Abstract
Left ventricular assist device (LVAD) support may facilitate myocardial recovery. We evaluated the impact of LVAD support on Fas expression in a cohort with end‐stage heart failure. Myocardial gene expression was assessed pre‐ and post‐LVAD by RNase protection assay and compared to control donor hearts. The expression of Fas is markedly elevated at the time of LVAD support and is tightly correlated with TNF expression. While interleukin (IL)‐6 was significantly reduced by LVAD support, the impact of support on Fas was highly variable and tightly linked to tumor necrosis factor (TNF). The role of Fas in predicting recovery after LVAD support requires further investigation.
Keywords: apoptosis, cytokines, myocardium, heart failure, transplantation
Introduction
Progressive hemodynamic deterioration in severe heart failure (HF) induces alterations in myocardial gene expression. 1 , 2 , 3 For subjects with end‐stage cardiomyopathy, hemodynamic support with a left ventricular assist device (LVAD) frequently serves as an important bridge to cardiac transplantation (BTT). 4 , 5 In addition, there is an increasing interest in the potential use of LVAD support as a “bridge to recovery” (BTR) to facilitate myocardial recovery without transplantation. 6 Themajority of BTR subjects thus far reported have had self‐limited inflammatory cardiomyopathy or myocarditis. 6 The potential for BTR in chronic cardiomyopathy remains more uncertain.
Apoptosis, or programmed cell death, is evident in the myocardium of subjects with end‐stage HF who undergo cardiac transplantation, 7 , 8 and the resultant loss of cardiac myocytes contributes to HF progression. 9 , 10 The apoptotic program is triggered by the activation of Fas, a transmembrane receptor belonging to the TNF receptor superfamily. 11 Cross‐linking of Fas by Fas ligand (FasL), a TNF‐α‐related cytokine, promotes apoptosis and/or transcription factor activation in a highly cell type‐specific manner. 12 , 13 Soluble Fas can be detected in circulating plasma, and its levels are elevated in patients with HF and correlate with disease severity and clinical outcomes. 14
The production of prointflammatory cytokines in the myocardium is greater in subjects with end‐stage HF rather than at the time of initial diagnosis 1 and appears to be a late event in HF progression. 1 Studies have shown elevated myocardial TNF‐α, interleukin (IL)‐1β, FasL, and IL‐6. 15 , 16 , 17 , 18 , 19 Cytokines have been proposed to play an important role in the remodeling of the myocardium and may facilitate apoptosis and HF progression. 10 , 11 In myocardial cells, the apoptotic signal is transmitted to the IL‐1β‐converting enzyme (ICE) like protease effector cascade by a cytosolic complex of activated Fas with Fas‐associated death domain (FADD) protein and FADD‐like ICE (FLICE). 2
Given the potential role of an LVAD to promote myocardial recovery, a number of investigators have evaluated the impact of support on gene expression. Recently, we have demonstrated 20 that among subjects with recent‐onset cardiomyopathy, low Fas expression predicts a greater likelihood of myocardial recovery. The impact of LVAD support on the myocardial expression of Fas and related cytokines in more chronic cardiomyopathy may assist in the determination of the potential for myocardial recovery. We sought to evaluate the efect of LVAD placement on Fas and cytokine expression in a cohort with end‐stage HF.
Method
Study population
Fifeen subjects who underwent LVAD placement at the University of Pittsburgh as a “bridge to cardiac transplantation” (BTT) were investigated. Consent was obtained, and subjects were enrolled in the LVAD/transplant tissue bank. The institutional review board for human studies approved the study, and all subjects gave written and informed consent. Myocardial tissue excised from the left ventricular apex was obtained at the time of LVAD placement, and again at the time of cardiac transplantation (Tx). Myocardial expression in a group of donor hearts that were not used for transplantation (n= 8; 44 ± 16 years) served as the nonfailing controls.
Myocardial gene expression
Myocardial samples obtained at the time of LVAD placement or at the time of explant of the native heart and LVAD during cardiac transplantation and at the time of donor harvest for control samples were immediately frozen in liquid nitrogen and stored at –80°C until analysis. Myocardial gene expression was assayed as previously described. 1 , 20 Total ribonucleic acid (RNA) was extracted from the frozen tissue using an acid guanidinium thiocyanate‐phenol‐chloroform method. To evaluate transcript levels in the myocardium, a commercially available multiprobe RNase protection assay kit (Riboquant; PharMingen, San Diego, CA, USA) was used, with the assay performed according to the manufacturer's protocol. The transcript levels of Fas, FasL, FLICE, TNF, TNF receptor (p55), IL‐6, and IL‐1β were assessed. The value of each hybridized probe was quantifed by a phosphoimager using the Image Quant software (Molecular Dynamics, Inc., Sunnyvale, CA, USA) and normalized to that of glyceraldehyde phosphate dehydrogenase (GAPDH) included in each template set as an internal control. The transcript levels were expressed as % GAPDH level: expression gene X = (X quantifed by phosphoimager/GAPDH quantified by phosphoimager) multiplied by 100. L32, an additional constitutively active gene was used as an internal control to assess for errors.
Statistical analysis
For each gene analyzed, the mean expression in patients with end‐stage HF (at the time of LVAD implant) was compared to donor controls and to levels of expression after LVAD unloading (at the time of transplantation). In addition, the correlation of Fas expression levels to cytokine expression was examined. All statistical analyses were performed using SPSS (SPSS, Inc., Chicago, IL, USA). The results are presented as mean ± SD. Student's t‐test was used to compare each variable in the groups, and a one‐way analysis of variance (ANOVA) was performed for more than two group analyses with LSD post hoc test. Pearson's correlation coefcient r was used as a measure of linear association between two variables. Statistical analyses between the related subgroups to assess the change in expression at LVAD and at the time of transplant was performed using the Wilcoxon signed‐rank test. The differences were considered significant at a value of p < 0.05.
Results
The cohort (n= 15; age 47 ± 12 years) was 73% male and 33% ischemic. All were NYH A class 4 at the time of LVAD implantation, with a mean LVEF of 0.19 ± 0.08 ( Table 1 ). The mean duration of CHF symptoms was 71 ± 44 months, and the duration of LVAD implant was 89 ± 66 days. Pre‐LVAD, 6 patients (40%) received an intraaortic balloon pump (6 ± 6 days) and 6 (40%) received biventricular support (mean duration 44 ± 85 days). The patients were implanted with commercially available pulsatile flow LVADs: 66% Novacor (World Heart Corporation, CA, USA) and 33% Toratec (Toratec Corporation, MA, USA).
Table 1.
Demographics of the LVAD cohort and controls.
| LVAD | Control | |
|---|---|---|
| n | 15 | 8 |
| Age (years) | 47 ± 12 | 44 ± 16 |
| Male (%) | 73 | 50 |
| Ischemic (%) | 33 | not applicable |
| lVEF | 0.19 ±−0.08 | |
| PCWP (mmHg) | 27 ± 6 | |
| PA mean (mmHg) | 37 ± 8 | |
| Cardiac index (L/min) | 2.1 ± 0.6 | |
| IABP (%) | 40 | |
| RVAD (%) | 40 | |
| Duration of lVAD (days) | 89 ± 66 |
Gene expression in end‐stage HF: time of LVAD implantation
At the time of LVAD implantation, the expression of myocardial cytokines was significantly elevated ( Figure 1 ) when compared to donor controls including TNF (0.38 ± 0.18 vs. 0.10 ± 0.06, p= 0.0002), IL‐6 (1.41 ± 1.36 vs. 0.13 ± 0.08, p= 0.01), and IL‐1β (1.2 ± 0.9 vs. 0.24 ± 0.18, p= 0.008), with a trend toward increased TNFR55 (9 ± 6 vs. 6.2 ± 2.9, p= 0.09). The expression of the Fas receptor (0.89 ± 0.44 vs. 0.38 ± 0.06, p= 0.004) and FLICE (0.73 ± 0.6 vs. 0.22 ± 0.08, p= 0.02) was significantly elevated, with a trend toward increased FasL expression (0.05 ± 0.07 vs. 0.01 ± 0.03, p= 0.08).
Figure 1.
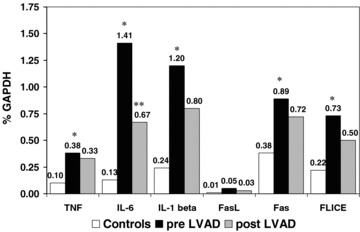
Cytokines and apoptotic gene expression in controls, pre‐LVAD, and post‐LVAD. Transcript levels expressed as % GAPDH level: expression gene X = (X quantifed by phosphimager/GAPDH quantifed by phosphoimager) multiplied by 100. *Pre‐LVAD mean levels significantly higher than control levels, p < 0.05. **Post‐LVAD mean levels significantly lower than pre‐LVAD, p < 0.05.
Myocardial expression post‐LVAD support
At the time of transplantation, with LVAD support, the expression of IL‐6 was markedly decreased (post‐LVAD 0.67 ± 1.2, p= 0.036). In contrast to IL‐6, the mean levels of myocardial TNF (0.33 ± 0.28, p= 0.53), IL‐1β (0.80 ± 0.8, p= 0.3), Fas (0.72 ± 0.55, p= 0.4), and FasL (0.03 ± 0.03, p= 0.9) remained elevated. The impact of VAD support on individual subjects myocardial expression of Fas and FLICE was markedly heterogeneous ( Figure 2 ).
Figure 2.
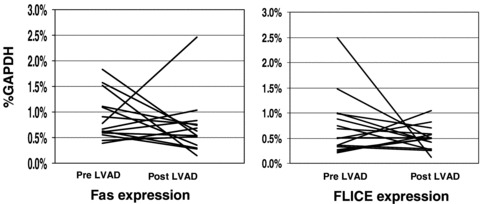
Impact of LVAD support on Fas and FLICE expression. Transcript levels expressed as % GAPDH level. Changes in gene expression from pre‐LVAD to post‐LVAD for each individual subject represented by a solid line.
Relationship of Fas and TNF expression pre‐ and post‐LVAD
Correlations of Fas to cytokine expression are displayed in Table 2 . The expression of Fas was strongly linked to TNF expression at the time of implantation (r=0.72, p= 0.001). While individual changes in both TNF and Fas post implantation were markedly heterogeneous, the correlation remained strong and was unafected by LVAD support (r= 0.82, p < 0.001). Indeed, changes in Fas correlated with changes in TNF (r= 0.81, p= 0.001; Figure 3 ). In contrast, myocardial expression of TNF was more weakly correlated with the TNF receptor, TNFR1 (pre‐LVAD: r= 0.45, p= 0.04; post‐LVAD: r= 0.48, p= 0.03). In a similar fashion, FasL was only weakly correlated with Fas (pre‐LVAD: r= 0.57, p= 0.01; post‐LVAD: r= 0.46, p= 0.04).
Table 2.
Pearson's r correlates among cytokines and apoptosis genes at the time of LVAD and transplant.
| FAS | TNFR55 | FLICE | |
| TNF‐α | |||
| Pre‐LVAD | 0.72*** | 0.45 * | 0.36 |
| Post‐LVAD (Tx) | 0.82*** | 0.48 * | 0.76*** |
| Fas ligand | |||
| Pre‐lVaD | 0.57** | 0.75*** | 0.82*** |
| Post‐LVAD (Tx) | 0.46* | 0.18 | 0.58** |
| IL‐Iβ | |||
| LVAD | 0.73*** | 0.91*** | 0.90*** |
| Post‐LVAD (Tx) | 0.49* | 0.61** | 0.41* |
| IL‐6 | |||
| LVAD | 0.39 | 0.4 | 0.28 |
| Post‐LVAD (Tx) | 0.27 | 0.55* | 0.2 |
All values are r (p value: *<0.05, **<0.01, ***<0.001).
Figure 3.
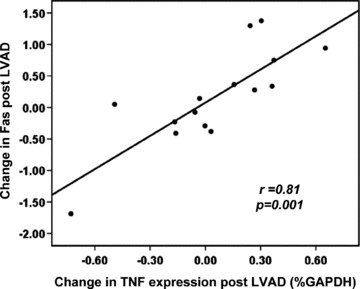
Scatter plot of the relationship of the change in Fas expression after LVAD implantation. Fas expression in% GAPDH on the y‐axis, with the change in TNF expression on the x‐axis. The change in Fas significantly correlates with the change in TNF (r= 0.81, p < 0.001).
Discussion
In the current study, the expression of Fas, TNF, and other cytokines was markedly elevated in the myocardium of subjects with end‐stage HF. When reassessed post‐LVAD at the time of transplantation, Fas and TNF expression remained elevated compared to donor controls. While the response of Fas expression to hemodynamic unloading was highly variable, it remained tightly linked to TNF expression, suggesting that TNF expression may be an important driver of apoptosis.
A report by Torre‐Amione et al. was among the first to evaluate the impact of LVAD support on gene expression. 21 In contrast to the current results, this investigation of 8 subjects by immunohistochemistry pre‐ and post‐LVAD implantation demonstrated significant reductions of myocardial TNF. However, this study included 4 subjects who were BTR as well as 4 who were BTT, unlike our current data, which is limited to 15 BTT subjects. Consistent with the current data, a comparison of the BTR and BTT subjects demonstrated significantly more TNF reduction in BTR than in BTT subjects, leading the investigators to suggest that TNF expression may predict which subjects have greater potential for recovery. In a study of 14 BTT subjects by Taegtmeyer and colleagues, the response of TNF expression to hemodynamic unloading by LVAD was highly variable, with few clinical predictors. 22 The current analysis suggests this variable response is closely correlated to changes in Fas expression, and through this mechanism, may identifiy subjects with greater potential for recovery.
Previous studies 8 evaluated myocardial cytokine expression in a cohort undergoing LVAD implantation and compared this to a cohort that received transplant without LVAD support. This investigation demonstrated significantly increased myocardial cytokine expression in LVAD subjects. In addition, this study evaluated caspases as regulators of apoptosis and found higher levels of caspase‐9 in LVAD subjects. The investigators concluded that in LVAD subjects, myocardial cytokines had resulted in alterations in the apoptotic pathway. Indeed, they argued that anticytokine therapeutics might delay disease progression.
Among the cytokines assessed, only IL‐6 was significantly reduced by LVAD support. A comparison of subjects with compensated cardiomyopathy with those requiring an LVAD 1 demonstrated that IL‐6 expression was not evident in the myocardium of compensated subjects but was abundantly expressed in the myocardium in end‐stage HF. The use of an LVAD markedly decreases myocardial wall stress in the left ventricle (LV), and the significant reductions in IL‐6 suggest that the change in expression is driven by the unloading of the LV. In contrast, the absence of significant reductions in TNF with LVAD support suggests its expression may not be refecting hemodynamic decompensation but rather a separate primary pathogenic process.
There is an increasing interest in the use of ventricular support to facilitate myocardial recovery. Hall et al. reported on the changes in integrin signaling by microarray analysis in 6 patients who underwent LVAD as BTR. 23 The changes in cytokelatal proteins appear distinctly different in BTR subjects when compared to subjects who require transplantation. 24 The LVAD Working Group reported that myocardial recovery can occur within 30 days on LVAD support; however, the degree of left ventricular recovery is sufcient to allow explantation in only a minority of subjects. 25 Adjunctive therapy may allow LVAD support to be more effective as a BTR, and a recent report suggests that treatment with the β2 agonist clenbuterol may facilitate recovery. 26
Anticytokine and antiapoptotic therapeutic interventions may also have a beneficial impact on myocardial recovery. Skudicky et al. 27 demonstrated a significant reduction of TNF and Fas and an improvement in left ventricular function in a study of subjects treated with the phosphodiesterase inhibitor, pentoxyphylline. In contrast, a large trial of the soluble TNF receptor, enbrel, as an anticytokine strategy in HF 28 failed to demonstrate any significant clinical benefit. It remains to be determined whether strategies to block cytokines or apoptosis in subjects requiring LVAD support will potentially facilitate myocardial recovery.
The current study demonstrated the variable impact of LVAD support on both cytokine and apoptotic gene expression. We have previously demonstrated in subjects with recent‐onset cardiomyopathy that lower Fas expression predicts a greater likelihood of myocardial recovery during follow‐up. 20 Tough the assessment of Fas gene expression may assist decisions on recovery potential, the current study is limited by its restriction to BTT subjects, and investigation of gene expression in subjects with LVAD support for BTR will be critically important. In addition, while the relationship of TNF and Fas in subjects undergoing LVAD support is clear, additional investigations are required to confrm the correlation of Fas to TNF expression, with histologic assessment of apoptosis. For many subjects who receive LVAD support, left ventricular dysfunction has reached the end‐stage of the disorder and myocardial recovery may not be a reasonable goal. Further investigations of the molecular signature of left ventricular dysfunction should eventually lead to an assay or a profle that indicates when myocardial recovery is still feasible. For subjects with an appropriate recovery profle, explanation of the LVAD without cardiac transplantation should remain an important goal of therapeutic intervention.
A limitation of the current study is that protein levels were not assayed, only transcript levels were. In addition, transcriptional analysis utilized RNase protection assay (RPA) rather than quantitative PCR. An important long‐term objective of the current myocardial expression analysis is the determination of recovery potential. For the transcriptional profiling to become clinically useful for predicting recovery, it must be performed on the limited tissue obtained by routine endomyocardial biopsy. While tissue availability was not a factor in the current study, a methodology was purposefully chosen that has been performed previously by our group on biopsy samples. 1 Indeed, this same analytical technique has been performed on a single endomyocardial biopsy sample 20 in subjects with recent‐onset cardiomyopathy and has demonstrated that low Fas expression by RPA predicted increased recovery potential.
In conclusion, a marked elevation of myocardial Fas expression is present in end‐stage HF and correlates with the expression of TNF. The relationship of Fas expression to TNF is independent of hemodynamic loading and is not altered by LVAD support. Indeed, cytokine expression in end‐stage HF may trigger an irreversible apoptosis effector cascade. Adjunctive therapies designed to interrupt this cytokine‐apoptosis pathway may be necessary to assist LVAD recovery strategies and allow expansion of BTR to more chronic progressive cardiomyopathy.
Acknowledgments
This study was supported in part by grants from the National Heart, Lung and Blood Institute, contracts VO1 HL66948 (Maninder S. Bedi), R01 HL75038 (Dennis M. McNamara), and K24 HL69912 (Dennis M. McNamara).
References
- 1. Kubota T, Masayuki M, Alvarez R, Kormos R, Rosenblum W, Demitris A, Semigran M, Dec GW, Holubkov R, McTiernan CF, Mann DL, Feldman AM, McNamara DM. Expression of prointflammatory cytokines in the failing human heart: comparison of recent onset and end stage heart failure. J Heart Lung Trans. 2000; 19(9): 819–824. [DOI] [PubMed] [Google Scholar]
- 2. Chen QM, Tu VC. Apoptosis and heart failure. Am J Cardiovasc Drugs. 2002; 2(1): 43–57. [DOI] [PubMed] [Google Scholar]
- 3. Mann DL. Inflammatory mediators and the failing human heart. Past present and the foreseeable future. Circ Res. 2002; 91: 988–998. [DOI] [PubMed] [Google Scholar]
- 4. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsy W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson RG, Ronan NS, Meier P. Long term use of a left ventricular assist device for end stage heart failure. N Engl J Med. 2001; 345(20): 1435–1443. [DOI] [PubMed] [Google Scholar]
- 5. DeRose JJ, Argenziano M, Sun BC, Reemtsma K, Oz MC, Rose EA. Implantable left ventricular assist devices: an evolving long term cardiac replacement therapy. Ann Surg. 1997; 226(4): 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simon MA, Kormos RL, Murali S, Nair P, Heffernan M, Gorcsan J, Winowich S, McNamara DM. Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation. 2005;112 (9 Suppl): I32–I36. [DOI] [PubMed] [Google Scholar]
- 7. Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw B‐A. Apoptosis in myocytes in end‐stage heart failure. N Engl J Med. 1996; 335: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 8. Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, Mullen AJ, Khaghani A, Barton PJR, Polak JM, Pepper JR, Banner NR, Yacoub MH. Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation. 2001; 104(12 Suppl): I233–I240. [DOI] [PubMed] [Google Scholar]
- 9. Bennet MR. Apoptosis in the cardiovascular system. Heart. 2002; 87(5): 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall D, Sack MN. Apoptosis: a pivotal event or an epiphenomenon in the pathophysiology of heart failure. Heart. 2000; 84(4): 355–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schumann H, Moraweitz H, Hakim K, Zerkowski HR, Holtz J, Darmer D. Alternative splicing of the primary Fas transcript in the failing ventricular myocardium. Biochem Biophys Res Commun. 1997; 239(3): 794–798. [DOI] [PubMed] [Google Scholar]
- 12. Valgimigli M, Curello S, Ceconi C, Agnolett L, Comini L, Bachetti T, Merli E, Ferrari R. Neurohormones, cytokines, and programmed cell death in heart failure. Cardiovasc Drugs Ther. 2001; 15: 529–537. [DOI] [PubMed] [Google Scholar]
- 13. Wollert KC, Heineke J, Westermann J, Ludde M, Fiedler B, Zeirhut W, Laurent D, Bauer MK, Schulze‐Osthoff K, Drexler H. The cardiac FAS (APO1/CD95) receptor/FAS ligand system: relation to diastolic wall stress in volume overload hypertrophy in vivo and activation of the transcription factor AP1 in cardiac myocytes. Circulation. 2000; 101(10): 1172–1178. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi S, Yamaoka M, Okuyama M, Nitoube J, Fukui A, Shirakabe M, Shirakawa K, Nakamura N, Tomoike H. Elevated circulating levels and cardiac secretion of soluble Fas ligand in patients with congestive heart failure. Am J Cardiol. 1999; 83: 1500–1503. [DOI] [PubMed] [Google Scholar]
- 15. Blum A, Miller H. Role of cytokines in heart failure. Am Heart J. 1998; 135: 181–186. [DOI] [PubMed] [Google Scholar]
- 16. Satoh M, Nakamura M, Tamura G, Makita S, Segawa I, Tashiro A, Satodate R, Hiramori K. Inducible nitric oxide synthase and tumor necrosis factor alpha in human dilated cardiomyopathy. J Am Coll Cardiol. 1997; 39: 716–724. [DOI] [PubMed] [Google Scholar]
- 17. MacGowan GA, Mann DL, Kormos RL, Feldman AM, Murali S. Circulating interleukin‐6 in severe heart failure. J Am Coll Cardiol. 1997; 79: 1128–1131. [DOI] [PubMed] [Google Scholar]
- 18. Torre‐Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and function of TNF receptors in the human myocardium. Circulation. 1995; 92: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 19. Kapadia S, Torre‐Amione G, Yokoyama T, Mann DL. Soluble tumor necrosis factor proteins modulate the negative inotropic effect of TNF alpha in vitro. Am J Physiol. 1995; 37: H517–H525. [DOI] [PubMed] [Google Scholar]
- 20. Sheppard R, Bedi M, Kubota T, Semigran MJ, Dec W, Holubkov R, Feldman AM, Rosenblum WD, McTiernan CF, McNamara DM; IMAC investigators . Myocardial expression of Fas and recovery of left ventricular function in patients with recent‐onset cardiomyopathy. J Am Coll Cardiol. 2005; 46(6): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 21. Torre‐Amione G, Stetson S, Youker K, Durand J, Radovancevic B, Delgado RM, Frazier OH, Entman ML, Noon G. Decreased expression of tumor necrosis factor‐a in failing human myocardium after mechanical circulatory support: a potential mechanism for cardiac recovery. Circulation. 1999; 100(11): 1189–1193. [DOI] [PubMed] [Google Scholar]
- 22. Razeghi P, Mukhopadhyay M, Myers TJ, Williams JN, Moravec CS, Fraizier OH, Taegtmeyer H. Myocardial tumor necrosis factor‐alpha expression does not correlate with indices of heart failure in patients on left ventricular device support. Ann Thorac Surg. 2001; 72(6): 2044–2050. [DOI] [PubMed] [Google Scholar]
- 23. Hall JL, Birks EJ, Grindle S, Cullen ME, Barton PJ, Rider JE, Lee S, Harwalker S, Mariash A, Adhikari N, Charles NJ, Felkin LE, Polster S, George RS, Miller LW, Yacoub MH. Molecular signature of recovery following combination left ventricular assist device (LVAD) support and pharmacologic therapy. Eur Heart J. 2007; 28(5): 522–524. [DOI] [PubMed] [Google Scholar]
- 24. Birks EJ, Hall JL, Barton PJ, Grindle S, Latif N, Hardy J P, Rider JE, Banner NR, Khaghani A, Miller LW, Yacoub MH. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular‐assist device support. Circulation. 2005; 112(9 Suppl): I57–I64. [DOI] [PubMed] [Google Scholar]
- 25. Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, Miller LW, Margulies K, McRee S, Frazier OH, Torre‐Amione G, for the LVAD Working Group . Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007; 115(19): 2497–2505. [DOI] [PubMed] [Google Scholar]
- 26. Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006; 355(18): 1873–1884. [DOI] [PubMed] [Google Scholar]
- 27. Skudicky D, Sliwa K, Bergermann A, et al Reduction in the FAS/APO‐1 plasma levels correlate with improvement in left ventricular function in patients with idiopathic dilated cardiomyopathy treated with pentoxyphyline. Heart. 2000; 84: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bozkurt B, Torre‐Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL. Results of targeted anti‐tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001; 103(8): 1044–1047. [DOI] [PubMed] [Google Scholar]


