St. Louis, Missouri, sits astride the confluence of the Mississippi and Missouri Rivers, a position that made the city the “Gateway to the West.” Its most famous monument, the Gateway Arch, memorializes the millions of pioneers who traveled to the city seeking transportation to the frontier lands of the West, and who were outfitted with the tools and knowledge they needed on the journey to these new lands of opportunity. The Clinical & Translational Science Awards (CTSA) program has enabled Washington University (WU) and its regional partners to create a gateway of another sort, aimed at helping researchers toward new opportunities and discoveries in clinical and translational research.
The Washington University Institute of Clinical and Translational Sciences (ICTS) was founded in September 2007 to serve as the intellectual and physical home for clinical and translational research and clinical research training at WU and its partner institutions. ICTS was founded on WU's 4 prior clinical research and training grants—GCRC, K12, K30, and T32—and on an existing university‐wide strategic initiative in multidisciplinary collaborative research emphasizing genome sciences, biological imaging, and clinical investigation. The formation of the ICTS resulted in a new level of regional partnership with other academic, health care, and industrial and community partners. In its second year of existence, the ICTS is increasingly able to integrate the different strengths of its partners to reach its goals of reinventing and reinvigorating CTS research and training.
In designing the ICTS, we used a conceptual model that identified important phases in the progress of a clinical or translational research project ( Figure 1 ). For each phase, we identifed needed resources and potential barriers to completion, and have structured the ICTS in a way that attempts to provide needed resources and expertise for each phase of research from development and refinement of research ideas through translation of results to clinical practice and community implementation. Education, training, and career development are at the heart of our ICTS, and are linked to all research phases and programmatic activities. Our ICTS has 15 key Program Functions, each linked to 1 or more research phases, and each with specific goals, milestones, and evaluation criteria for various services, cores, and programs. We will highlight 2 new and important program functions below: our community‐based research efforts and our programs in biomedical informatics.
Figure 1.
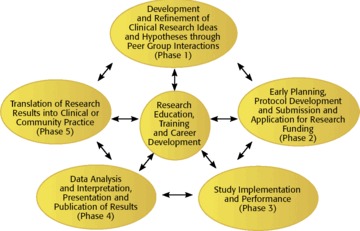
Phases in the evolution of clinical research projects.
The 3 CCBR arms serve as a model for community participation to improve research relevant to public health.
From the Medical Center to the Community
A central transformative element offour ICTS was the establishment of the Center for Community‐Based Research (CCBR), which has initiated community engagement ef orts in 3 important groups: community organizations, community‐based health care providers, and individuals from medically underserved populations. Each of the 3 arms is designed to solicit community input to increase the relevance of research to community needs, and to encourage partnerships between academic researchers and community groups. Our Community Advisory Board, established by the 3 arms, meets quarterly, to ensure relevance of the CCBR programs and the ICTS programs as a whole.
The Community Organizations program has focused on cultivating partnerships with community organizations and conducting community needs assessments. This new model has spearheaded a series of conferences with our Regional Health Commission to address how our academic medical center can better serve the needs of our local community and improve the public health. In the fall of 2009, we will begin a series of shared conferences with community organizations highlighting successful community‐based participatory research projects. These conferences will include joint presentations by academics and community partners and will strive to facilitate the formation of new partnerships. To further stimulate new community‐based participatory research projects, we will soon initiate a research grants program, supported by a regional coalition of universities and health care institutions, to fund research projects that include a high degree of cooperation between community organizations and academic researchers. The program has also initiated a traveling poster presentation forum that translates data in a community relevant fashion.
The Community Health Care Practitioners program seeks to expand community networks of practitioners to increase opportunities for clinical and translational (T2 and T3) research. One such effort is the Washington University Pediatric and Adolescent Ambulatory Research Consortium (WU PAARC). Currently comprised of approximately 60 community pediatricians and 5 pediatric nurse practitioners, the network provides a formal structure to develop research ef orts that identify best practices and translate research findings into practice. Community pediatricians participate in the consortium in different ways, from contributing ideas and receiving study updates to helping develop study protocols and recruit patients from their practices. Some studies are initiated by WU PAARC physicians, such as a recent study on a Telephone Asthma Program that resulted from physicians’ frustration with patients not using their asthma maintenance medications. For this randomized controlled trial, 362 families with asthmatic children were recruited from 95 community pediatric offices over 13 months. The 12‐month telephone asthma coaching intervention helped parents better manage their child's asthma care and improved children's asthma control and parents’ quality‐of‐life scores. An upcoming study will compare 2 corticosteroid treatments for croup, a disease commonly seen in community practice but never before studied in this setting.
In addition to WU PAARC‐sponsored studies, community pediatricians have opportunities to participate in other studies developed by ICTS researchers including participating in study development and implementation. An example is a prevalence study for community‐acquired methicillin‐resistant Staph aureu s for which 1,300 children were recruited from 11 WU PAARC practices over an 8‐month period. WU PAARC serves as a model for cooperative research between academic and community physicians, and these examples show how those involved have a real opportunity to improve the health of children and adolescents. As our ICTS matures, we plan to expand our practice‐based research network activities to include new patient and provider populations.
The CCBR has also started HealthStreet, a project linking populations that have been underrepresented in research to health information, referrals to social, medical, and psychiatric services, as well as opportunities to participate in research studies. HealthStreet utilizes Community Health Workers and a comprehensive outreach approach to establish trust between the lay and medical communities. The hub of CCBR activity is a new storefront facility in an underserved neighborhood near our medical center where community members can receive free health screenings, health referrals, and information about WU clinical studies. Community Health Workers also reach people in sites such as beauty shops, laundromats, bus stops, and community agencies to spread the word about services offered, and opportunities for research.
The 3 CCBR arms serve as a model for community participation to improve research relevant to public health. CCBR investigators are actively training and engaging other ICTS investigators to collaborate on community‐based projects with the goal of addressing “outcomes that matter.”
The Center for Biomedical Informatics
Major institutional goals for improving CTS research are the creation of efficient communication and coordination across key function activities; the creation of an informatics infrastructure capable of integrating ICTS program components for data sharing; and the development of electronic resources of existing clinical and biospecimen data that may be used to develop new clinical studies, identify potential research participants, and execute novel molecular correlative studies. The ICTS Center for Biomedical Informatics (CBMI) is addressing these complex core issues through industry‐standard hardware infrastructure and software tools to store, integrate, query, analyze, and visualize complex clinical and molecular data sets. Among the several activities of CBMI are the integration and implementation of the research management tool ClinPortal, development and management of biospecimen tracking tools, and creation of the Clinical Investigation Data Exploration Repository (CIDER), a research repository of medical records.
ClinPortal is a research management tool that allows investigators and study coordinators to integrate the data collection and management needs of a clinical study with critical regulatory compliance requirements. Investigators create case report forms using data elements (or variables) that are defined using controlled vocabularies, and ClinPortal then automatically generates the backend database schema as well as the forms to enter the data and conduct queries. Use of ClinPortal offers a number of advantages to the institution and to the investigator. By default, data stored in ClinPortal is available for sharing while following all HIPAA regulations, although the investigator that owns the data may turn of sharing if so desired. As a result, once an investigator has established his or her study within the ClinPortal system, there is potential for sharing relevant data with and from other studies. Data access is controlled both by investigator permissions and by regulatory requirements. Future phases include automated access to clinical data from our hospital and outpatient systems. Assuming appropriate release by the patient and access rights for the researcher, investigators using ClinPortal will eventually have access to clinical data from hospital and clinic sources for their study participants.
Tissue Suite, a Web‐based biospecimen inventory, tracking, and annotation tool, may be used for study‐based biospecimen collection efforts and by other non‐study investigators interested in identifying existing biospecimens throughout the medical campus that may be available for translational research. The ICTS Translational Pathology and Molecular Phenotyping (TPMP) Core supports the physical storage of biospecimens, while Tissue Suite is the application supported by both the CBMI and the TPMP to provide the informatics to manage data about those biospecimens. Investigators who are managing their own biospecimen collection efforts outside of the TPMP Core may still make use of the Tissue Suite application to manage their inventory in compliance with caBIG standards. Web accessibility, the ability to share biospecimens and biospecimen‐related data, advanced search functionality, and capture of clinical, pathological, and quality assurance data for collected biospecimens are key features of Tissue Suite. Together, ClinPortal and Tissue Suite facilitate collaboration between investigators and data integration across studies. As clinical data becomes available through the ClinPortal tool, biospecimens referenced in the complementary Tissue Suite tool will gain increasing clinical annotation, making them even more valuable for translational research studies.
Another major focus of the CBMI has been the development of a comprehensive research patient data warehouse. CIDER contains a wealth of data from the electronic medical records of most inpatients and outpatients seen at the WU medical center and affiliated hospitals in the BJC HealthCare system. This system will become available to ICTS investigators in 2009 and will initially contain data on patient encounters, laboratory testing, and medications. We sought community input in designing the patient notification and opt‐out procedures and in strictly designating access and use of this information by qualified investigators. We expect that CIDER will become a very useful tool for many clinical and translational researchers to perform a review preparatory to research, identify potential participants to recruit to active clinical studies both by querying CIDER and as they are encountered in the health care system, and implement quality assurance and improvement initiatives.
In addition to these highlighted functions, the CBMI is working on other ways to simplify communication and tracking within the ICTS. One new project is the creation of an electronic Research Concierge function to serve as the focal point to help clinical and translational researchers navigate through the study planning, design, review, and execution processes. This Research Concierge function will be added to WU's existing site that currently provides single sign‐on access to the resources necessary to propose, manage, and close research projects and satisfy compliance requirements for research. In a nod to St. Louis history, these resources can be found at the WU Research Gateway Web site (http://research.wustl.edu). More information about the WU ICTS can be found at our main site http://icts.wustl.edu.


