Abstract
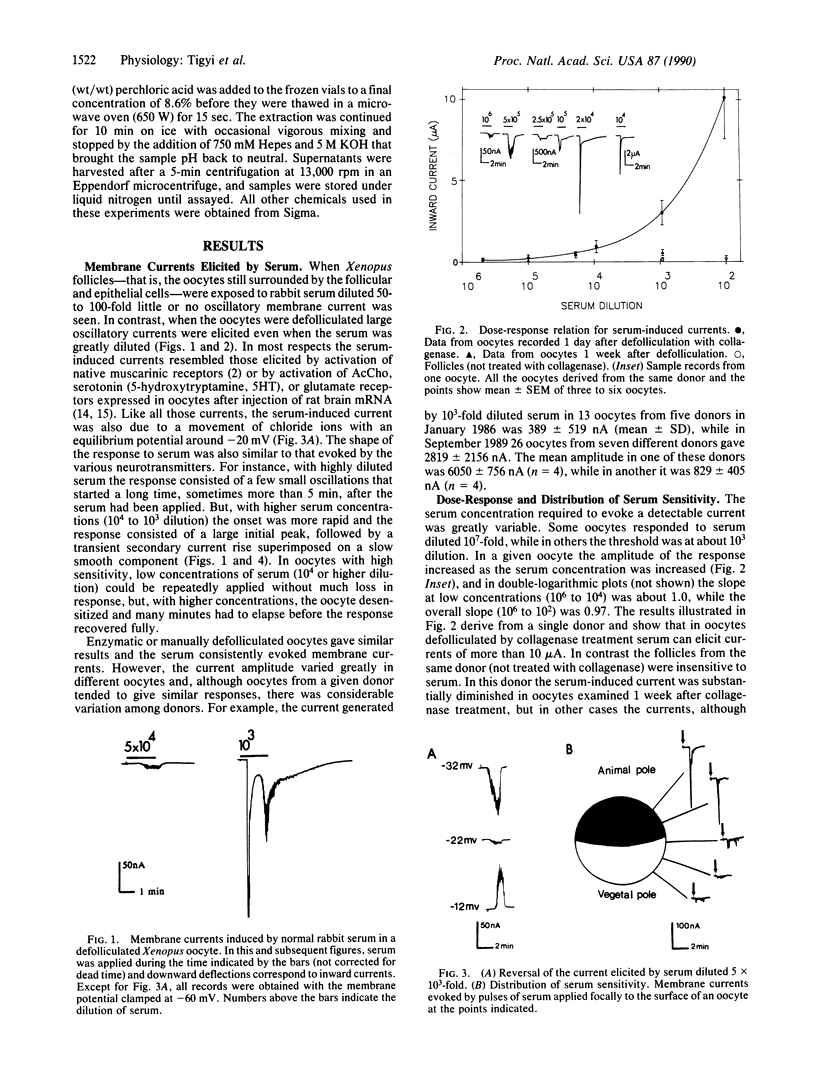
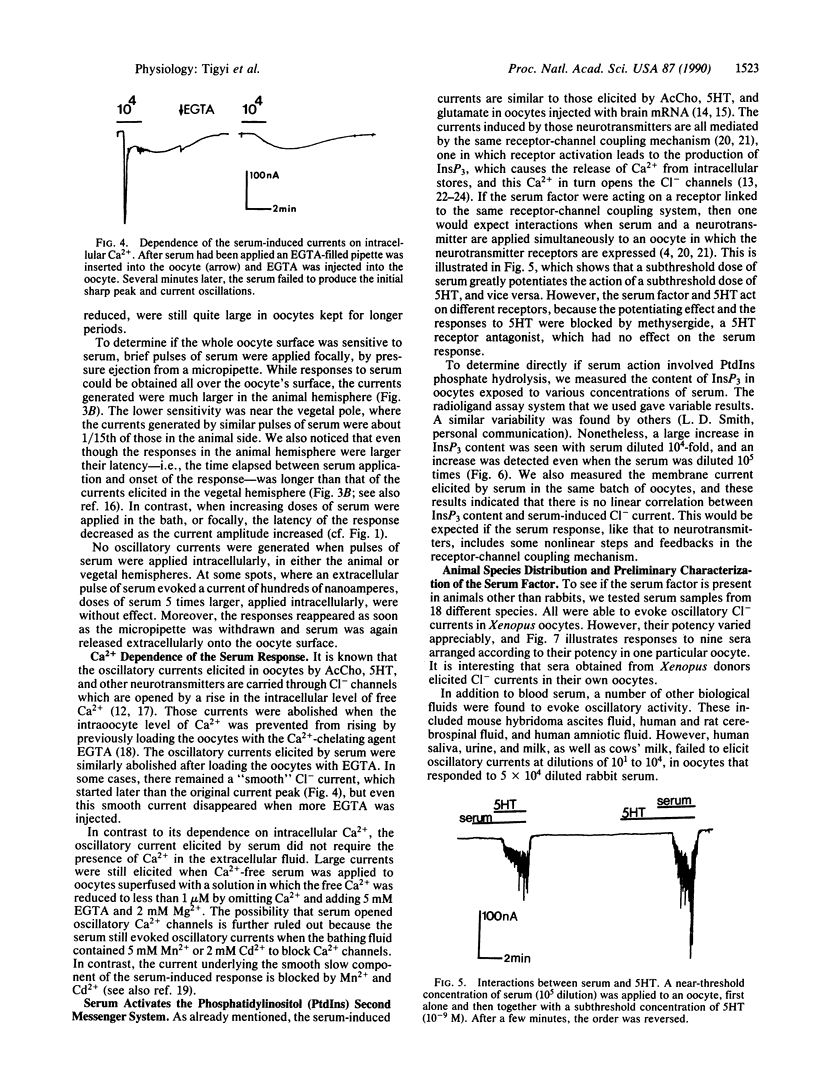
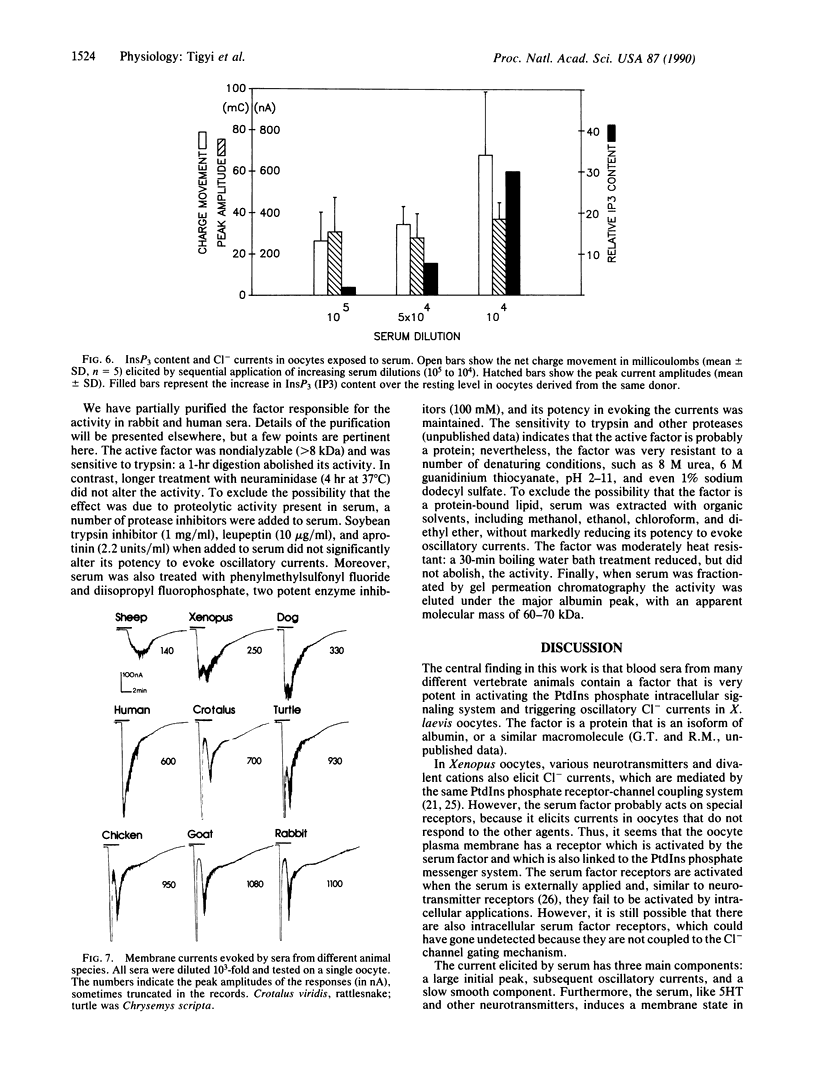
Blood sera from many vertebrate species elicit large oscillatory chloride currents in oocytes from the frog Xenopus laevis. Rabbit serum was active at dilutions as great as one part in 10 million. Intracellularly applied serum was ineffective, and externally applied serum failed to trigger oscillatory currents when the intracellular level of ionized calcium was prevented from rising by loading the oocyte with EGTA. The serum also caused an increase of inositol 1,4,5-trisphosphate in the oocyte. We conclude that serum contains a factor which activates a membrane receptor that is coupled to the phosphatidylinositol second messenger system. The active factor is a protein with an apparent molecular mass of 60-70 kDa in gel permeation chromatography. Although the normal function of the serum factor is still unknown, it may have far-reaching implications, because it acts on the multifunctional phosphatidylinositol phosphate signaling system. Also, because of its great potency the serum factor and Xenopus oocytes are very useful for probing the operation of the phosphatidylinositol system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Boton R., Dascal N., Gillo B., Lass Y. Two calcium-activated chloride conductances in Xenopus laevis oocytes permeabilized with the ionophore A23187. J Physiol. 1989 Jan;408:511–534. doi: 10.1113/jphysiol.1989.sp017473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N., Brummett A. R. Oogenesis in Xenopus laevis (Daudin). V. Relationships between developing oocytes and their investing follicular tissues. J Morphol. 1978 Jan;155(1):73–97. doi: 10.1002/jmor.1051550106. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Takahashi T., Miledi R. Calcium entry induced by acetylcholine action on snail neurons. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):55–62. doi: 10.1098/rspb.1985.0050. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Latencies of membrane currents evoked in Xenopus oocytes by receptor activation, inositol trisphosphate and calcium. J Physiol. 1989 Aug;415:189–210. doi: 10.1113/jphysiol.1989.sp017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Woodward R. M. Membrane currents elicited by divalent cations in Xenopus oocytes. J Physiol. 1989 Oct;417:173–195. doi: 10.1113/jphysiol.1989.sp017796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Membrane currents elicited by prostaglandins, atrial natriuretic factor and oxytocin in follicle-enclosed Xenopus oocytes. J Physiol. 1989 Sep;416:623–643. doi: 10.1113/jphysiol.1989.sp017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger C. Early sedentary economy in the basin of Mexico. Science. 1979 Jan 12;203(4376):131–142. doi: 10.1126/science.203.4376.131. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kaneko S., Kato K., Yamagishi S., Sugiyama H. Inositol phosphate formation and chloride current responses induced by acetylcholine and serotonin through GTP-binding proteins in Xenopus oocyte after injection of rat brain messenger RNA. Brain Res. 1987 Jul;388(2):113–123. doi: 10.1016/s0006-8993(87)80004-5. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):279–292. doi: 10.1098/rspb.1985.0002. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Intracellular Ca2+-dependent and Ca2+-independent responses of rat brain serotonin receptors transplanted to Xenopus oocytes. Neurosci Res. 1985 Aug;2(6):491–496. doi: 10.1016/0168-0102(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. On the orientation of foreign neurotransmitter receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Dec 23;226(1244):263–269. doi: 10.1098/rspb.1985.0095. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Injection of inositol 1,3,4,5-tetrakisphosphate into Xenopus oocytes generates a chloride current dependent upon intracellular calcium. Proc R Soc Lond B Biol Sci. 1987 Oct 22;232(1266):59–70. doi: 10.1098/rspb.1987.0061. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Inositol trisphosphate activates a voltage-dependent calcium influx in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):27–36. doi: 10.1098/rspb.1987.0033. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Activation of a common effector system by different brain neurotransmitter receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):37–45. doi: 10.1098/rspb.1987.0034. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Hormonal activation of ionic currents in follicle-enclosed Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Membrane currents elicited by porcine vasoactive intestinal peptide (VIP) in follicle-enclosed Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Sep 22;231(1265):489–497. doi: 10.1098/rspb.1987.0057. [DOI] [PubMed] [Google Scholar]
- van den Hoef M. H., Dictus W. J., Hage W. J., Bluemink J. G. The ultrastructural organization of gap junctions between follicle cells and the oocyte in Xenopus laevis. Eur J Cell Biol. 1984 Mar;33(2):242–247. [PubMed] [Google Scholar]


