Abstract
The current lack of a validated intraspinal delivery approach precludes translation of promising cell or viral‐based therapeutics for treatment of varied spinal cord afflictions. We have developed a stabilized cervical microinjection platform with the intent of precise delivery to intraspinal sites of interest. Nine 30–40 kg female swine underwent coordinate‐based microinjection AAV2‐GFP at three injected volumes (10, 25, and 50 μL (n= 3/group)) and matched infusion rates (1.0, 2.5, and 5.0 μL/min) over a period (t= 10 minutes). Preliminary validation is provided by behavioral and targeting data demonstrating safe delivery of a viral vector carrying a fluorescent reporter gene to the cervical spinal cord ventral horn.
Keywords: targeted delivery, gene therapy, cervical spinal cord, adeno‐associated viral vector, pig
Introduction
Afflictions of the spinal cord, whether neurodegenerative, oncologic, or traumatic, pose unique and significant challenges towards the end of achieving effective therapeutic intervention. Specific pathologic entities, including amyotrophic lateral sclerosis (ALS) 1 , 2 or spinal muscular atrophy (SMA), 3 intramedullary cord tumors, 4 and spinal cord injury (SCI) 5 , 6 may be amenable to cell or viral‐based therapeutic approaches tailored to address the fundamental dysfunction within the local neuronal microenvironment. One of the most significant barriers towards clinical translation and assessment of these molecular therapeutic strategies in Phase 1 clinical trials remains validation of a safe, accurate therapeutics delivery approach to the spinal cord gray matter. To our knowledge, the closest related studies have examined either convection enhanced delivery of macromolecules to the adjacent white matter 7 and of multiple substrates to the heterogeneous pontine region. 8 , 9 We have previously published preliminary data demonstrating our ability to deliver cellular grafts to the lumbar 10 or cervical 11 ventral horn utilizing a prototype stabilized microinjection platform. Herein, we extend our prior experiences to a gene therapy delivery protocol (AAV2‐GFP) characterized by a (t= 3 weeks) survival period.
Methods
Surgical procedure
We have described the surgical procedure in detail. 10 , 11 Briefly, the surgical field was prepared in the standard sterile fashion. A 22 cm midline incision was performed between the occiput and the C7 spinous process. An avascular midline dissection of the posterior spinal elements was then performed followed by a cervical laminectomy at the C3‐C4 levels. The dura was incised with a No. 15 blade scalpel and a pial opening created with microbipolar coagulation followed by use of a No.11 blade to assure hemostasis. Figure 1A demonstrates the microinjection platform fabricated by the Cleveland Clinic Prototype Laboratory. This was used in combination with a Narishige M0‐97 hydraulic microdrive (East Meadow, NY), capable of submillimetric depth adjustment. Figure 1B demonstrates an in vivo view of the platform and microdrive. The microinjection cannula (0.015″ OD, 0.011″ ID; 27.5 Gauge) was inserted to a depth of 4 mm, withdrawn to a depth of 3.5 mm, and adeno‐associated viral vector serotype 2 (AAV2‐GFP) delivery (4e12 vg/mL, Ceregene, Inc., San Diego, CA) commenced with aid of a Harvard p99 (Harvard Apparatus Inc., Boston, MA) programmable infusion pump. Table 1 introduces the study design. Briefly, microinjection occurred over a range of matched volumes (10, 25, and 50 μL (n= 3/group)) and infusion rates (1.0, 2.5, and 5.0 μL/minute), respectively. A constant intraspinal residence time was present between groups, including 10 minutes for delivery and an additional 5 minutes to prevent reflux along the cannula track. The dura was closed in a running watertight fashion with 4‐0 Nurulon®. A four‐layer closure included deep musculature (0 Vicryl®), fascia (0 Vicryl®), subdermal (2‐0 Vicryl®), and skin (3‐0 Ethilon®).
Figure 1.
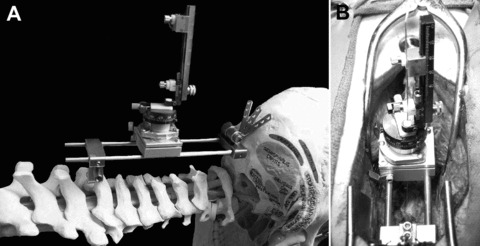
Stabilized cervical intraspinal microinjection platform, in situ and in vivo. (A) The microinjection platform is demonstrated while attached rostrally to the occiput and caudally to the spinous process of C7. (B) The platform and microdrive are demonstrated following a C3/4 laminectomy and dural opening prior to unilateral microinjection into the porcine cervical spinal cord.
Table 1.
Microinjection group study design.
| Swine number | Injection volume (μl) | |||
|---|---|---|---|---|
| 10 | 25 | 50 | ||
| Injection | 1 | Group 1 (n= 3) | ||
| Rate (μL/minute) | ||||
| 2.5 | Group 2 (n= 3) | |||
| 5 | Group 3 (n= 3) | |||
Behavioral assessment
A modification of the Tarlov scale, used to assess a combination of motor function and ambulation status in small animal studies, was adapted for this study. Table 2 demonstrates the modified Tarlov scoring system. Animals were assessed preoperatively, followed by serial postoperative scoring at Days 3, 14, and prior to euthanasia at Day 21.
Table 2.
Modified Tarlov scoring system.
| 0 | No voluntary movements |
| 1 | Perceptible movements at joints |
| 2 | Good movements at joints but inability to stand |
| 3 | Ability to get up and stand with assistance <1 minute |
| 4 | Ability to get up with assistance and stand unassisted <1 minute |
| 5 | Ability to get up with assistance and stand unassisted >1 minute |
| 6 | Ability to get up and stand unassisted >1 minute |
| 7 | Ability to walk <1 minute |
| 8 | Ability to walk >1 minute |
| 9 | Full recovery and normal walking |
Histological analysis
Following euthanasia, spinal cords were excised between the C2 and C5 levels and placed in 4% paraformaldehyde for a period of 7 days. The tissue specimens were dehydrated in a 30% sucrose solution for 1–2 days and subsequently frozen for histological analysis. Injection site identification was aided by placement of a blue 6‐0 Prolene stitch in the dura overlying the microinjection site. A cryostat (Jung Frigocut 2800N, Leica Microsystems, Inc., Bannockburn, IL) was used to attain 30 μm thickness sections for analysis. Specimens were left unstained for GFP visualization.
Results
Behavioral outcomes
At preoperative baseline, each animal retained an intact modified Tarlov score. As demonstrated in Figure 2 , eight of nine swine recovered to baseline status by POD3 while one animal demonstrated continued improvement with return to baseline function by POD21. During necropsy, a resolving epidural hematoma was discovered. On POD 15, one Group 2 animal was found deceased in its cage following a rapid, progressive decline. A rigid, distended abdomen was observed. Necropsy revealed intestinal volvulus. Neither the observed morbidity nor the mortality was attributable to cannulation of the spinal cord or microinjection.
Figure 2.
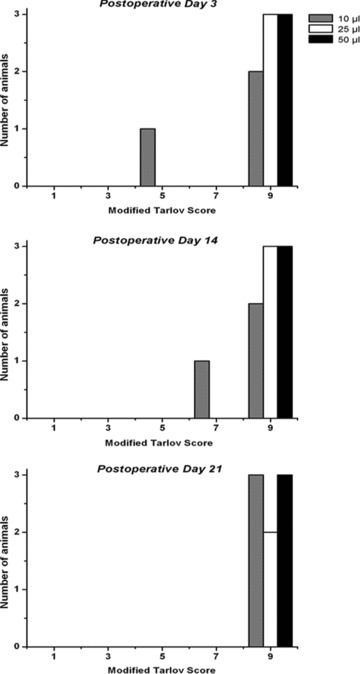
Postmicroinjection behavioral outcomes. Top panel: A maximal Tarlov score was achieved in all but one animal at postoperative Day 3. The affected swine was in Group 1 (10 μL injection group). Middle panel: By Day 14, behavioral improvement was noted in this animal. Bottom panel: At euthanasia, the affected swine from Group 1 had recovered to preoperative baseline. On Day 15, one animal from Group 2 was found deceased in its cage following a rapidly deteriorating course. Necropsy demonstrated the presence of intestinal volvulus
Ventral horn targeting
Ventral horn targeting and GFP transgene expression were achieved in each micro‐injection group. Though quantification of vector biodistribution was not the primary study endpoint, Figure 3 demonstrates a “dose‐dependent” and “targeted” GFP expression between groups. Observance of increasing GFP expression with microinjection volume and rate serves as evidence for a lack of payload reflux along the cannula track. Multilevel injection may further augment vector biodistribution along the neuraxis.
Figure 3.
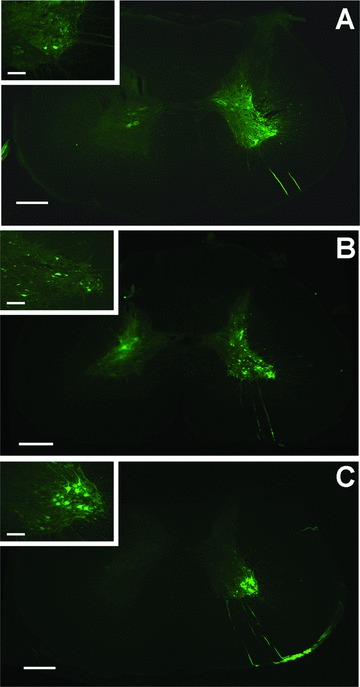
AAV‐GFP ventral horn expression. Specimens from each group demonstrate GFP expression 3 weeks after injections. Accuracy of the injection and a dose‐dependent increase in fluorescence can be observed. (A) Group 1–10 μL, 1.0 μL/minute; (B) Group 2–25 μL, 2.5 μL/minute; (C) Group 3–50 μL, 5.0μL/minute. Scale bars: 500 μm (A)–(C); 200 μm (A)–(C) inset.
Discussion
The data presented above extend previous findings from our laboratory demonstrating an ability to safely cannulate a specific intraspinal site of interest for therapeutics delivery. Figure 2 demonstrates a return to baseline function by POD3 in eight of nine animals, with all animals eventually achieving a return to neurologic baseline. Neither the episode of forelimb dysfunction nor the mortality was attributable to intraspinal cannulation, infusion, or viral delivery. Figure 3 demonstrates accurate transgene delivery to the ventral horn, irrespective of infusion volume. Ventral horn targeting, localized gene distribution, lack of tissue damage at the injection site, and a lack of association between behavioral outcomes and infusion parameters over a range of volumes and infusion rates were noted.
Our prior studies have utilized both stabilized lumbar 10 and cervical 11 approaches to deliver cell‐based grafts to the ventral horn, utilizing microelectrode recording and coordinate‐based microinjection targeting strategies, respectively. To our knowledge, these efforts represented the first reported attempts tocannulate and deliver a viral vector to a specific target within the spinal cord of a large animal, for the purpose of clinical translation. We extend the potential generalizability for the safety of stabilized microinjection to delivery of viral vectors engineered to achieve therapeutic effect.
Acknowledgments
We would like to thank Shujun Zhou for her contribution to this work and Ceregene, Inc. for providing the viral vector. This research was supported by the ALS Association.
References
- 1. Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, Roskelley EM, Treleaven CM, Rizo L, Martin H, Kim SH, Kaspar R, Taksir TV, Griffiths DA, Cheng SH, Shihabuddin LS, Kaspar BK. Delivery of AAV‐IGF‐1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008; 16(6): 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF‐1 prolongs survival in a mouse ALS model. Science. 2003; 301(5634): 839–842. [DOI] [PubMed] [Google Scholar]
- 3. Corti S, Nizzardo M, Nardini M, Donadoni C, Salani S, Ronchi D, Saladino F, Bordoni A, Fortunato F, Del Bo R, Papadimitriou D, Locatelli F, Menozzi G, Strazzer S, Bresolin N, Comi GP. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J Clin Invest. 2008; 118(10): 3316–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colak A, Goodman JC, Chen SH, Woo SL, Grossman RG, Shine HD. Adenovirus‐mediated gene therapy for experimental spinal cord tumors: Tumoricidal efficacy and functional outcome. Brain Res. 1995; 691(1–2): 76–82. [DOI] [PubMed] [Google Scholar]
- 5. Pallini R, Vitiani LR, Bez A, Casalbore P, Facchiano F, Di Giorgi Gerevini V, Falchetti ML, Fernandez E, Maira G, Peschle C, Parati E. Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery. 2005; 57(5): 1014–1025. [DOI] [PubMed] [Google Scholar]
- 6. Deshpande DM, Kim YS, Martinez T, Carmen J, Dike S, Shats I, Rubin LL, Drummond J, Krishnan C, Hoke A, Maragakis N, Shefner J, Rothstein JD, Kerr DA. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006; 60(1): 32–44. [DOI] [PubMed] [Google Scholar]
- 7. Lonser RR, Gogate N, Morrison PF, Wood JD, Oldfield EH. Direct convective delivery of macromolecules to the spinal cord. J Neurosurg. 1998; 89(4): 616–622. [DOI] [PubMed] [Google Scholar]
- 8. Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, Morrison PF, Brechbiel MW, Oldfield EH. Successful and safe perfusion of the primate brainstem: In vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg. 2002; 97(4): 905–913. [DOI] [PubMed] [Google Scholar]
- 9. Lonser RR, Warren KE, Butman JA, Quezado Z, Robison RA, Walbridge S, Schiffman R, Merrill M, Walker ML, Park DM, Croteau D, Brady RO, Oldfield EH. Real‐time image‐guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J Neurosurg. 2007; 107(1): 190–197. [DOI] [PubMed] [Google Scholar]
- 10. Riley J, Butler J, Baker KB, McClelland S, III , Teng Q, Yang J, Garrity‐Moses M, Federici T, Boulis NM. Targeted spinal cord therapeutics delivery: stabilized platform and microelectrode recording guidance validation. Stereotact Funct Neurosurg. 2008; 86(2): 67–74. [DOI] [PubMed] [Google Scholar]
- 11. Riley J, Federici T, Park J, Suzuki M, Franz C K, Tork C, McHugh J, Teng Q, Svendsen S, Boulis N. Cervical spinal cord therapeutic delivery: Preclinical safety validation of a stabilized microinjection platform. Neurosurgery. In press, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


