Introduction
Loss of vision due to corneal disease or trauma affects over 10 million individuals worldwide. For many, corneal trans‐plantation is the only treatment, but the supply of good quality donor tissue cannot meet the demand, especially in the developing world. To address this need, several corneal substitutes have been proposed; 1 , 2 however, to date, the only substitutes clinically tested in humans have been fully synthetic prostheses (keratoprostheses). 3 , 4 Although improving, complications with corneal prostheses, including retroprosthetic membrane formation, calcification, infection, and glaucoma, 2 , 4 , 5 have limited their use to cases where human donor tissue fails; 1 , 2 so, prostheses do not alleviate the primary need for human donor corneas.
An alternative approach is to support the inherent regenerative capacity of the human cornea to promote the in vivo reconstruction of healthy, viable corneal tissue. In this regard, tissue‐engineered biomimetic materials designed to mimic the corneal extracellular matrix (ECM) have been proposed as scaffolds for endogenous tissue regeneration. 6 We have previously reported on a biomimetic corneal substitute composed of porcine atelocollagen cross‐linked by 1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide (EDC) and N‐hydroxysuccinimide (NHS) to give robust, implantable, cornea‐shaped scaffolds. 7 Subsequently we adapted this cross‐linking strategy to recombinantly produced human collagen, either type I or III (RHCI or RHCIII), that gave similar, stable host‐graft integration and regeneration of corneal cells and nerves in pigs. 8 Although both recombinant collagens gave similar results, RHCIII implants had superior optical properties. 8 Recombinant human collagen promises a source of safe, predictable, and chemically defined material for tissue engineering, 9 while minimizing the risk of disease transmission or immune reaction that could result after implantation of animal‐source collagen. 8
Following the successful implantation of biomimetic corneas in animal models, we report here early results from the first clinical study to surgically implant a biomimetic corneal substitute in human subjects.
Methods
Following approvals from the Swedish Medical Products Agency and the Regional Ethical Review Board in Linköping, Sweden, and following the tenets of the Declaration of Helsinki with informed consent given by each patient, we conducted a Phase I clinical study of 10 patients with vision loss from keratoconus or scarring as indications for corneal transplantation. The cohort comprised eight males and two females, aged 43.6 ± 18 years (mean ± SD, range: 18‐75 years) at the time of surgery. RHCIII (13.7% wt/wt) was cross‐linked with EDC‐NHS and fabricated into substitutes with the dimensions of a human cornea. 8 Using anterior lamellar keratoplasty (ALK) 10 under general or local anesthesia, a button (6.0–6.5 mm diameter, 370–400 μm thick) of host corneal tissue was manually excised, leaving a recipient bed of posterior stromal tissue and an intact endothelium. Patients received biomimetic substitutes (6.25–6.75 mm diameter, 500 μm thick) anchored with three to four overlying 10–0 nylon sutures and covered with a bandage lens. Antibiotic eye drops (chloramphenicol 0.5%, MINIMS® Chauvin Pharmaceuticals, London, UK) and topical steroid drops (dexamethasone 0.1% without preservatives, Opnol®, Clean Chemical Sweden, Borlänge, Sweden) were given postoperatively (one drop of each, three times daily), typically for 5 weeks. Bandage lenses and sutures were typically removed 5 weeks after surgery. Patients received either Chloromycetin® eye ointment (chloramphenicol 1%, Pfizer, Sollentuna, Sweden) applied two to three times daily, or Terracortil® with Polymyxin B eye drops (oxytetracycline 0.5%, hydrocortisone acetate 1.5%, and polymyxin B sulphate 10,000 units, Pfizer, Sollentuna, Sweden) applied one to two drops daily, for a duration of typically one month following suture removal.
Results
After 6–7 months, the corneal substitutes were well integrated in all 10 recipients, without adverse effects or complications such as neovascularization, inflammation, or rejection. Remodeling of the thick substitutes occurred, as previously observed in animal studies, resulting in restoration of a seamless host–graft interface and a corneal surface becoming progressively smoother over time ( Figure 1A and B ). Visually, the operated eye was indistinguishable from the patient's unoperated eye. Mean central corneal thickness after 6–7 months was 420 ± 99 μm (mean ± SD, range: 307–581 μm) as determined by ultrasound pachymetry. Tear film production was normal in seven operated eyes, as measured by Schirmer's test (>15 mm in 5 minutes), while the remaining operated eyes exhibited moderate wetting (9–12 mm in 5 minutes). To avoid applying direct force to the corneal surface, intraocular pressure at 6 months was assessed by manual palpation. All patients exhibited a normal pressure response by this method.
Figure 1.
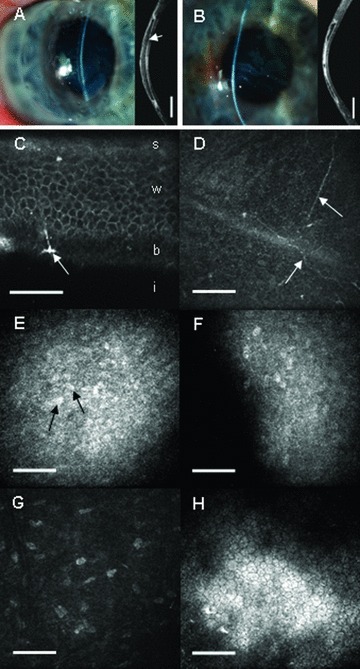
Representative slit‐lamp photographs, optical coherence tomography images (ASOCT, Visante, Carl Zeiss Meditec, Jena, Germany), and in vivo confocal microscopy images (HRT3, Heidelberg Engineering, Heidelberg, Germany) of corneas 7 months after lamellar keratoplasty. (A) Operated cornea of a 36‐year‐old male, which is transparent with a smooth corneal surface. In the OCT image, the boundary of the biomimetic substitute (arrow) is distinct, with initial remodeling evident (bright areas) at the peripheral host‐to‐graft interface (OCT image width = 9.2 mm, scale bar = 1.5 mm). (B) Operated cornea of a 57‐year‐old male with a slight central haze. The OCT image indicates remodeling of the central region of the implanted tissue by wound‐healing stromal fibroblasts (bright areas). Detailed in vivo micro‐morphology (C–H) of corneas at 7 months postoperative, with the depth of each image from the corneal surface given in parentheses. (C) Oblique section showing regenerated stratified epithelium with superficial (s), wing (w), and basal (b) epithelial cells, overlying the implant (i). New, sprouting nerves were observed at the basal epithelium (arrow), scale bar = 30 μm. (D) Fine, regenerating nerves (arrows) at the regenerated basal epithelium‐implant boundary (depth 43 μm), scale bar = 100 μm. (E) Nuclei of stromal cells (arrows) that have migrated into the implant from the host's cornea. The bright background represents endogenous wound‐healing fibroblast activity in the anterior part of the implanted tissue (depth 76 μm), scale bar = 100 μm.(F) Advancing front of endogenous stromal cells deep within the implant, with as yet unpopulated, cell‐free areas appearing dark (depth 192 μm), scale bar = 100 μm. (G) Host keratocyte nuclei within the host's preserved posterior stroma (depth 322 μm), scale bar = 100 μm. (H) Preserved host endothelium (depth 493 μm), scale bar = 100 μm.
Regeneration of host corneal epithelium to cover the implant surface was observed in all patients, with the time to full epithelial cover ranging from 1 to 3 months in nine patients and 5 months postoperatively in one patient (who received an amniotic membrane patch after 3 months to facilitate epithelialization). In vivo confocal microscopy (IVCM) showed that the regenerated epithelium exhibited a normal, stratified morphology with superficial, wing, and basal epithelial cells ( Figure 1C ), which has remained stable at seven months. Fine subepithelial nerves were observed ( Figure 1D ) and corresponded to a partial return of sensitivity as determined by Chochet‐Bonnet aesthesiometry (20–50 mm in the center of all operated corneas; normal 60 mm). Varying degrees of stromal cell migration and activity ( Figure 1E and F ) were observed within the implanted region by IVCM. The residual posterior stromal bed and endothelium in each patient's operated cornea maintained a normal morphology ( Figure 1G and H ). Vision continues to change as the stromal wound healing activity subsides and the ocular surface quality improves; however, even at this early stage best‐spectacle‐corrected visual acuity improved from preoperative values in four patients, remained unchanged in four patients, and decreased slightly in two patients ( Table 1 ).
Table 1.
Visual acuity and refraction at 6‐month postoperative follow‐up. For Patient 5, uncorrected visual acuity did not improve with refractio.
| Patient no. | Best‐spectacle‐corrected visual acuity | Refraction (sph/cyl axis) | |
|---|---|---|---|
| Preop | 6 months postop | 6 months postop | |
| 1 | <20/200 | 20/100 | +8/−2 130 |
| 2 | <20/200 | <20/200 | ±0/−5 85 |
| 3 | 20/200 | 20/200 | ±0/−6 50 |
| 4 | <20/200 | 20/200 | ±0/−4 90 |
| 5 | <20/200 | 20/200 | n/a |
| 6 | 20/67 | 20/100 | +4/−3 100 |
| 7 | 20/67 | 20/100 | +2.5/−2.5 65 |
| 8 | 20/67 | 20/67 | +1/−10 70 |
| 9 | <20/200 | 20/200 | ±0/−8 80 |
| 10 | 20/200 | 20/200 | ±0/−6 10 |
Discussion
The results of this study are encouraging. To our knowledge, this is the first report of the regeneration of corneal epithelium, stroma, and nerves in humans implanted with a corneal substitute. The clinical data support the hypothesis that a cell‐free corneal substitute mimicking the ECM can support endogenous regeneration of corneal tissue without rejection, inflammation, or neovascularization. The absence of adverse reactions in the first small patient group provides initial clinical evidence of the safety of RHC and the cross‐linking method used. Unlike most cross‐linkers that become chemically bound into the final collagen product with a subsequent propensity for breakdown products causing in vivo toxicity, neither EDC nor NHS are incorporated into the final product, 7 circumventing the possibility of toxic substance release from cross‐link breakdown.
Conclusion
Visual acuity, ocular surface quality, and corneal sensitivity are continuously improving in the first patients to receive a biomimetic corneal substitute. Such substitutes could find use as temporary or emergency corneal replacements, where human tissue is unavailable and prostheses are undesirable. The prospect for biomimetic substitutes to serve as longer‐term corneal replacements, and therefore, as an alternative to the use of human donor corneas for transplantation, awaits a longer‐term follow‐up in a large patient population.
Acknowledgments
We thank Dr. Bruce Jackson for helpful comments in our preparation of this manuscript, Cooper Vision for fabrication of the corneal implants for this study, and various colleagues in Ottawa, the EU, and Japan for their previous, invaluable contributions to this project. This study was supported by grants from the Swedish Research Council and the County of Östergötland to Per Fagerholm and the Canadian Stem Cell Network to May Griffith.
References
- 1. Griffith M, Fagerholm P, Liu W, McLaughlin CR, Li F. Corneal regenerative medicine: corneal substitutes for transplantation In: Reinhard T, Larkin F, eds. Cornea and External Disease, Essentials in Ophthalmology. Heidelberg , Germany : Springer; 2008: 37–53. [Google Scholar]
- 2. Myung D, Koh W, Bakri A, Zhang F, Marshall A, Ko J, Noolandi J, Carrasco M, Cochran JR, Frank CW, Ta CN. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed Microdevices. 2007; 9: 911–922. [DOI] [PubMed] [Google Scholar]
- 3. Hicks CR, Crawford GJ, Dart JK, Grabner G, Holland EJ, Stulting RD, Tan DT, Bulsara M. AlphaCor – Clinical outcomes. Cornea. 2006; 25: 1034–1042. [DOI] [PubMed] [Google Scholar]
- 4. Khan BF, Harissi‐Dagher M, Khan DM, Dohlman CH. Advances in Boston keratoprosthesis: enhancing retention and prevention of infection and inflammation. Int Ophthalmol Clin. 2007; 47: 61–71. [DOI] [PubMed] [Google Scholar]
- 5. Allan B. Artificial corneas. BMJ. 1999; 318: 821–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008; 60: 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Gan L, Carlsson DJ, Fagerholm P, Lagali N, Watsky MA, Munger R, Hodge WG, Priest D, Griffith M. A simple, cross‐linked collagen tissue substitute for corneal implantation. Invest Opthalmol Vis Sci. 2006; 47: 1869–1875. [DOI] [PubMed] [Google Scholar]
- 8. Merrett K, Fagerholm P, McLaughlin CR, Dravida S, Lagali N, Shinozaki N, Watsky MA, Munger R, Kato Y, Li F, Marmo CJ, Griffith M. Tissue‐engineered recombinant human collagen‐based corneal substitutes for implantation: Performance of type I versus type III collagen. Invest Ophthalmol Vis Sci. 2008; 49: 3887–3894. [DOI] [PubMed] [Google Scholar]
- 9. Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. The application of recombinant collagen in tissue engineering. BioDrugs. 2004; 18: 103–119. [DOI] [PubMed] [Google Scholar]
- 10. Ehrlich MI, Phinney RB, Mondino BJ, Pettit TH. Techniques of lamellar keratoplasty. Int Ophthalmol Clin. 1988; 28: 24–29. [DOI] [PubMed] [Google Scholar]


