Abstract
Background
Measurements of olfaction may serve as useful biomarkers of incipient dementia. Here we examine the improvement in diagnostic accuracy of Alzheimer’s disease (AD) and mild cognitive impairment (MCI) when assessing both cognitive functioning and odor identification.
Objective
To determine the utility of odor identification as a supplementary screening test in incipient AD.
Methods
Sniffin’ Sticks Odor Identification Test (SS-OIT) and the Montreal Cognitive Assessment (MoCA) were administered in 262 AD, 174 MCI [150 amnestic (aMCI), and 24 non-amnestic (naMCI)], and 292 healthy older adults (HOA).
Results
Odor identification scores were higher in HOA relative to MCI or AD groups, and MCI outperformed AD. Odor identification scores were higher in aMCI single domain than aMCI multiple domain. Complementing MoCA scores with the SS-OIT significantly improved diagnostic accuracy of individuals with AD and MCI, including within MCI subgroups.
Discussion
Odor identification is a useful supplementary screening tool that provides additional information relevant for clinical categorization of AD and MCI, including those who are at highest risk to convert to AD.
Keywords: Alzheimer’s disease, mild cognitive impairment, Montreal Cognitive Assessment, odor identification, smell, Sniffin’ Sticks Olfactory Identification Test
INTRODUCTION
Alzheimer’s disease (AD) is a debilitating neurodegenerative disease and the leading cause of disability in old age [1]. Early identification of individuals likely to develop AD dementia is crucial for preventative or mitigating interventions. Current research efforts are focused on mild cognitive impairment (MCI), a cognitive syndrome enriched in individuals with prodromal AD [2]. Individuals with MCI, in particular those with amnestic MCI, are at heightened risk for developing dementia [3], with annual conversion rates to AD between 8-15%, with most conversions within three years of presentation [4].
Early and accurate detection of cognitive and other neurological or psychiatric impairments in MCI that are indicative of a risk for progression to dementia can enhance clinical management as well as lead to better understanding of individual differences in disease progression. To this effect, recent studies of cognitive function in MCI are aimed at early detection and prevention strategies. Recent work [5] confirms and extends prior findings on the diagnostic utility of detailed neuropsychological inventories and cognitive screens in AD and MCI. However, challenges remain in efficiently identifying the prodromal stages of MCI that lead to AD. Poor differentiation is likely due to several factors including: 1) heterogeneity of the MCI diagnosis; 2) variable progression rates from MCI to AD; 3) sensitivity and specificity of cognitive tests; and 4) the limited use of non-cognitive screening measures to capture other dimensions of neurodegeneration. The last point should not be minimized as other neurological domains are affected in AD and MCI (e.g., motor function, olfactory function). In fact, sensory deficits may prove useful in the early detection of dementia and may contribute to the functional decline of AD [6, 7].
Measurements of olfaction may serve as useful biomarkers of incipient dementia [6, 8, 9]. Olfactory deficits in AD and MCI are reliably observed in multiple olfactory domains, including odor detection threshold, identification, and recognition [10]. Olfactory deficits precede the onset of illness [11], distinguish patients with prodromal symptoms from healthy older adults [12, 13], and may predict which vulnerable individuals go on to develop frank dementia [2, 11, 12]. Impaired odor identification and detection is found in AD [14] and MCI amnestic type [13]. In fact, combining olfactory testing with cognitive screening (e.g., the Mini-Mental State Examination (MMSE)) leads to improved diagnostic classification [14]. Moreover, a recent, prospective population-based study found olfactory impairment is associated with incident amnestic MCI and with progression from amnestic MCI to AD dementia [15]. Odor identification was also found to be superior to episodic memory deficits in predicting cognitive decline in cognitively intact individuals [16].
Given the cumulative evidence implicating abnormal olfactory function and structure in the pathogenesis of dementia, we propose that olfactory screening, when combined with well-validated cognitive screening, can improve the clinical specificity and diagnostic accuracy of individuals with MCI and AD, and specifically those at highest risk for conversion to AD. Here, we tested the hypotheses that: 1) AD and MCI have lower odor identification scores than healthy older adults; 2) amnestic MCI individuals have lower odor identification scores than other MCI subgroups; and 3) odor identification scores improve diagnostic classification of individuals with MCI above and beyond cognitive screening.
MATERIALS AND METHODS
Subject selection
Participants were recruited from the Penn Memory Center and Clinical Core of the University of Pennsylvania’s Alzheimer’s Disease Center between 2005-2015. Participants consisted of 262 individuals with expert consensus clinical diagnoses of AD, 174 individuals with MCI [80 amnestic MCI single domain (aMCIsd), 70 amnestic MCI multiple domain (aMCImd), 24 non-amnestic (naMCI)], and 292 healthy older adults (HOA). Recruitment and subject assessment procedures were described previously [5]. Briefly, diagnostic assessments included medical history and physical and neurologic examinations conducted by experienced clinicians, including the review of neuroimaging, neuropsychological testing, and laboratory data. A consensus diagnosis was established using established clinical criteria for AD, MCI, or other neurologic or psychiatric conditions presenting with cognitive impairment [5]. All tests were administer by a trained technician or clinician.
Three subtypes of MCI are defined: 1) naMCI: those without objective memory impairment; 2) aMCIsd: those with isolated memory impairment; and 3) aMCImd: those with impairments in other cognitive domains beyond memory. Amnestic individuals [17, 18], in particular individuals with aMCImd [19, 20], are most likely to progress AD. Subtypes of MCI were determined according to the Petersen criteria [21] and psychometric testing as described by the National Alzheimer’s Coordinating Center (NACC) Uniform Dataset (UDS2) [22, 23]. HOA were recruited and assessed identically to the patients. Informed consent was obtained from all persons, in accord with University of Pennsylvania institutional review board.
Cognitive screening for dementia
Most, but not all, participants completed the Montreal Cognitive Assessment (MoCA) [24]. In the case of missing MoCA scores, but a valid MMSE score, MoCA scores were generated using the previously published MMSE to MoCA conversion [5]. MoCA scores can range from 0-30 and mean MoCA scores are presented for each diagnostic group in Table 1. Typically, the MoCA takes 10-15 minutes to administer. We acknowledge that this can be a significant burden on the clinician. Thus, we recently published a valid brief version of the MoCA, called the s-MoCA [25]. This brief version is 8 questions long and takes approximately 5 minutes to administer.
Table 1.
Demographic characteristics, clinical, cognitive and olfactory performance scores for HOA, MCI, and AD
| HOA | MCI | AD | |
|---|---|---|---|
| n | 292 | 174 | 262 |
| Age, mean (SD) in years | 70.96 (8.74)† | 72.46 (8.57)† | 75.18 (8.22)*‡ |
| Sex, n | |||
| Male | 89‡ | 82*† | 98‡ |
| Female | 203‡ | 92*† | 164‡ |
| Race, n | |||
| White | 180†‡ | 122* | 201* |
| African American | 97†‡ | 38* | 35* |
| Other | 15†‡ | 14* | 26* |
| Education, mean (SD) in years | 15.62 (2.96) † | 15.02 (3.61)† | 14.10 (3.83)*‡ |
| Clinical Dementia Rating, mean (SD)§ | 0.02 (0.10)†‡ | 0.47 (0.15)*† | 0.81 (0.41)*‡ |
| Functional Rating Scale, mean (SD)¶ | 0.54 (1.27)†‡ | 5.00 (4.15)*† | 13.59 (7.16)*‡ |
| Geriatric Depression Scale, mean (SD)# | 0.94 (1.69)†‡ | 2.24 (2.61)* | 2.50 (2.68)* |
| MoCA, mean (SD) | 25.98 (2.74)†‡ | 21.32 (3.97)*† | 15.27 (5.24)*‡ |
| Sniffin’ Sticks Test, mean (SD) | 12.43 (2.53)†‡ | 9.94 (3.28)*† | 7.82 (3.46)*‡ |
| CERAD-NB, mean (SD)** | 84.29 (8.81)†‡ | 66.07 (10.55)* † | 47.84 (14.30)*‡ |
HOA, healthy older adults; MCI, mild cognitive impairment; AD, Alzheimer’s disease,
p<0.05 difference from HOA;
p<0.05 difference from AD;
p<0.05 difference from MCI,
Clinical Dementia Rating (CDR): n = HOA (285), MCI (155), AD (228),
Functional Rating Scale (FRS): n = HOA (268), MCI (168), AD (259),
Geriatric Depression Scale (GDS): n = HOA (285), MCI (160), AD (227),
CERAD-NB: n = HOA (292), MCI (174), AD (256),
HOA, MCI, and AD: HOA and MCI were younger and attained higher levels of education than AD. The proportion of females was higher in the HOA than in the MCI group. HOA, AD, and MCI groups included more Caucasians than African Americans and more African Americans than other races. As expected, there were systematic group differences in overall neuropsychological function and clinical ratings: CERAD-NB, MoCA, CDR, FRS, and GDS. In addition, the CDR, FRS, and GDS were administered to many individuals.
Olfactory testing
Olfaction was measured using the Sniffin’ Sticks Odor Identification Test (SS-OIT) [26]. The SS-OIT is a commercially available test with highly reproducible results [27]. During this task, the subject is presented with 16 odors via felt-tipped pen dispensers. For each odor, the subject is asked to identify the odor from four given choices. SS-OIT scores can range from 0-16 and mean SS-OIT scores are presented for each diagnostic group in Table 1. Administration of the SS-OIT takes between 5-8 minutes.
Statistical analyses
Demographic characteristics were compared across diagnostic groups using Pearson χ2 or one-way analysis of variance (ANOVAs) with post-hoc t-tests. Odor identification across diagnostic groups was evaluated using a one-way ANOVA with sex, race, education years, and age included in the model. Post-hoc t-tests were performed and were corrected for unequal variance using the Welch approximation. Pearson correlation coefficients were calculated for the overall sample, and each diagnostic subsample, to show the relationship between MoCA score and the SS-OIT. Statistical significance was defined as an alpha level less than 0.05.
Overall accuracy of the SS-OIT to differentiate diagnoses was assessed using the receiver operating characteristic (ROC) curve analysis. Area-under-the-curve (AUC) was also determined for the SS-OIT. Classification accuracy of the MoCA and SS-OIT was calculated by establishing a cut-off score for each measure that best differentiated diagnostic group, determined using the Youden Index [28], which maximizes the tradeoffs between sensitivity and specificity. This cut-off was then applied to the data to obtain diagnostic classification accuracy.
We used a two-stage analysis to determine if SS-OIT improved diagnostic accuracy above and beyond the MoCA. In Stage 1, the previously generated MoCA cut-off scores from Roalf et al. [5] were used to differentiate AD from HOA (MoCA = 23); AD from MCI (MoCA = 19), including all subtypes; and MCI from HOA (MoCA = 25). Individuals incorrectly classified by their MoCA scores were then identified. In Stage 2, the olfactory cut-off score generated using the SS-OIT was then applied to individuals misclassified by their MoCA score and diagnostic classification was determined on this subset. All correctly identified individuals (true positive or true negative using either MoCA or SS-OIT) are reported. Multinomial ROC analyses and Delong’s tests for two ROC curves were used to compare overall models. All statistical analyses were performed using R (version 3.0.2) software.
RESULTS
Participant characteristics
Participant characteristics are displayed by diagnosis (AD, MCI, HOA) in Table 1A. Groups differed by age [F(2,433.5)=17.46, p < 0.0001], years of education [F(2,413.3)=13.36, p < 0.0001], sex [χ2 = 13.01, p = 0.001], and race [χ2 = 32.39, p < 0.0001]. Group specific comparisons are detailed in Table 1A and in the Supplementary Material.
Olfactory performance in AD, MCI, and HOA
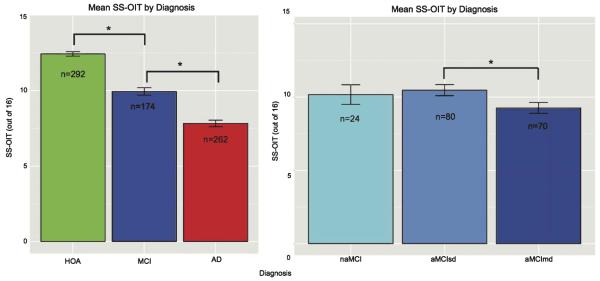
Odor identification (SS-OIT) differed between diagnostic groups [F = 230.1, p < 0.0001], after controlling for sex, race, age, and education (Fig. 1A). SS-OIT performance was better in HOA relative to MCI [t(295.2)=8.60, p < 0.0001] and AD [t(473.7)=17.72, p < 0.0001]. SS-OIT performance was better in MCI as compared to AD [t(383.4)=6.46, p < 0.0001]. There was a significant, but small correlation between MoCA and SS-OIT in HOA [r = 0.14, n = 292, p = 0.013], AD individuals [r = 0.30, n = 230, p < 0.0001], and MCI individuals [r = 0.16, n = 109, p = 0.03].
Fig. 1.
A) Mean SS-OIT scores with standard error bars by diagnosis (HOA, healthy older adults; MCI, mild cognitive impairment; AD, Alzheimer’s disease; *p < 0.0001). B) Mean SS-OIT scores with standard error bars by diagnosis (naMCI, mild cognitive impairment non-amnestic; aMCIsd, mild cognitive impairment amnestic single domain, aMCImd, mild cognitive impairment multiple domain; *p < 0.023).
Olfactory performance in MCI subgroupings
Performance across MCI subgroups was measured in an exploratory analysis. Participant characteristics of MCI individuals are displayed by diagnostic subgroup (aMCImd, aMCIsd, naMCI) in Table 1B. naMCI attained higher education than aMCImd [t(47.3)=2.37, p < 0.022].
MCI subgroups did not differ in MoCA score. aMCIsd had higher SS-OIT than aMCImd [t(147.6)=2.31, p < 0.023] (Fig. 1B). naMCI performance was intermediate between aMCIsd and aMCImd, and did not statistically differ from either. MoCA and SS-OIT performance was correlated in aMCIsd [r = 0.24, n = 80, p = 0.031], but not naMCI [r = 0.07, n = 24, p = 0.74] or aMCImd [r = 0.07, n = 70, p = 0.55].
ROC analyses of odor identification
Diagnostic classification using odor identification scores alone
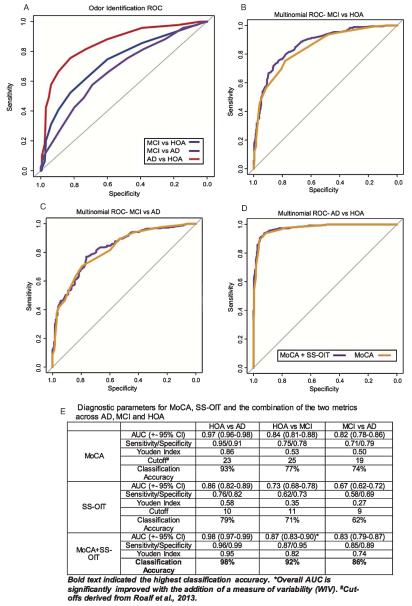
ROC analyses were performed using the SS-OIT to determine optimal cut-off scores for diagnostic classification accuracy (Fig. 2). SS-OIT best differentiated AD from HOA individuals [AUC = 0.855], then HOA from MCI [AUC = 0.731], and then MCI from AD individuals [AUC = 0.67]. Details are presented in Fig. 2E.
Fig. 2.
ROC curves for SS-OIT. A-D) Comparison of multinomial AUC (MoCA + SS-OIT) to MoCA only AUC for diagnostic accuracy. The addition of SS-OIT to the MoCA significantly improved overall prediction between MCI and HOA. E) AUC, sensitivity and specificity, Youden index, optimal cut-off score, and diagnostic classification accuracy for the MoCA, SS-OIT, and MoCA + SS-OIT.
Multinomial ROC analysis
Overall, using both MoCA and SS-OIT to classify individuals was significantly better for differentiating MCI from HOA [Z = 2.65, p = 0.008], marginally better for differentiating AD from HOA [Z = 1.90, p = 0.057], but no better than the MoCA alone for differentiating AD from MCI [Z = 1.46, p = 0.143]. Details are presented in Fig. 2E.
Diagnostic classification combining MoCA and odor identification scores
In practice, diagnostic cut-off scores are more useful than continuous scores. Thus, we used previously established cut-off scores [5] for the MoCA and the newly derived SS-OIT (see above) cut-offs to determine the percent improvement of diagnostic classification when the SS-OIT is used to complement the MoCA (Fig. 3 & Supplementary Material).
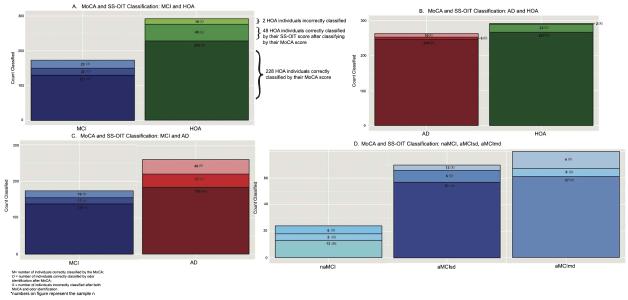
Fig. 3.
Classification accuracy of MoCA and SS-OIT scores by diagnosis. The bottom portion of each bar represents the number of individuals correctly classified by the optimal MoCA score (M). The middle portion of each bar indicates the number of individuals that were misidentified by MoCA score, but correctly identified by SS-OIT score (O). The top portion of each bar represents the number of individuals misidentified by both MoCA and SS-OIT score (X).
AD versus HOA
The use of both the MoCA and SS-OIT cut-off scores resulted in correct classification of 96% of AD and 99% of healthy individuals (Fig. 3A), an improvement of 1% and 8% over the MoCA alone, respectively.
MCI versus HOA
The use of both the MoCA and SS-OIT resulted in correct classification of 87% of MCI and 95% of healthy individuals (Fig. 3B), an improvement of 12% and 17% over the MoCA alone, respectively.
MCI versus AD
The use of both the MoCA and SS-OIT resulted in correct classification of 89.1% of MCI and 85% of AD individuals (Fig. 3 C), an improvement of 9.8% and 14% over the MoCA alone, respectively.
MCI subtypes
The MoCA had moderate classification accuracy for differentiating MCI subgroups from HOA, misclassifying 23.8% (19 of 80) aMCIsd, 18.6% (13 of 70) aMCImd, and 46% (11 of 24) naMCI. Subsequent use of SS-OIT scores correctly classified 31.6% (6 of 19) aMCIsd, 69.2% (9 of 13) aMCImd, and 45.6% (5 of 11) naMCI. Thus, the use of both the MoCA and SS-OIT resulted in correct classification of 84% aMCIsd, 94% aMCImd and 75% naMCI (Fig. 3D), an improvement of 8%, 13%, and 21%, respectively.
Olfactory screening in healthy older adults with worrisome MoCA scores
We considered, in an exploratory manner, that HOAs with worrisome MoCA scores might exhibit more olfactory deficits than those with no appreciable MoCA deficits. Thus, we determined the odor identification scores of HOA with MoCA scores at or above the reported MCI versus HOA cut-off (25). Normal MoCA performers were grouped in High (29-30), Middle (27-28), and Low (25-26) performers. The overall effect of MoCA performance group on odor identification was significant [F(2,225)=3.056, p < 0.05]. Pairwise comparisons indicated that High MoCA performers [mean(sd): 13.38 (1.63), n = 44] had significantly better SS-OIT score than Middle MoCA [mean(sd): 12.27 (2.54), n = 96; p = 0.04)]performers and marginally better performance than Low MoCA performers [mean(sd): 12.53 (2.76), n = 88; p = 0.09]. Furthermore, more Low and Middle MoCA performers performed below the SS-OIT cut-off score of 11 : 16% of Low MoCA individuals, 20% of Middle MoCA individuals, but only 7% of High MoCA individuals performed below this score (Fig. 4).
Fig. 4.

Percentage of HOA individuals with normal MoCA scores falling below the odor identification threshold. Normal MoCA performers were grouped in High (29-30), Middle (27-28), and Low (25-26) performers. Individuals with the lower MoCA scores were more likely to perform poorly on odor identification.
DISCUSSION
In this study, we report clinically useful cut-offs for a popular, simple-to-administer odor identification test; and we confirm recent reports of the utility of odor identification as a useful marker for incipient dementia that should be used for clinical screening in conjunction with traditional cognitive screening. In a clinically ascertained sample, poorer odor identification performance was associated with AD and MCI, particularly in the amnestic multiple domain subtype of MCI. Odor identification alone was a significant predictor of clinical status. When combined with the MoCA—a common screen of global cognitive functioning—identification of individuals with AD and MCI improved significantly. Determination and use of clinically valid cut-off scores for the SS-OIT indicate that using this psychophysical olfactory test as a supplementary measure to the MoCA improves diagnostic accuracy in incipient dementia, particularly in patients with aMCImd subtype, those most likely to transition to AD dementia.
We confirm previous work indicating olfactory impairment is a regular feature of AD dementia and MCI [13, 16]. Notably, we extend these findings by providing useful clinical cut-offs for the SS-OIT. SS-OIT scores below 10 were indicative of AD as compared to HOA, scores under 11 were associated with MCI as compared to HOA, while scores below 9 were indicative of AD as compared to MCI. However, olfactory scores alone were not as robust as the MoCA for clinical categorization. Given the small range of cut-off scores between frank dementia and MCI, the prodromal stage of AD, we used odor identification scores as a supplementary screening measure to the MoCA. Multinomial analyses indicated improved clinical classification when olfactory scores were considered with MoCA scores, an effect that was more robust in MCI than AD. The minimal correlation between SS-OIT and MoCA scores argues that each of these tests is tapping unique variance in these disorders, and the improvement in clinical classification bolsters support for the addition of olfactory testing as a screening measure. That is, it appears that the use of a supplemental olfactory assessment can hone in on a comorbid sensory deficit that goes undetected with the use of traditional cognitive screening measures. Importantly, olfactory screening is routine [29], reliable [30], and quick and easy to administer [31]. Moreover, our findings are consistent with recent work by Devanand et al. [16] suggesting superiority of olfactory testing over an episodic verbal memory test in predicting cognitive decline. Finally, our findings corroborate those of Velayudhan et al. [14] who report a 10% increase in diagnostic accuracy of AD versus HOA when using both the University of Pennsylvania Smell Identification Test (UPSIT) and the MMSE.
More specifically, our use of derived clinical cut-off scores for the MoCA and SS-OIT significantly improved both sensitivity and specificity. In the comparison of AD and HOA, a large number of HOA individuals misclassified by MoCA scores were correctly identified by SS-OIT scores, but relatively few AD patients were reclassified using the supplementary SS-OIT score. In the comparison of MCI and HOA, more MCI and HOA individuals were subsequently reclassified correctly after considering their olfactory scores. When differentiating MCI and AD, a moderate number of MCI and AD individuals were subsequently reclassified correctly after considering their olfactory scores. Importantly, we also find that HOAs with imperfect cognitive screening scores are more likely to exhibit olfactory deficits. This further underscores the potential utility of olfactory testing in the screening of individuals at potential risk very early on for developing dementia. As suggested by Roberts et al. [15], we show that the combination of olfactory and cognitive testing is useful in screening individuals for early cognitive decline that may lead to AD.
The heterogeneity of MCI makes early identification difficult. To this effect, understanding the disease course of distinct MCI subtypes may aid in early identification of those at highest risk for developing AD compared to those for whom stability is predicted. Not only do we find olfactory impairment in the general MCI cohort, we find significantly more impairment in individuals with amnestic multiple domain MCI as compared to those with MCI amnestic single domain. This deficit is consistent with prior findings in the literature [13, 15] and suggests that when the disease burden includes other domains beyond memory, the relevance of odor identification deficits increases. Moreover, this suggests a distributed neuropathological state in those where deficits extend to multiple domains and is in agreement with studies finding higher conversion rate to AD in this MCI subtype [32, 33].
Our use of derived clinical cut-off scores for SS-OIT to correctly classify individuals misclassified by MoCA scores improved classification of all MCI subgroups. In the comparison of MCI subgroups, a higher percentage of aMCImd individuals were subsequently reclassified correctly after considering their olfactory scores. This suggests that utilizing SS-OIT cut-off scores as a supplement to MoCA is most useful as a clinical tool for those at highest risk for converting to AD. Olfactory deficits were similar between a small sample of non-amnestic and single and multiple domain amnestic individuals in agreement with limited previous work [13, 34], further indicating that MCI is etiologically a heterogeneous group. Finally, longitudinal studies with larger samples should further examine olfactory ability within this subtype. Deficits in olfactory performance denote fundamental neuroanatomic and neurophysiologic abnormalities that are specific to the peripheral olfactory system [8, 35], olfactory bulb and/or primary olfactory cortices [36]. Olfactory dysfunction is correlated with the global level of AD pathology on postmortem examination [1], biopsy of the olfactory epithelium indicates the presence of AD pathology (e.g., amyloid-β, tau) in pathologically verified AD patients [37], and the presence of tau protein has been reported in nasal secretions of AD individuals with olfactory deficits [38]. Finally, poorer olfactory ability is associated with structural brain changes in the hippocampus and entorhinal cortex, two regions prominently affected in early stages of AD [39–41]. Thus, the olfactory deficits in AD may arise throughout the olfactory system. Additional work remains necessary to elucidate the sequential neurobiological mechanisms responsible for olfactory deficits in MCI and AD dementia.
We note a few limitations to the current study. First, as is common among olfactory studies, only odor identification was measured. Other studies have identified deficits in odor detection threshold and odor recognition memory in MCI [9, 42], and the utility of these measures of olfactory functioning also warrant further investigation. The study also only utilized one form of odor identification testing, the SS-OIT; however, this test is a reliable clinical assessment tool with large normative basis [30] that can be performed quickly given the few number of items. We acknowledge that there is the need for adequate and effective cognitive and sensory screening given the rapid growth of the elderly population. As such, adding additional tests comes at some time cost to clinicians. Here we report data from both the full MoCA and SS-OIT, which in total, take between 15-25 minutes. However, we recently published a short version of the MoCA (s-MoCA) that only takes 5 minutes to administer [25]. Additionally, short, nonforced choice versions of the SS-OIT are available and validated for clinical use; however, more work needs to be done to validate this in AD and MCI samples. Furthermore, similar results are found utilizing the B-SIT [15], UPSIT [8], and the Motol Hospital Smell Test [13]. The cross-sectional design of the study did not allow us to make precise conclusions about the conversion and disease trajectory of our MCI patients; however, follow-up studies are planned. ROC classification analyses were not performed in the MCI subtypes due to relatively small sample sizes.
We conclude that odor identification deficits are evident in AD and MCI subtypes. Importantly, the SS-OIT is a useful classification tool for MCI, and more specifically aMCImd, when used in conjunction with the MoCA.
Supplementary Material
Table 2.
Demographic characteristics, clinical, cognitive, and olfactory performance scores for MCI subtypes
| naMCI | aMCIsd | aMCImd | |
|---|---|---|---|
| n | 24 | 80 | 70 |
| Age, mean (SD) in years | 72.25 (8.67) | 72.50 (8.74) | 72.49 (8.48) |
| Sex, n | |||
| Male | 12 | 37 | 33 |
| Female | 12 | 43 | 37 |
| Race, n | |||
| White | 18 | 61 | 43 |
| African American | 5 | 15 | 18 |
| Other | 1 | 4 | 9 |
| Education, mean (SD) in years | 16.38 (3.03)‡ | 15.00 (3.70) | 14.59 (3.63)* |
| Clinical Dementia Rating, mean (SD)§ | 0.35 (0.24)†‡ | 0.49 (0.12)* | 0.50 (0.13)* |
| Functional Rating Scale, mean (SD)¶ | 6.26 (5.51) | 5.00 (3.98) | 4.57 (3.76) |
| Geriatric Depression Scale, mean (SD)# | 2.20 (2.44) | 2.38 (2.53) | 2.12 (2.76) |
| MoCA, mean (SD) | 23.04 (3.50) | 21.36 (3.75) | 20.67 (4.22) |
| Sniffin’ Sticks Test, mean (SD) | 10.17 (3.28) | 10.46 (3.37)‡ | 9.26 (3.10)† |
| CERAD-NB, mean (SD) | 71.21 (7.05)†‡ | 66.94 (10.50)*‡ | 63.31 (10.88)*† |
naMCI, mild cognitive impairment non-amnestic; aMCIsd, mild cognitive impairment amnestic single domain; aMCImd, mild cognitive impairment amnestic multiple domain,
p<0.05 difference from naMCI;
p<0.05 difference from aMCIsd;
p<0.05 difference from aMCImd,
Clinical Dementia Rating: n = naMCI (20), aMCIsd (71), aMCImd (64),
Functional Rating Scale: n = naMCI (23), aMCIsd (77), aMCImd (68),
Geriatric Depression Scale: n = naMCI (20), aMCIsd (71), aMCImd (69).
ACKNOWLEDGMENTS
The authors express appreciation to the research participants and staff of the Penn Memory Center/Clinical Core of the University of Pennsylvania Alzheimer’s Disease Center.
This work was supported by NIMH [K01 MH102609 (DRR)]; NIA [P30 AG10124]; and the University of Pennsylvania Center of Excellence for Research on Neurodegenerative Diseases (CERND).
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0842r1).
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-160842.
REFERENCES
- [1].Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Conti MZ, Vicini-Chilovi B, Riva M, Zanetti M, Liberini P, Padovani A, Rozzini L. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch Clin Neuropsychol. 2013;28:391–399. doi: 10.1093/arclin/act032. [DOI] [PubMed] [Google Scholar]
- [3].Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- [4].Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- [5].Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement (Amst) 2013;9:529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- [7].Del Campo N, Payoux P, Djilali A, Delrieu J, Hoogendijk EO, Rolland Y, Cesari M, Weiner MW, Andrieu S, Vellas B, Study MAPT/DSA, Group Relationship of regional brain beta-amyloid to gait speed. Neurology. 2016;86:36–43. doi: 10.1212/WNL.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- [10].Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: A meta-analysis. Behav Brain Res. 2012;231:60–74. doi: 10.1016/j.bbr.2012.02.047. [DOI] [PubMed] [Google Scholar]
- [11].Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- [12].Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- [13].Vyhnalek M, Magerova H, Andel R, Nikolai T, Kadlecova A, Laczo J, Hort J. Olfactory identification in amnestic and non-amnestic mild cognitive impairment and its neuropsychological correlates. J Neurol Sci. 2015;349:179–184. doi: 10.1016/j.jns.2015.01.014. [DOI] [PubMed] [Google Scholar]
- [14].Velayudhan L, Gasper A, Pritchard M, Baillon S, Messer C, Proitsi P. Pattern of smell identification impairment in Alzheimer’s disease. J Alzheimers Dis. 2015;46:381–387. doi: 10.3233/JAD-142838. [DOI] [PubMed] [Google Scholar]
- [15].Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73:93–101. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Masurkar A, Stern Y, Mayeux R, Doty RL. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78:401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- [19].Han JW, Kim TH, Lee SB, Park JH, Lee JJ, Huh Y, Park JE, Jhoo JH, Lee DY, Kim KW. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement. 2012;8:553–559. doi: 10.1016/j.jalz.2011.08.007. [DOI] [PubMed] [Google Scholar]
- [20].Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De-Kosky ST. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- [22].Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. Exploring the recognition memory deficit in Parkinson’s disease: Estimates of recollection versus familiarity. Brain. 2006;129:1768–1779. doi: 10.1093/brain/awl115. [DOI] [PubMed] [Google Scholar]
- [23].Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [25].Roalf DR, Moore TM, Wolk DA, Arnold SE, Mechanic-Hamilton D, Rick J, Kabadi S, Ruparel K, Chen-Plotkin AS, Chahine LM, Dahodwala NA, Duda JE, Weintraub DA, Moberg PJ. Defining and validating a short form Montreal Cognitive Assessment (s-MoCA) for use in neurodegenerative disease. J Neurol Neurosurg Psychiatry. 2016 doi: 10.1136/jnnp-2015-312723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: Screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- [27].Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257:205–211. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
- [28].Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [29].Schriever VA, Mori E, Petters W, Boerner C, Smitka M, Hummel T. The “Sniffin’ Kids” test–a 14-item odor identification test for children. PLoS One. 2014;9:e101086. doi: 10.1371/journal.pone.0101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- [31].Doty RL. Olfactory dysfunction and its measurement in the clinic. World J Otorhin. 2015;1:28–33. doi: 10.1016/j.wjorl.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP. A 10-item smell identification scale related to risk for Alzheimer’s disease. Ann Neurol. 2005;58:155–160. doi: 10.1002/ana.20533. [DOI] [PubMed] [Google Scholar]
- [34].Westervelt HJ, Bruce JM, Coon WG, Tremont G. Odor identification in mild cognitive impairment subtypes. J Clin Exp Neuropsychol. 2008;30:151–156. doi: 10.1080/13803390701287408. [DOI] [PubMed] [Google Scholar]
- [35].Davies DC, Brooks JW, Lewis DA. Axonal loss from the olfactory tracts in Alzheimer’s disease. Neurobiol Aging. 1993;14:353–357. doi: 10.1016/0197-4580(93)90121-q. [DOI] [PubMed] [Google Scholar]
- [36].Vasavada MM, Wang J, Eslinger PJ, Gill DJ, Sun X, Karunanayaka P, Yang QX. Olfactory cortex degeneration in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2015;45:947–958. doi: 10.3233/JAD-141947. [DOI] [PubMed] [Google Scholar]
- [37].Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, Lee VM, Trojanowski JQ. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67:462–469. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Passali GC, Politi L, Crisanti A, Loglisci M, Anzivino R, Passali D. Tau protein detection in anosmic Alzheimer’s disease patient’s nasal secretions. Chem Percept. 2015;8:201–206. [Google Scholar]
- [39].Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: Prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- [40].Marigliano V, Gualdi G, Servello A, Marigliano B, Volpe LD, Fioretti A, Pagliarella M, Valenti M, Masedu F, Di Biasi C, Ettorre E, Fusetti M. Olfactory deficit and hippocampal volume loss for early diagnosis of Alzheimer disease: A pilot study. Alzheimer Dis Assoc Disord. 2014;28:194–197. doi: 10.1097/WAD.0b013e31827bdb9f. [DOI] [PubMed] [Google Scholar]
- [41].Growdon ME, Schultz AP, Dagley AS, Amariglio RE, Hedden T, Rentz DM, Johnson KA, Sperling RA, Albers MW, Marshall GA. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology. 2015;84:2153–2160. doi: 10.1212/WNL.0000000000001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.