z=0.44, p=0.66). Follow-up analyses are detailed in the online supplementary materials.
MCI subtype
Analysis of the five MCI subtypes did not reveal any differences between aMCI, aMCI+, sdMCI, mdMCI or MCI Mixed groups (QB(4)=0.94, p=0.92).
Demographic characteristics
Sex
Analysis of sex composition of the samples revealed that studies with a larger proportion of men showed a greater magnitude of olfactory deficit (k=55, Z=−6.88, p<1.0×10−5).
Age
The average age of MCI subjects was 72.83 (range 64.10–82.00) years and meta-regression revealed a somewhat counterintuitive relationship between higher age and less olfactory deficit (k=57, Z=2.39, p=0.017). Further examination of the scatterplot, however, revealed six outliers where the mean patient age was >79 years and the samples comprised a higher proportion of female participants (~70% female, on average). Indeed, when these outliers were removed from the analysis, an inverse relationship between age and olfactory performance was observed with higher age being associated with poorer olfactory performance (k=51, Z=−4.25, p<2.0×10−5). The latter analysis suggests an interaction between age and sex on olfactory performance.
Education
Overall, years of education (mean=12.5) revealed no significant effect on the observed olfactory deficit (k=35, Z=−0.92, p=0.36). Follow-up analyses are detailed in the online supplementary materials.
Publication bias
Analysis for the presence of possible response bias revealed an asymmetric funnel plot and significant Begg (p=7.0×10−5) and Egger (p=2.0×10−5) tests. Considering potential ‘file drawer’ and/or publication bias in MCI literature, we calculated the potential missing studies using the Duval and Tweedie34 ‘trim and fill’ method. This procedure indicated that no studies were missing from analysis and generated a point estimate (−0.75) that was nearly identical to the original estimate. Finally, calculation of a fail-safe N revealed that 8738 ‘null’ studies would need to be found and incorporated into the analysis to negate the observed effect. As such, these methods support the notion that the current meta-analytic data accurately represent the extant literature concerning olfactory function in patients with MCI and that actual publication bias is unlikely.
DISCUSSION
This meta-analytic review extends the current literature on olfactory functioning in patients with MCI by quantifying the magnitude of olfactory deficit relative to healthy comparison subjects, as well as specifying potential moderator variables that influence psychophysical olfactory performance in this population. It is notable that the overall effect size was relatively large (d=−0.76, CI −0.87<δ<−0.64), although considerably smaller than the large olfactory deficits seen in frank AD. Tests of odour identification generally yielded larger deficits as opposed to odour discrimination, odour memory and odour detection threshold.
The current findings are generally consistent with a prior meta-analysis in AD, where AD patients showed robust deficits across olfactory domain.5 For example, in a meta-analysis by Mesholam et al,2 mean effect size as measured by Cohen’s d across olfactory domains was 3.36 in the AD group; a significant psychophysical impairment. Similarly, in a more recent meta-analysis, Rahayel et al5 also reported an extremely large composite effect size, where Cohen’s d=1.73. As expected, however, the magnitude of olfactory deficit in MCI appears to fall between that of healthy controls and that seen in AD patients. Odour identification deficits in MCI were larger relative to detection threshold sensitivity and deficits in odour recognition memory. A relatively greater deficit in odour identification in MCI is similar to findings in AD and is relevant as it likely denotes impairment that is different from typical age-related olfactory deficits, since HOA are more impaired on odour detection threshold rather than odour identification.16 Thus, our findings re-emphasise recent calls for the inclusion of tests of odour identification in the screening of individuals at risk for developing dementia.5,23
Somewhat unexpectedly, our results indicate no significant association between olfactory measures and MMSE score—a pre-eminent cognitive screening tool. Several studies35–37 propose that odour identification and recognition ability rely on high-order cognitive resources (eg, executive function, verbal or semantic memory) whereas odour detection relies more on basic perceptual processing.4,38 In fact, one empirical study found discrete differentiation of two odour-processing domains using a principal component analysis—(1) odour detection threshold and (2) odour identification or discrimination—suggesting relatively independent processing.39 In addition, at least one study reports a strong association (r=−0.34 (left nostril); r=−0.49 (right nostril)) between MMSE score and olfactory semantic errors across 37 individuals that were cognitively normal, AD or MCI.40 Fundamentally, odour identification is a complex task that relies on intact sensory perception and higher order semantic processing.41 As AD is associated with the latter, it is plausible that odour identification ability might be associated with deficits in semantic processing and present in individuals with incipient dementia. In this light, a weak, non-significant association of odour identification or memory with MMSE scores in the current meta-analysis could be considered surprising.
Yet, there are several possible explanations for the weak association. First, the MMSE was the only cognitive test consistently used in the studies included in the meta-analysis. While the MMSE is perhaps the most widely used screening measure, it has a limited range of scores and is far less informative than other recent screening measures (eg, Montreal Cognitive Assessment (MoCA)) and multidimensional neuropsychological inventories, particularly when attempting to differentiate MCI and normal cognitive ageing.1 Even our recent work42 found that performance on the MoCA, typically considered to be more comprehensive and difficult than the MMSE, shows only weak associations with odour identification in MCI individuals (r=0.16, n=109, p=0.03), but a much stronger association in AD (r=0.30, n=230, p<0.0001). Moreover, several studies show that the use of an odour identification test improves diagnostic classification in dementia, above and beyond a cognitive screen.3,43 The fact that odour identification tests can explain additional variance when included with cognitive screening tests or other neuropsychological inventories argues that these abilities are somewhat orthogonal. Finally, it is also possible that response methods for odour identification tasks bypass the need for semantic retrieval via the use of multiple choice formats. That is, the use of simple forced-choice responses included in the UPSIT and Sniffin’ Sticks may not be ideal for differentiating sensory and cognitive difficulties in incipient dementia. Use of more sophisticated olfactory testing and analysis approaches, such as using odour targets with increasingly similar semantic properties between targets and foils40 may better elucidate the relationship between cognition and odour identification in AD and MCI. Given the heterogeneity of the MCI diagnosis and the relatively small difference in cognitive screening scores typically reported between MCI and cognitively HOA, it is not surprising that we only find a weak association between MMSE and odour identification. Taken altogether, the stronger deficits found in odour identification may thus be interpreted as the sum of perceptual and cognitive processes. As such, olfactory testing may serve as an additional independent assessment tool that could enhance screening sensitivity.
Surprisingly, odour memory performance was relatively unimpaired in MCI, which deviates from reported effects in AD.5 Unfortunately, there are relatively few studies of odour memory in MCI and the methodology used in these studies is inconsistent. Together these studies suggest small deficits in odour memory performance in MCI. Yet, it remains to be seen whether MCI patients are unimpaired on odour memory tests, or conversely, if older adults are, in general, poorer on this specific type of olfactory test.40 Odour memory may therefore be a salient olfactory domain to monitor over time, as it may differentiate those at risk from those with frank dementia, and changes over time may signal disease progression. Recent evidence indicates that impaired olfaction is associated with the progression from MCI to dementia,23 which corroborates other longitudinal studies,19,44,45 but, these studies are limited to odour identification. If AD is signified by the loss of odour identification and odour recognition memory, additional longitudinal studies of odour memory are needed for determining the utility of this deficit as a potential biomarker of transition.
As can be seen in the extant literature on MCI, there is considerable variation in diagnostic criteria for MCI and possible subtypes as well as the nomenclature used to define them. The results of this meta-analysis suggest that there are few differences in the magnitude of olfactory impairment between MCI subtypes as we defined them, but clearly more work needs to be done. First, in reviewing articles it was clear that while generally accepted diagnostic criteria for MCI were followed, the approach of further subtyping varied considerably. Indeed, in many cases, no subtypes were reported, with a likely heterogeneous mix of MCI patients with varying neurocognitive deficits being merged in a composite diagnosis. In the few instances where subtypes were reported, the classification criteria used and specific cognitive domains affected in sdMCI and mdMCI were not reported or lacking. Along this line, there has been considerable debate as to how exactly MCI should be defined, and whether it somehow ‘medicalises’ cognitive changes that simply characterise the normal ageing process.46 This is relevant as individuals with non-amnestic MCI may actually have a disorder other than AD.47 For example, olfactory impairments are also common in Dementia with Lewy bodies (DLB),48,49 and, in fact, more pronounced than what is typically seen in AD.49,50 It is possible that many MCI individuals with significant olfactory impairment will progress to DLB and not AD. At the very least, future studies should detail the methods used to arrive at subtype classification as well provide additional details of the specific types and number of affected cognitive domains, especially in the sdMCI and mdMCI categories. Better classification along with longitudinal studies will allow for improved sensitivity and specificity of olfactory dysfunction.
Moderator analysis of other variables revealed an unexpected and counterintuitive relationship between increasing age and less olfactory deficit. This relationship appeared to be driven by six outlying effects comprised of older and predominantly female samples. Sex differences in olfactory function have been well demonstrated, with women outperforming men on most tests of psychophysical olfactory function.51 Women also have longer life expectancies than men52 and in the current analysis, studies whose average age is higher tend to have a higher proportion of women to men. Despite this, there have been studies that show that women experience a higher rate of progression in MCI and AD,53,54 and they tend to exhibit higher morbidity (at a younger age) than men. It is possible that the six studies noted above are not representative of the MCI-to-AD continuum. As noted above, women tend to have higher morbidity at a younger age, but these six studies include a larger number of older women who have not converted to AD. These effects underscore the need for more emphasis on the effect of sex—and its interaction with age—in AD and MCI to improve our understanding of olfactory performance deficits and, more generally, disease detection and treatment.55
Limitations
The current study has some limitations that are worthy of discussion. First, there were a number of clinical and demographic moderators that we were unable to examine. For example, data on smoking history were often not available or were not even mentioned. While the effects of smoking on olfaction are significant, they are typically smaller than the impact of age and sex.56 Regardless, future research could help detail the impact of smoking on olfactory functions by clearly detailing patients’ smoking status as well as the overall burden of smoking through the calculation of pack years or days.56 Second, there are an unequal number of studies using each olfactory test type and the specific olfactory tests used varied from study to study (see table 1). Different numbers of studies in each domain likely influence the power to detect subtle differences that may be relevant in incipient dementia. Comparing results from studies that employ different olfactory tests is challenging, but the available evidence suggests that these tests are comparable,57 at least for the commercially available tests. While a global screening measure of cognition was not related to olfactory functioning in this current analysis, future studies using a broader neuropsychological battery may help explore any possible relationship with memory dysfunction or other neurocognitive deficit. Finally, as can be seen by the relative paucity of papers in the literature on this topic, the research on olfactory dysfunction in MCI is still in its infancy.
CONCLUSION
In conclusion, olfactory deficits are present and robust in MCI. Odour identification is most impaired in MCI, which parallels the most prominent deficit in Alzheimer’s type dementia. As such, simple-to-administer tests of odour identification warrant inclusion in the screening of individuals at risk for developing AD.
Supplementary Material
Acknowledgments
Funding This work was supported by National Institute of Mental Health (K01 MH102609) and National Institute on Aging (P30 AG10124).
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/jnnp-2016-314638).
Contributors They had full access to all data and take responsibility of the integrity of the data and the accuracy of the data analysis. MJM and DRR conceptualised and designed the study. Acquisition, analysis or interpretation of data was carried out by PJM, DRR, LB, MJM and SK. DRR, MJM and PJM drafted the manuscript. DRR, MJM, BIT, LB, SK, DAW and PJM critically revised the manuscript for intellectual content. Statistical analysis was performed by MJM and DRR. DRR, MJM and DAW obtained the funding. Administrative, technical or material support was provided by MJM and DRR. DRR and MJM supervised the study.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement This is a meta-analysis of published work. All data are in the public domain in the form of manuscripts. Any and all data that we report are presented in either the body of the manuscript or the online supplemental material. We are happy to share the final collated data set.
REFERENCES
- 1.Roalf DR, Moberg PJ, Xie SX, et al. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement (Amst) 2013;9:529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesholam RI, Moberg PJ, Mahr RN, et al. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djordjevic J, Jones-Gotman M, De Sousa K, et al. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res. 2012;231:60–74. doi: 10.1016/j.bbr.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Davies DC, Brooks JW, Lewis DA. Axonal loss from the olfactory tracts in Alzheimer’s disease. Neurobiol Aging. 1993;14:353–357. doi: 10.1016/0197-4580(93)90121-q. [DOI] [PubMed] [Google Scholar]
- 7.Vasavada MM, Wang J, Eslinger PJ, et al. Olfactory cortex degeneration in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2015;45:947–958. doi: 10.3233/JAD-141947. [DOI] [PubMed] [Google Scholar]
- 8.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 9.Marigliano V, Gualdi G, Servello A, et al. Olfactory deficit and hippocampal volume loss for early diagnosis of Alzheimer disease: a pilot study. Alzheimer Dis Assoc Disord. 2014;28:194–197. doi: 10.1097/WAD.0b013e31827bdb9f. [DOI] [PubMed] [Google Scholar]
- 10.Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology. 2015;84:2153–2160. doi: 10.1212/WNL.0000000000001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesson DW, Levy E, Nixon RA, et al. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold SE, Lee EB, Moberg PJ, et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67:462–469. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passali GC, Politi L, Crisanti A, et al. Tau protein detection in anosmic Alzheimer’s disease patient’s nasal secretions. Chem Percept. 2015;8:201–206. [Google Scholar]
- 14.Schriever VA, Mori E, Petters W, et al. The “Sniffin’ Kids” test--a 14-item odour identification test for children. PLoS One. 2014;9:e101086. doi: 10.1371/journal.pone.0101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttenbrink KB. Disorders of smell and taste. Standard and recent methods in diagnosis and therapy. Laryngorhinootologie. 1997;76:506–514. doi: 10.1055/s-2007-997469. [DOI] [PubMed] [Google Scholar]
- 16.Hummel T, Kobal G, Gudziol H, et al. Normative data for the “Sniffin’ Sticks” including tests of odour identification, odour discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 17.Eibenstein A, Fioretti AB, Lena C, et al. Olfactory screening test: experience in 102 Italian subjects. Acta Otorhinolaryngol Ital. 2005;25:18–22. [PMC free article] [PubMed] [Google Scholar]
- 18.Doty RL. Olfactory dysfunction and its measurement in the clinic. World J Otorhin. 2015;1:28–33. doi: 10.1016/j.wjorl.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devanand DP, Michaels-Marston KS, Liu X, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- 20.Morgan CD, Nordin S, Murphy C. Odour identification as an early marker for Alzheimer’s disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol. 1995;17:793–803. doi: 10.1080/01688639508405168. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Schneider JA, Arnold SE, et al. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 22.Vyhnalek M, Magerova H, Andel R, et al. Olfactory identification in amnestic and non-amnestic mild cognitive impairment and its neuropsychological correlates. J Neurol Sci. 2015;349:179–184. doi: 10.1016/j.jns.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RO, Christianson TJ, Kremers WK, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73:93–101. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti MZ, Vicini-Chilovi B, Riva M, et al. Odour identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch Clin Neuropsychol. 2013;28:391–399. doi: 10.1093/arclin/act032. [DOI] [PubMed] [Google Scholar]
- 25.Sun GH, Raji CA, MacEachern MP, et al. Olfactory identification testing as a predictor of the development of Alzheimer’s dementia: a systematic review. Laryngoscope. 2012;122:1455–1462. doi: 10.1002/lary.23365. [DOI] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from The National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement (Amst) 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delano-Wood L, Bondi MW, Sacco J, et al. Heterogeneity in mild cognitive impairment: differences in neuropsychological profile and associated White matter lesion pathology. J Int Neuropsychol Soc. 2009;15:906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark LR, Delano-Wood L, Libon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc. 2013;19:635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioural sciences. 2nd. Hillsdale, New Jersey: Erlbaum; 1988. [Google Scholar]
- 31.Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic press; 1985. [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–1101. [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 35.Dulay MF, Gesteland RC, Shear PK, et al. Assessment of the influence of cognition and cognitive processing speed on three tests of olfaction. J Clin Exp Neuropsychol. 2008;30:327–337. doi: 10.1080/13803390701415892. [DOI] [PubMed] [Google Scholar]
- 36.Royet JP, Koenig O, Paugam-Moisy H, et al. Levels-of-processing effects on a task of olfactory naming. Percept Mot Skills. 2004;98:197–213. doi: 10.2466/pms.98.1.197-213. [DOI] [PubMed] [Google Scholar]
- 37.Luzzi S, Snowden JS, Neary D, et al. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Hedner M, Larsson M, Arnold N, et al. Cognitive factors in odour detection, odour discrimination, and odour identification tasks. J Clin Exp Neuropsychol. 2010;32:1062–1067. doi: 10.1080/13803391003683070. [DOI] [PubMed] [Google Scholar]
- 39.Lötsch J, Reichmann H, Hummel T. Different odour tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2008;33:17–21. doi: 10.1093/chemse/bjm058. [DOI] [PubMed] [Google Scholar]
- 40.Bahar-Fuchs A, Moss S, Rowe C, et al. Olfactory performance in AD, aMCI, and healthy ageing: a unirhinal approach. Chem Senses. 2010;35:855–862. doi: 10.1093/chemse/bjq094. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson RJ, Boakes RA. A mnemonic theory of odour perception. Psychol Rev. 2003;110:340. doi: 10.1037/0033-295x.110.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Quarmley M, Moberg PJ, Mechanic-Hamilton D, et al. Odour identification screening improves diagnostic classification in incipient dementia. J Alzheimers Dis. 2016;55(4) doi: 10.3233/JAD-160842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roalf DR, Quarmley M, Elliott MA, et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devanand DP, Lee S, Manly J, et al. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78:401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanciu I, Larsson M, Nordin S, et al. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc. 2014;20:209–217. doi: 10.1017/S1355617713001409. [DOI] [PubMed] [Google Scholar]
- 46.Hughes TF, Snitz BE, Ganguli M. Should mild cognitive impairment be subtyped? Curr Opin Psychiatry. 2011;24:237. doi: 10.1097/YCO.0b013e328344696b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boeve BF. Mild cognitive impairment associated with underlying Alzheimer’s disease versus Lewy body disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S41–S44. doi: 10.1016/S1353-8020(11)70015-3. [DOI] [PubMed] [Google Scholar]
- 48.Yoon JH, Kim M, Moon SY, et al. Olfactory function and neuropsychological profile to differentiate dementia with Lewy bodies from Alzheimer’s disease in patients with mild cognitive impairment: a 5-year follow-up study. J Neurol Sci. 2015;355:174–179. doi: 10.1016/j.jns.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Williams SS, Williams J, Combrinck M, et al. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatr. 2009;80:667–670. doi: 10.1136/jnnp.2008.155895. [DOI] [PubMed] [Google Scholar]
- 50.Sato T, Hanyu H, Kume K, et al. Difference in olfactory dysfunction with dementia with Lewy bodies and Alzheimer’s disease. J Am Geriatr Soc. 2011;59:947–948. doi: 10.1111/j.1532-5415.2011.03380.x. [DOI] [PubMed] [Google Scholar]
- 51.Doty RL, Cameron EL. Sex differences and reproductive hormone influences on human odour perception. Physiol Behav. 2009;97:213–228. doi: 10.1016/j.physbeh.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barford A, Dorling D, Davey Smith G, et al. Life expectancy: women now on top everywhere. BMJ. 2006:332. doi: 10.1136/bmj.332.7545.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin KA, Choudhury KR, Rathakrishnan BG, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 2015;1:103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15:451. doi: 10.1016/S1474-4422(16)00067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- 57.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odour identification, odour discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257:205–211. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.