Abstract
Biofilm formation on representative implantable medical devices using a known human pathogen (Staphylococcus aureus) was significantly reduced (p < 0.01) at all time points measured (24,48, and 72 hours) by employing a novel antibacterial envelope (AIGIS Rx™). The result was demonstrated using a standard US Centers for Disease Control (CDC) bioreactor model and the results were confirmed by Scanning Electron Microscopy (SEM). The antibacterial envelope used in the study is coated with a proprietary combination broad spectrum antibiotics (rifampin and minocycline) embedded in a resorbable polymeric coating. The antibiotics are designed to elute out of the coating over a multi‐day period for controlled, site‐specific drug delivery.
The infection rate for patients receiving pacemakers and defibrillators is increasing faster than the rate of new implants and the growing resistance of S. aureus strains suggests that conventional, systemic antibiotic prophylaxis may have limited future utility. Moreover, emerging evidence suggests that bacterial biofilms result in infections of implantable medical devices. These findings demonstrate the in vitro efficacy of a new means to address potential biofilm‐derived Hospital Acquired Infections (HAIs) related to implantable medical devices composed of titanium inclusive of pacemakers and defibrillators by means of a locally delivered, low dose, combination antibacterial treatment.
Keywords: cardiovascular implantable electronic device, CIEDs, cardiac device infection, CDI, antibacterial envelopes, staphylo‐cocci, biofilms
Introduction
In modern medical practice, cardiovascular implantable electronic devices (CIEDs), including pacemakers (PM), implantable cardioverter‐defibrillator (ICD), cardiac resynchronization device (CRT), implantable loop recorder (ILR), and implantable cardiovascular monitor (ICM), are used to manage a variety of cardiac conditions. 1 Like other implanted devices, 2 , 3 CIEDs (composed of a generator, a header, and intravascular leads) can sometimes become colonized by bacteria or fungi that are introduced during implantation surgery. This colonization may lead to cardiac device infections (CDIs), 4 which, if not treated properly, can lead to serious life‐threatening conditions. CDI rates range from 1% to 7% of all implanted cardiac devices. 5 Recent reports indicate that the number of CDIs is rising as the number of patients receiving implanted devices continues to increase and as the number of patients requiring CIED change‐outs (replacement) increases. 6 , 7
The clinical presentation of CDIs can range from postsurgical, superficial wound infections at the implantation site to serious systemic infections that can result in pacemaker infective endocarditis (PMIE). 7 Although PMIE is uncommon—infection rates range from 0.38% to 1% in patients with permanent pacemakers 8 , 9 —the condition has a mortality rate approaching 66%. 10
Nearly one‐half to two‐thirds of all CDIs 11 , 12 are caused by coagulase‐negative staphylococci (Staphylococcus epidermidis) and S. aureus. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 The emergence of antibiotic‐resistant staphylococci, most notably methicillin‐resistant S. aureus (MRSA), has made it increasingly costly and difficult to treat CDIs using conventional antibiotic treatment regimens. 13 , 14
There is a growing body of evidence that suggests that the formation of bacterial biofilms on implanted CIEDs is responsible for many infections. 11 , 15 It has been suggested that biofilms that form on or around the generator portions of implanted CIEDs can result in intravascular lead infections, which, in turn, may develop into potentially life‐threatening PMIE. 15 Further, bacteria growing as biofilms are hundreds to thousands of times more tolerant to antibiotics than identical bacteria grown in a liquid culture, which may also hinder conventional antibiotic treatment of device‐related infections. 16
Strategies to limit the incidence of CDIs during device implantation include: (1) proper use of sterile techniques, 11 (2) minimization of the amount of implanted hardware, 17 and (3) administration of intravenous antibiotics during and after device implantation. 11 , 17 , 18 , 19 Another strategy has been the use of antibiotic‐coated implantable devices that can reduce or eliminate the bacterial burden during implantation surgery. These types of devices were found to reduce the infection rates of central venous catheters and urology and cosmetic surgery implantable devices. 3 , 20 , 21
In the present study, a controlled‐release polypropylene envelope impregnated with the antibiotics rifampin and minocycline (AIGISRx™, TyRx Pharma, Inc., NJ, USA) was assessed for efficacy using a standard model. The AIGISRx™ device is commercially available in the United States, where the device has been labeled to indicate that it may reduce the likelihood of subsequent generator and pocket wound infections. In addition, the present study evaluated the ability of AIGISRx™ (antibacterial) envelopes to prevent biofilm formation by S. aureus on “mock CIEDs” using an in vitro biofilm model system. 22 The results of this study showed that the antibacterial envelope significantly reduced the ability of S. aureus to form biofilms on mock CIEDs.
Methods
Bacteria
S. aureus ATCC strain 33591 was used in this study. Unless otherwise specified, strain 33591 was grown in tryptic soy broth (TSB; Fisher Scientific Waltham, MA, USA) or on TSB agar plates. Bacteria were grown at room temperature for all biofilm experiments and at 37°C for plate count experiments.
Description of the CDC biofilm reactor system
The CDC biofilm reactor (CDC‐BR) system was developed by the Centers for Disease Control and Prevention, Atlanta, Georgia, to assess biofilm formation and prevention on surfaces and devices. 22 Two CDC‐BR model CBR90 bioreactors (Biosurface Technologies Corporation, Bozeman, MT, USA) were used in this study.
The CDC‐BR consists of a 1‐L bioreactor vessel with eight polypropylene coupon holders that can accommodate three 0.5‐inch diameter sample coupons suspended from the reactor lid ( Figure 1 ). A liquid growth medium enters through the top of the vessel lid and exits via a side‐arm discharge port. A magnetic stir bar incorporating a mixing blade provides fluid mixing and surface shear ( Figure 1 ). In the current study, the CDC‐BR system, which forms the basis of a standard method for the growth of Pseudomonas aeruginosa biofilms on polycarbonate surfaces, 22 was adapted to test the ability of S. aureus to form biofilms on titanium coupons (mock CIEDs) in antibacterial envelope or nonantibiotic‐containing control envelopes.
Figure 1.
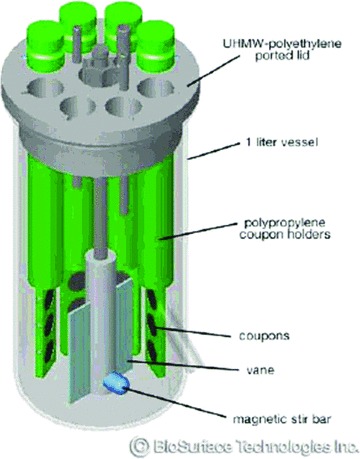
Schematic rendering of the CDC‐BR, model 90.
Bioreactor experimental design
Two bioreactors were used in the experiments described in this study. All experiments in the study were repeated at least three times.
One reactor (experimental) was used to evaluate biofilm formation on titanium coupons enclosed in the antibiotic‐containing envelope, whereas the other (control) was used to assess biofilm formation on coupons in control envelopes that contained no antibiotics. It was necessary to use two separate reactors for these experiments because the antibiotics in the antibacterial envelope are slowly released and easily diffuse in aqueous solutions.
In the experimental reactor, two sterile titanium coupons were enclosed in the antibacterial envelopes and two others were left unenclosed. Likewise, in the control reactor, two titanium coupons were left exposed, whereas the remaining two coupons were enclosed in nonantibiotic‐containing envelopes made from the same polypropylene as used in the antibacterial envelope. Unused ports in the lid assemblies of both reactors were plugged with sterile rubber stoppers.
Biofilm formation experiments
Inocula for the bioreactors were prepared by inoculating 800 mL of sterile 10%‐strength TSB with overnight cultures of S. aureus strain ATCC 33591 and by growing these cultures at room temperature, with continuous stirring for 24 hours. After incubation, 400 mL of the culture was added to a sterile experimental reactor and the remaining 400 mL was added to a second sterile control reactor. Sterile 1% TSB was continuously added to each reactor at a rate of 2.7 mL/min using a peristaltic pump.
Reactor fluid samples were removed via the exit ports from both reactors after incubation at room temperature for 24, 48, and 72 hours. After 72 hours of incubation, lid assemblies from both reactors ( Figure 1 ) were removed and transferred to beakers containing 400 mL of sterile phosphate buffered saline (PBS) and stirred (washed) for 5 minutes at room temperature. After the wash, the control and experimental coupons and envelopes were removed for microbiological analysis.
Microbiological analysis
After removal from the reactors, individual control and experimental envelopes or titanium coupons were placed in 10 mL of PBS and sequentially vortexed (30 seconds at the maximum setting of Maxi max II Barnstead/Thermolyne stirrer [Dubuque, IA, USA]), sonicated for 2 minutes, and then vortexed again to disaggregate biofilms and create bacterial suspensions. The bacterial suspensions were serially diluted in sterile PBS and the dilutions were spread on Tryptic Soy Agar plates that were incubated at 37°C for 24 hours. The number of colony forming units (CFU) per sample was determined using standard microbial spread plate count methods. The number of CFU associated with the titanium coupons was expressed as CFU/cm2, the number of bacteria associated with the control and experimental envelopes as CFU/envelope, and the number of bacteria in the reactor fluids as CFU/mL. Statistical analysis 23 of bacterial counts obtained from the control and treatment reactors was conducted using a 2‐tailed, Student's t‐test.
Scanning electron microscopy (SEM)
Exposed and unexposed titanium coupons and experimental and control envelopes were fixed with neutral buffered formalin, dehydrated with a graded ethanol series, and then air‐dried. The envelopes were sputter‐coated with iridium to make them conductive for SEM analyses. SEM examination of the coupons and envelopes was performed using a Zeiss Supra 55VP field‐emission SEM (Thornwood, NY, USA).
Results
Biofilm formation with S. aureus 33591 ATCC strain 33591
The results from the SEM experiments revealed that S. aureus ATCC strain 33591 formed biofilms in the CDC‐BR model system ( Figure 2 ). This verified the utility of using the CDC‐BR model system to evaluate the effects of the antibacterial envelope on the ability of strain 33591 to form biofilms on mock CIEDs.
Figure 2.
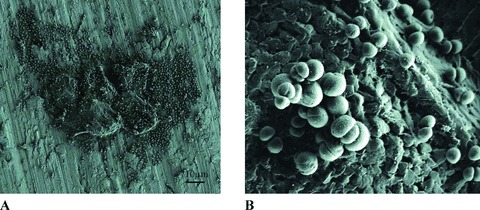
SEM micrographs at different magnifi cations of S. aureus biofilm formation on an exposed titanium coupon from a control reactor after 72 hours of incubation at room temperature. (A) 500x; (B) 9,000x.
Microbiological analysis
The analysis of the antibacterial and control envelopes after 72 hours of incubation ( Figure 3 ) revealed that there was a marked and statistically significant difference (p < 0.01) between the number of staphylococci associated with the antibacterial envelope (6.04 log10 CFU/envelope) as compared with the nonantibiotic‐containing control envelopes (8.89 log10 CFU/envelope). Similarly, there was a statistically significant difference (p < 0.01) in the number of bacteria associated with the titanium coupons enclosed in the antibacterial and control envelopes (mean log10 difference of 2.24 CFU/cm2). Likewise, there was a statistically significant difference (log10 difference of 3.77 CFU/cm2, p < 0.01) between the number of bacteria associated with the unenclosed titanium coupons taken from the experimental and control reactors after 72 hours of incubation ( Figure 4 ).
Figure 3.
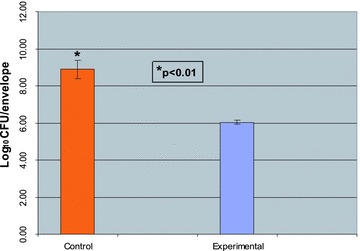
Numbers of bacteria associated with experimental (AIGISRx™) and control envelopes after 72 hours at room temperature. 111 control and experimental envelopes were removed from the reactors after 72 hours of incubation at room temperature and processed as described in the “Methods” section to create bacterial suspensions and determine the number of bacteria associated with each envelope. The numbers of viable bacteria associated with the control and experimental envelopes are expressed as log10 CFU/envelope.
Figure 4.
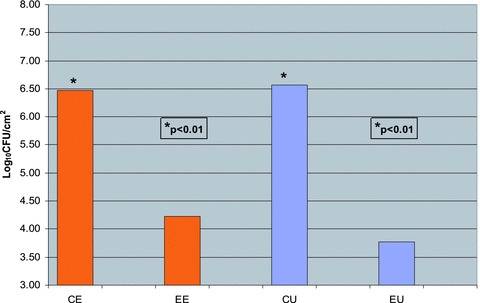
Number of bacteria associated with enclosed and unenclosed titanium coupons from experimental and control reactors. Titanium coupons were removed from the reactors ater 72 hours of incubation at room temperature and processed as described in the “Methods” section to create bacterial suspensions and determine the number of bacteria associated with the titanium coupons. The numbers of viable bacteria associated with the control and experimental coupons are expressed as log10 CFU/cm2. CE = control/enveloped coupons; EE = experimental/enveloped coupons; CU = control/unenveloped (exposed) coupons; EU = experimental/unenveloped (exposed) coupons.
Finally, there was a statistically significant difference (p < 0.01) in the number of bacteria found in the experimental and control reactor fluids after 24,48, and 72 hours of incubation ( Figure 5). The mean log10 difference (in CFU/mL) between the experimental and the control samples at 24, 48, and 72 hours was 3.52, 4.31, and 3.42, respectively.
Figure 5.
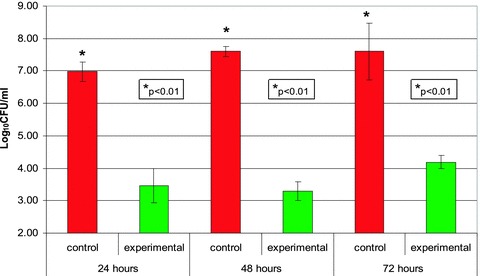
Number of bacteria in control and experimental reactor fluids after 24,48, and 72 hours at room temperature. Fluids from the control and experimental reactors were removed after 24, 48, and 72 hours of incubation at room temperature and the number of viable bacteria in the samples was determined. The numbers of viable bacteria found in the control and experimental reactor fluids are expressed as log10 CFU/mL.
The reduction (in CFU/m/L) in reactor fluid samples taken from the experimental and control reactors was confirmed by visual inspection of the reactors after 72 hours of incubation. As shown in Figure 6 , there was a marked reduction in bacterial growth (turbidity) in the experimental reactor as compared with the control reactor.
Figure 6.
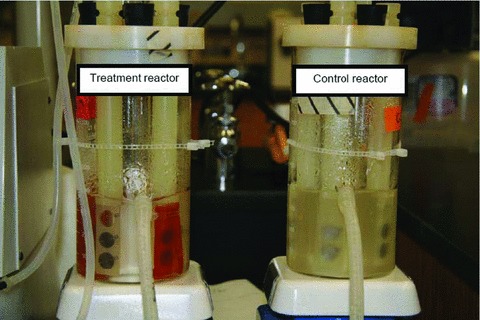
Differences in bacterial growth (turbidity) in experimental and control reactors after 72 hours of incubation at room temperature.
SEM analysis
The SEM analysis revealed that after 72 hours of incubation, there was an observable difference in the staphylococcal biofilms formed on the titanium coupons and envelopes from the control and experimental reactors. Exposed titanium coupons taken from the control reactors were colonized by large numbers of actively dividing staphylococci that formed biofilms ( Figure 2 ). Biofilms also formed on the control‐enveloped titanium coupons, but to a lesser extent. Far fewer bacteria were found on both exposed and enveloped titanium coupons from the experimental reactors (data not shown).
The differences in biofilm formation on the experimental (antibacterial envelope) and control envelopes were more pronounced. The control envelopes were heavily colonized with grape‐like clusters of dividing staphylococci ( Figure 7A and B ). In marked contrast, staphylococci were rarely observed on the antibacterial envelopes, and when present, they were typically observed as single, isolated bacterial cells. ( Figure 7C and D ).
Figure 7.
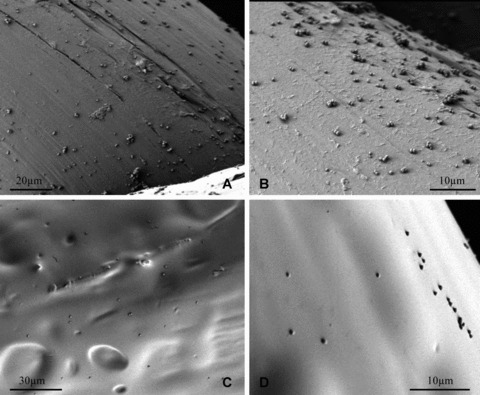
S. aureus biofilm formation on control and experimental envelopes at different magnifications after 72 hours of incubation at room temperature. (A) Control, 500x; (B) Control, 1,500x; (C) Experimental, 500x; (D) Experimental, 1,500x.
Discussion
The results from the present study demonstrate that the antibacterial envelope significantly inhibited the ability of S. aureus to form biofilms on mock CIEDs. Although we used an in vitro biofilm system in the present study, our results may have important clinical implications for CIED implant surgery.
The incidence of CIED infections among cardiac patients is rising. 6 , 7 This is likely because of increases in the rates of device implantation, generator change‐outs, and increasing comorbidities and illnesses of the patients who receive cardiac devices. 7 Attempts to control infections using more rigorous aseptic surgical techniques and the perioperative administration of antibiotics have met with limited success. Although antibiotic‐impregnated devices have been shown to reduce the incidence of implantable device infections, 3 , 20 , 21 there are currently, as demonstrated by a review of the Food and Drug Administration (FDA) product approvals, no FDA‐sanctioned antibiotic‐containing CIEDs on the market.
Approximately 69% of patients with CDIs present with localized pocket infections and about 10% of these infections progress to PMIE. 24 Several studies have found that untreated pocket infections can result in intravascular lead contamination, which can increase the likelihood of systemic infection or PMIE in people with permanent CIEDs 11 , 15 , 25 Because pocket site infections are the most common type of infection among CIED recipients, it is generally thought that bacterial contamination occurs during implantation. The perioperative use of antibiotics with CIED implantation and the concomitant reduction in the incidence of CDIs tend to support this idea. 18 Unfortunately, the growing numbers of methicillin‐ and vancomycin‐resistant strains of S. aureus suggest that conventional perioperative antibiotic prophylaxis may have limited utility in the future to treat staphylococcal device infections.
There is emerging evidence that suggests that many implantable device infections are caused by bacterial biofilms—communities of bacteria enmeshed in secreted polymers attached to a device surface. 26 As mentioned previously, biofilms are extremely difficult to treat with conventional antibiotic treatments 26 because of a number ot tactors, including the avascular nature ot the CIED implant pocket, which, when infected, may increase the risk of endocarditis. To lessen the likelihood of biofilm formation on CIEDs—and ultimately reduce the incidence of infections—we assessed a rifampin and minocycline‐containing envelope, called the antibacterial envelope, into which CIEDs can be placed during implant surgery. The results of the present study suggest that the antibacterial envelope markedly reduced the ability of S. aureus to form biofilms on mock CIEDs in an in vitro model biofilm system. 22 This is important, because staphylococci are the major pathogens that are responsible for most CDIs 2 , 5 , 9 , 27 and nosocomial infective endocarditis. 10 Also, the emergence of MRSA and, more recently, vancomycin‐resistant staphylococci (VRSA) 28 has made device infections caused by these organisms more costly and difficult to treat. 13 Finally, there is strong evidence that implicates biofilm formation as a major factor in the etiology of staphylococcal wound infections, including CDIs. 29 , 30
Other implantable devices impregnated with rifampin and minocycline—most notably central venous catheters 20 —exhibited a lower incidence of device‐related bacterial infections following implantation. 3 , 31 , 32 Moreover, the combination of rifampin and minocycline has been cleared for general use in medical devices by regulatory agencies, and there are no reports of bacterial resistance or toxicity associated with any implanted devices containing these antibiotics. Together, these observations suggest that the antibacterial envelope may reduce the incidence of CDIs if used at the time of CIED implant surgery.
The microbiological ( Figure 3 ) and SEM analysis ( Figure 7 ) revealed that the combination of rifampin and minocycline in the antibacterial envelope was sufficient to effectively inhibit biofilm formation on mock CIEDs after 72 hours of incubation in the CDC‐BR system. Moreover, unpublished results from recent in vivo experiments using a novel device infection model developed in the rabbit showed that the antibacterial envelope eliminated the ability of staphylococci and other bacteria to colonize implanted CIEDs and reduced the colonization of surgical implant pockets ( Figure 8 ). Finally, the antibacterial envelope recently received regulatory clearance from the U.S. FDA for use during CIED implant surgery 34
Figure 8.
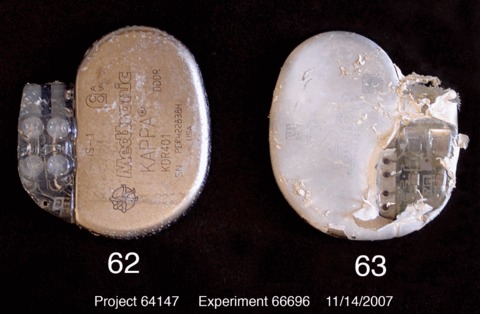
Representative biofilm formation at 7 days on pacemakers with or without the AIGISRx™ envelope after implantation in a rabbit model of infectivity using Acinetobacter baumanii. Left: with AIGISRx™ envelope; right: without AIGISRx™ envelope (Hansen et al. 33 by permission).
Conclusion
Despite improvements in aseptic surgical practices, perioperative use of antibiotics, and better lead and management strategies for CIEDs, 2 , 7 , 11 , 17 the incidence of CDIs continues to rise. The results of the present study showed that the antibacterial envelope significantly inhibited the ability of S. aureus to form biofilms on mock CIEDs. This suggests that the antibacterial envelope's potent antimicrobial activity, coupled with its ease of use and cost‐effectiveness, supports additional in vivo studies to better demonstrate the ability of the antibacterial envelope to help reduce the incidence of CDIs and its potential to provide better long‐term medical outcomes for people with permanent CIEDs.
Conflict of Interest
Mark Citron is an employee of TyRx Pharma, Inc. Drs. Oussama Wazni and Bruce D. Wilkoff are not affiliated with TyRx and did not receive any honoraria, consulting fees, grants, or funds from the company. The work performed by Drs. Alessandra Agostinho and Garth James in the study was funded by a contract between the Center for Biofilm Engineering at Montana State University and TyRx Pharma, Inc.
References
- 1. Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Hewlett JG, Kautzner J, Love A, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs) description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008; 5(6): 907–925. [DOI] [PubMed] [Google Scholar]
- 2. Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000; 133(8): 604–608. [DOI] [PubMed] [Google Scholar]
- 3. Hedrick TL, Adams JD, Sawyer RG. Implant‐associated infections: an overview. J Long Term Eft Med Implants. 2006; 16(1): 83–99. [DOI] [PubMed] [Google Scholar]
- 4. Klug D, Balde M, Pavin D, Hidden‐Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S. Risk factors related to infections of implanted pacemakers and cardioverter‐defibrillators: results of a large prospective study. Circulation. 2007; 116(12): 1349–1355. [DOI] [PubMed] [Google Scholar]
- 5. Karchmer AW, Longworth DL. Infections of intracardiac devices. Infect Dis Clin North Am. 2002; 16(2): 477–505, xii. [DOI] [PubMed] [Google Scholar]
- 6. Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR, Fowler VG, Jr . Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J. 2004; 147(4): 582–586. [DOI] [PubMed] [Google Scholar]
- 7. Uslan DZ, Baddour LM. Cardiac device infections: getting to the heart of the matter. Curr Opin infect Dis. 2006; 19(4): 345–348. [DOI] [PubMed] [Google Scholar]
- 8. Harcombe AA, Newell SA, Ludman PR, Wistow TE, Sharpies LD, Schofield PM, Stone DL, Shapiro LM, Cole T, Petch MC. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998; 80(3): 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rundstrom H, Kennergren C, Andersson R, Alestig K, Hogevik H. Pacemaker endocarditis during 18 years in Goteborg. Scand J Infect Dis 2004; 36(9): 674–679. [DOI] [PubMed] [Google Scholar]
- 10. Giamarellou H. Nosocomial cardiac infections. J Hosp Infect. 2002; 50(2): 91–105. [DOI] [PubMed] [Google Scholar]
- 11. Wilkoff BL. How to treat and identify device infections. Heart Rhythm. 2007; 4(11): 1467–1470. [DOI] [PubMed] [Google Scholar]
- 12. Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. Management and outcome of permanent pacemaker and implantable cardioverter‐defibrillator infections. J Am Coll Cardiol. 2007; 49(18): 1851–1859. [DOI] [PubMed] [Google Scholar]
- 13. Rubin RJ, Harrington CA, Poon A, Dietrich K, Greene JA, Moiduddin A. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg Infect Dis. 1999; 5(1) 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004; 350(14): 1422–1429. [DOI] [PubMed] [Google Scholar]
- 15. Klug D, Wallet F, Lacroix D, Marquie C, Kouakam C, Kacet S, Courcol R. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004; 90(8): 882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pichlmaier M, Marwitz V, Kühn C, Niehaus M, Klein G, Bara C, Haverich A, Abraham WR. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace 2008; 10: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 17. Borek PP, Wilkoff BL. Pacemaker and ICD leads: strategies for long‐term management. J Interv Card Electrophysiol. 2008; 23(1): 59–72. [DOI] [PubMed] [Google Scholar]
- 18. Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier A, Isaaz K, Touboul P. Antibiotic prophylaxis for permanent pacemaker implantation: a meta‐analysis. Circulation. 1998; 97(18): 1796–1801. [DOI] [PubMed] [Google Scholar]
- 19. Furuya EY, Lowy FD. Antimicrobial strategies for the prevention and treatment of cardiovascular infections. Curr Opin Pharmacol. 2003; 3(5): 464–469. [DOI] [PubMed] [Google Scholar]
- 20. Darouiche RO, Mansouri MD, Raad I, I. Efficacy of antimicrobial‐impregnated silicone sections from penile implants in preventing device colonization in an animal model. Urology. 2002; 59(2) 303–307. [DOI] [PubMed] [Google Scholar]
- 21. Darouiche RO, Raad, II , Heard SO, Thornby Jl, Wenker OC, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris RL, Mayhall G. A comparison of two antimicrobial‐impregnated central venous catheters. Catheter Study Group. N Engl J Med. 1999;340(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Donlan RM, Piede JA, Heyes CD, Sanii L, Murga R, Edmonds P, El‐Sayed I, El‐Sayed MA. Model system for growing and quantifying Streptococcus pneumoniae biofilms in situ and in real time. Appl Environ Microbiol. 2004; 70(8): 4980–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao PV. Statistical Research Methods in the Life Sciences. Pacific Grove , CA : Duxbuiy Press, 1998. [Google Scholar]
- 24. Arber N, Pras E, Copperman Y, Schapiro JM, Meiner V, Lossos IS, Militianu A, Hassin D, Shai A. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore). 1994; 73(6): 299–305. [DOI] [PubMed] [Google Scholar]
- 25. Klug D, Lacroix D, Savoye C, Goullard L, Grandmougin D, Hennequin JL, Kacet S, Lekieffre J. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation. 1997; 95(8): 2098–2107. [DOI] [PubMed] [Google Scholar]
- 26. Wolcott RD, Ehrlich GD. Biofilms and chronic infections. JAMA 2008; 299(22): 2682–2684. [DOI] [PubMed] [Google Scholar]
- 27. Karchmer AW, Longworth DL. Infections of intracardiac devices. Cardiol Clin. 2003; 21(2) 253–271, vii. [DOI] [PubMed] [Google Scholar]
- 28. Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. Infection with vancomycin‐resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003; 348(14): 1342–1347. [DOI] [PubMed] [Google Scholar]
- 29. Gotz F. Staphylococcus and biofilms. MolMicrobiol. 2002; 43(6): 1367–1378. [DOI] [PubMed] [Google Scholar]
- 30. Dy Chua J, Abdul‐Karim A, Mawhorter S, Procop GW, Tchou P, Niebauer M, Saliba W, Schweikert R, Wilkoff BL. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. Pacing Clin Electrophysiol. 2005; 28(12): 1276–1281. [DOI] [PubMed] [Google Scholar]
- 31. Carson CC, III . Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004; 171(4): 1611–1614. [DOI] [PubMed] [Google Scholar]
- 32. Darouiche RO, Meade R, Mansouri MD, Netscher DT. In vivo efficacy of antimicrobe‐impregnated saline‐filled silicone implants. Plast Reconstr Surg. 2002; 109(4): 1352–1357. [DOI] [PubMed] [Google Scholar]
- 33. Hansen L, Brown M, Johnson D, Palme D, Love C, Darouiche R. A novel in vivo model of human pathogen infection and demonstration of efficacy by an antimicrobial pouch for pacing devices. Pacing Clin Electrophysiol. In press, 2009. [DOI] [PubMed] [Google Scholar]
- 34. U.S. Department of Health and Human Services . U.S. Food and Drug Administration. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=23059. Accessed August 19,2008.


