Abstract
Immunization with the electrofusion product of tumor cells and dendritic cells (DCs) is a promising approach to cancer immunotherapy. Production of electrofusion vaccines currently requires the acquisition of tumor material and must be tailored to each individual. Alternative vaccine configurations were explored in this study. Results indicated that fusion vaccines with fully syngeneic, semi‐allogeneic or fully allogeneic components, were all effective in inducing specific, long‐lasting antitumor immunity. This previously undescribed activity of a fully allogeneic fusion product introduces the possibility of using defined allogeneic tumor and DC lines to simplify vaccine manufacturing.
Keywords: electrofusion, vaccine, melanoma, dendritic cells
Introduction
Overall, tumor cell‐based vaccines have largely lacked clinical efficacy despite attempts at increasing their immunogenicity via genetic modification, haptenation or combination with adjuvants (reviewed in Ref. 1 ). In recent years, several groups have reported that hybrid cells generated by the fusion of tumor cells and dendritic cells (DCs), using polyethylene glycol (PEG) or electrofusion, possess immunogenicity, and are able to induce protective immune responses against tumor cells. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 The fusion hybrids are believed to combine antigens from the tumor cells with the antigen‐presenting and costimulatory properties of DCs thus allowing for effective presentation of the full complement of potential antigens within the tumor, both known and unknown. While fusion vaccines hold considerable promise as cancer immunotherapy agents, their clinical application is complex and currently requires the generation of individually tailored vaccines generated with tumor cells and/or DCs from each patient. 11 , 12 , 13 , 14 , 15 , 16 In this study, the potential of alternative, more practical electrofusion vaccines generated with allogeneic material was explored.
Methods
Animals and cell lines
Six‐ to eight‐week‐old C57BT/6 and DBA/2 mice were purchased from Taconic Taboratories (Germantown, NY, USA). The B16‐F10 melanoma cell line was obtained from the National Cancer Institute (Bethesda, MD, USA). The M3 melanoma, P815 mastocytoma and the dendritic cell‐like JAWS II lines were purchased from the American Type Culture Collection (Manassas, VA, USA). All animal experiments were conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee at Genzyme Corporation.
Preparation of electrofusion vaccine
Primary DCs were derived from bone marrow by negative selection of precursors followed by culture in GM‐CSF‐containing medium as described previously 17 and were matured for 2 days by the addition of 50 ng/mL recombinant mouse TNF‐α to the culture medium (R&D Systems, Minneapolis, MN, USA). To generate primary tumor material for electrofusion, DBA/2 mice were injected subcutaneously (s.c.) with 1.5 × 104 M3 tumor cells and tumors were excised once they reached 25–50 mm2 in size. A single cell suspension was generated by digestion with collagenase (50 μg/mL) and DNase (25 μg/mL) followed by passage through a 100‐micron wire mesh strainer. For generation of the electrofusion product, DCs and tumor cells were each resuspended at 1 × 107/mL in a solution of 0.3M glucose in water, pH 7.0. Equal volumes of the DC and tumor cell suspensions were added to an electroporation cuvette and electrofused as described previously 6 The electrofusion product was then irradiated with 20,000 rads with an RS 2000 Biological Irradiator X‐ray irradiator (Rad Source Technologies Inc., Boca Raton, FL, USA) to ensure inactivation of the tumor cells and DCs. The irradiated product was injected without any further manipulation or enrichment. The dosing was based on the input number of cells that underwent the electrofusion process (e.g., a dose of 5 × 105 corresponds to the electrofusion product of 2.5 × 105 DCs and 2.5 × 105 tumor cells). The electrofusion efficiency was evaluated by flow cytometry on a FACS Calibur system (Becton Dickinson, San Diego, CA, USA) and was defined as the percentage of cells that stained positive for both a tumor cell marker (gp100) and a DC surface marker (CD11b). Fusion efficiencies typically ranged from 5% to 20% ( Figure 1 ).
Figure 1.
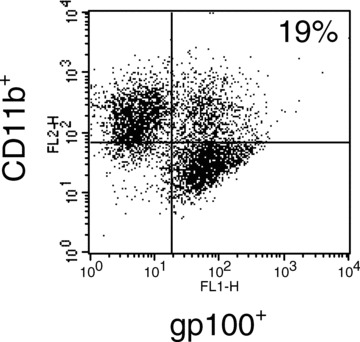
FACS characterization of electrofusion product. Fusion efficiency was evaluated by flow cytometry measuring the percentage of double positive cells expressing both gp 100 (tumor cell marker) and CD11 b (DC marker). A representative FACS plot is shown. Fusion efficiencies ranged from 5% to 20%.
Immunization with electrofusion vaccine and tumor challenge
Groups of 10 mice were immunized with 5.0 × 105 electrofused cells delivered intradermally in a total volume of 200 μL divided between two sites on the abdomen. In the pretreatment model, the animals were immunized twice, on days 0 and 14. The electrofusion product was prepared fresh each time. A lethal s.c. tumor challenge with M3 tumor cells (1.5 × 104) was performed on day 21. Rechallenge of surviving mice was performed with the same number of M3 tumor cells or with 2 × 105 P815 tumor cells. In the therapeutic model, tumor cells were injected on day 0 and vaccination with electrofusion product was performed on days 3, 7, and 14. Tumor size was measured twice a week with electronic digital calipers and animals were sacrificed when tumor size reached ⋛ 175 mm2. Kaplan‐Meier survival analysis was performed using the GraphPad Prism version 4.0 software (San Diego, CA, USA).
Immunization with gp100‐expressing allogeneic DCs
Bone marrow‐derived DCs from Balb/c mice were transduced with an adenovirus vector encoding gp100 17 at a multiplicity of infection of 500 for 24 h. Transduced DCs were injected s.c. into C57BL/6 mice (3 × 104 or 3 × 105 DCs in 100 μL, PBS). Two weeks later, pooled spleen cells from each group of DC‐immunized mice, or untreated mice as a negative control, were analyzed for gp100‐reactivity by ELISPOT as described previously 17 Briefly, spleen cells were plated in the wells of 96‐well nitrocellulose filter plates coated with rat antimouse interferon‐γ (IFN‐γ) capture antibody (Biosource International, Camarillo, CA, USA) and were stimulated for 48 hours with an MHC Class I‐restricted gp100 peptide (amino acid 25–33; KVPRNQDWL) or an irrelevant ovalbumin peptide as a negative control (amino acid 257–264; SIINFEKL). The cells were then removed by washing with PBS and the presence of IFN‐γ‐producing T cells was detected by the addition of biotinylated rat antimouse IFN‐γ (Pharmingen) followed by alkaline‐phosphatase‐conjugated streptavidin (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA). The number of stained spots was enumerated under a dissecting microscope.
Cytokine production
To assess the production of cytokines by tumor cells electrofused to DCs versus tumor cells undergoing the electrofusion process alone, irradiated fusion products were cultured in 12 well‐plates (1.5 × 106 cells/well in 1.5 mL), supernatants were collected after 72 hours and analyzed for cytokine content using the Millipore Multiplex cytokine analysis kit (St. Charles, MO, USA) and a Bioplex plate reader (Bio‐Rad, Hercules, CA, USA) according to the manufacturers instructions. Cultures were conducted in triplicate.
Results
Comparison of tumor cell lines and primary tumor cells as fusion partners
We have reported previously that electrofusion vaccines consisting of bone marrow‐derived DCs electrofused to cultured tumor cell lines induced significant levels of antitumor protection in murine tumor models. 6 Similar findings have been reported by others using a variety of tumor lines. 7 , 8 , 9 , 10 However, in clinical situations, the tumor cell component of electrofusion vaccines is commonly derived from an excised tumor mass containing several cell types in addition to tumor cells, including fibroblasts, endothelial cells, and stromal cells. 11 , 12 , 13 , 14 Therefore, the M3 melanoma tumor model was used to determine whether the use of tumor cell lines to generate electrofusion vaccines in animal models is an adequate substitute for the heterogeneous material commonly used in clinical vaccines. Single cell suspensions from primary M3 tumors or cultured M3 melanoma cells were fused to allogeneic DCs generated from H‐2b C57BL/6 mice and compared for their ability to induce antitumor protection. As shown in Figure 2, unvaccinated mice in the naïve group all developed progressively growing tumors. In contrast, immunization with electrofusion vaccines generated with either the tumor cell line or primary tumor cells provided significant antitumor protection resulting in a 90% to 100% survival rate (p < 0.0001). These results indicate that cells from a cultured tumor line and cells from primary tumor samples both represent adequate fusion partners for the production of electrofusion vaccines.
Figure 2.
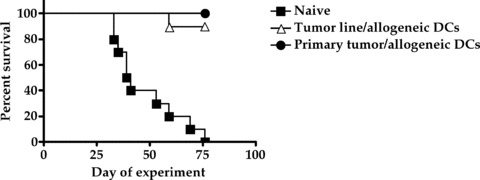
Comparison of tumor cell lines and primary tumor cells as fusion partners. Groups of 10 mice were immunized with 5.0 × 105 electrofused cells on days 0 and 14. A lethal subcutaneous tumor challenge with M3 tumor cells was performed on day 21. Tumor size was measured twice a week. Immunization with either of the electrofusion vaccines resulted in a statistically significant increase in survival compared to vehicle‐treated mice (p < 0.0001).
Comparison of syngeneic, semi‐allogeneic, and fully allogeneic electrofusion vaccines
One of the limiting factors in the production of electrofusion vaccines is the requirement for tumor material from the patient. Access to primary tumors or secondary metastases requires surgery and the amount of tumor cells recovered restricts the number of vaccinations that can be delivered to the patient. As demonstrated above, tumor cell lines are an adequate substitute for primary tumor cells and the use of a cultured allogeneic tumor cell line expressing tumor‐associated antigens shared by a given tumor type could circumvent the practical issues surrounding tumor acquisition. However, since direct MHC‐restricted presentation of tumor antigens has been generally believed to be provided by the fusion hybrids, generation of autologous DCs from the patient or an MHC‐matched donor would still be required. Testing of fully allogeneic hybrids, to our knowledge, has not been investigated. The M3 melanoma model was therefore used to test the potential of a fully allogeneic vaccine and compare its activity to the more “conventional” fusion vaccines where one or both fusion partners are syngeneic to the host. DBA/2 mice were immunized with fusion vaccines composed of either syngeneic (M3, H‐2d) or allogeneic (B16, H‐2b) melanoma tumor cells electrofused to either syngeneic (DBA/2, H‐2d) or allogeneic (C57BL/6, H‐2b) dendritic cells. After two administrations of vaccine (days 0 and 14), mice were challenged with a lethal s.c. dose of M3 tumor cells (day 21). As shown in Figure 3, immunization of mice with a fully syngeneic vaccine resulted in a strong level of protection from tumor challenge (62% rejection). In agreement with our previous findings, 6 a comparable level of protection was obtained with a vaccine consisting of syngeneic tumor cells electrofused to allogeneic DCs (67% rejection). Similarly, the combination of allogeneic tumor cells with syngeneic DCs resulted in a high level of protection from tumor challenge (100% rejection) as reported by others in various tumor systems. 9 , 10 , 18 , 19 Surprisingly, immunization with a fully allogeneic vaccine also gave rise to robust antitumor protection (100% rejection). Similar antitumor activity by a fully allogeneic vaccine was reproduced in two separate experiments. This result argues that direct MHC‐restricted antigen presentation by the fusion hybrids is not necessarily required for efficacy and that reprocessing of the vaccine by host antigen‐presenting cells is also likely to be occurring.
Figure 3.
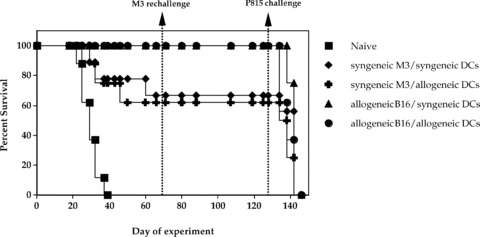
Comparison of syngeneic, semi‐allogeneic, and fully allogeneic electrofusion vaccines in the M3 tumor model. Groups of 8–10 DBA/2 mice were immunized with fusion vaccines composed of either syngeneic (M3, H‐2d) or allogeneic (B16, H‐2b) melanoma tumor cells electrofused to either syngeneic (DBA/2, H‐2d) or allogeneic (C57BL/6, H‐2b) DCs (5.0 × 105 electrofused cells) on days 0 and 14. The animals were then challenged with M3 tumor cells on day 21 and tumor size was measured twice a week. All vaccine configurations induced statistically significant antitumor protection compared to the unvaccinated control mice (p values ranging from <0.0001 to 0.0025). Although the two vaccines produced with allogeneic tumor cells (semi‐allogeneic and fully allogeneic) appeared to provide greater antitumor protection than the two vaccines prepared with syngeneic tumor cells (100% vs. 62%–67% survival), the difference in survival did not reach statistical significance (p= 0.0628). To assess specificity of the response, mice were rechallenged with M3 tumor cells on day 71 and challenged again on day 129 with PI 85, an unrelated syngeneic tumor line. As expected from a specific immune response, M3 tumor cells were rejected while the mice succumbed to challenge with unrelated P815 cells.
To ascertain whether a memory immune response developed as a result of vaccination, all surviving mice were rechallenged with a second dose of M3 tumor cells on day 71 ( Figure 3 ). In all groups, mice that rejected the primary M3 tumor cell challenge were resistant to rechallenge indicating that immunological memory was induced by all the vaccine configurations. To confirm the specificity of the immune response, mice were challenged again on day 129 with a lethal s.c. dose of P815 cells, an unrelated syngeneic tumor cell line ( Figure 3 ). All mice quickly succumbed to tumor challenge thereby verifying that the immune response induced by each of the vaccines was specific for shared M3/B16 melanoma‐associated antigens and that rejection of M3 tumor cells was not simply due to nonspecific immune activation. Taken together, these data demonstrate equivalent induction of specific antitumor immunity by syngeneic, semi‐allogeneic and, most interestingly, fully allogeneic electrofusion vaccines.
Therapeutic activity of an electrofusion vaccine generated with allogeneic tumor and DC cell lines
The potential use of a fully allogeneic electrofusion vaccine using tumor lines and donor DCs presents definite practical advantages but nevertheless still requires the use of human tissue to generate DCs. An attractive alternative would be to utilize both a tumor cell line and a DC line to produce fusion vaccines. Such a vaccine could be produced using well‐characterized cell lines without any requirement for blood or tumor tissues. This possibility was modeled in the M3 tumor model using the DC‐like JAWS II cell line and comparing its activity to bone marrow‐derived DCs as a fusion partner for tumor cell lines. Electrofusion vaccines were prepared with either syngeneic components (M3 tumor cells and DBA/2 DCs, H‐2d) or fully allogeneic components comprising cultured B16 melanoma tumor cells (H‐2b) electrofused to primary C57BL/6 DCs (H‐2b) or cells from the JAWS II line (H‐2b). In these studies, the vaccines were tested under a therapeutic as opposed to a prophylactic regimen to better reflect the clinical situation. DBA/2 mice were first challenged with a lethal dose of M3 tumor cells (day 0) and were subsequently treated with the electrofusion vaccines (days 3, 7, and 14). As expected, the degree of tumor growth inhibition of established tumor cells was not as great as that observed in a prophylactic setting but significant levels of tumor rejection and enhanced survival were obtained with all three vaccines compared to control (p < 0.0011, Figure 4 ). Interestingly, the vaccine generated with the two allogeneic lines produced the greatest degree of tumor growth inhibition although the difference in survival between the different vaccines did not reach statistical significance. These results suggest that fusion vaccines generated between well‐characterized tumor lines and a DC cell line may represent a viable, practical alternative to the current custom‐made, patient‐specific vaccines.
Figure 4.
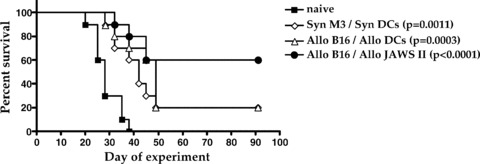
Therapeutic activity of an electrofusion vaccine generated with allogeneic tumor and DC cell lines. Electrofusion vaccines were prepared with either syngeneic components (M3 tumor cells and DBA/2 DCs, H‐2d) or fully allogeneic components comprising cultured B16 melanoma tumor cells (H‐2b) electrofused to primary C57BL/6 DCs (H‐2b) or cells from the JAWS II line (H‐2b; ATCC). Groups of 10 DBA/2 mice were first challenged with a lethal s.c. dose of M3 tumor cells and were then treated with 5.0 × 105 electrofused cells on days 3, 7, and 14. Tumor size was measured twice a week. All vaccine configurations induced statistically significant antitumor protection compared to the unvaccinated control mice (p values ranging from <0.0001 to 0.0011). The allogeneic cell line vaccine provided the greatest degree of protection (60% vs. 20% survival) but the difference with the other two vaccines was not statistically significant (p= 0.17).
Exploration of mechanism of action of allogeneic electrofusion vaccines
The ability of a fully allogeneic electrofusion vaccine to induce antitumor immunity suggests that reprocessing of the vaccine by host antigen‐presenting cells is taking place. This concept is supported by results from additional studies demonstrating the existence of cross‐presentation from allogeneic antigen‐containing DCs. As shown in Figure 5, C57BL/6 mice (H‐2b) immunized with fully allogeneic Balb/c (H‐2d) DCs transduced with an adenovirus vector expressing the gp 100 melanoma antigen were induced to develop a specific CD8+ T cell response against gp100 as determined by ELISPOT. The observed stimulation of antigen‐specific T cells by allogeneic DCs incapable of direct MHC‐restricted presentation implicates reprocessing and cross‐presentation of antigen as operative mechanisms in the host.
Figure 5.
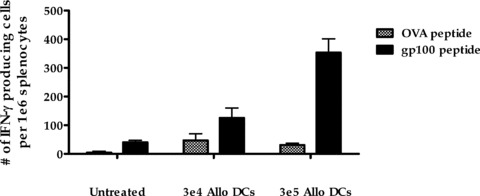
Antigen cross‐presentation by allogeneic DCs. Bone marrow‐derived DCs from Balb/c mice transduced with an adenovirus vector encoding gp 100 were injected s.c. into C57BL/6 mice. The induction of gp 100‐reactivity in the spleen was assessed 2 weeks later by ELI SPOT. Spleen cells were stimulated with an MHC Class l‐restricted gp 100 peptide or an irrelevant ovalbumin peptide as a negative control and the number of cells induced to produce IFN‐γ was enumerated. Results shown are the mean ± SEM of triplicate wells.
As reported by ourselves and others, the DC component of fusion vaccines is required for efficacy and the antitumor response induced is not simply due to immunization with inactivated tumor cells. 3 , 4 , 6 The actual role of the DC component in an allogeneic fusion vaccine that is reprocessed by the host remains to be defined. As described above, the allogeneic DCs can act as a source of antigen for cross‐presentation. It is also possible that the DCs (fused and unfused) may act by producing cytokines that deliver a “danger signal” and promote the development of cell‐mediated immune responses. To test this possibility, culture supernatants from B16 tumor cells electrofused with DCs, compared to B16 tumor cells undergoing the electrofusion process alone, were collected and analyzed for cytokine content. The results indicate that DCs electrofused with tumor cells do in fact produce significant amounts of pro‐inflammatory cytokines and chemokines with little or no cytokine release by tumors cells that underwent the electrofusion process alone ( Table 1 ).
Table 1.
Cytokine release (pg/mL ± SD) from electrofused cells.
| B16 cells alone | B16/DBADC | B16/C57 DC | |
|---|---|---|---|
| IL‐1 | 18 ± 5 | 1234 ± 64* | 6509 ± 242* |
| IL‐6 | 132 ± 42 | 100 ± 19 | 490 ± 49* |
| KC | 376 ± 184 | 1631 ± 261* | 8836 ± 87* |
| MCP‐1 | 22 ± 4 | 128 ± 29 | 3916 ± 209* |
| MIP‐1β | 32 ± 7 | 81 ± 19* | 1848 ± 21* |
| TNFα | 29 ± 4 | 345 ± 80* | 1647 ± 61* |
| IFN‐γ | 94 ± 6 | 103 ± 25 | 175 ± 23* |
| IL‐12 | 157 ± 18 | 138 ± 14 | 192 ± 34 |
*p < 0.05 versus B16 cells alone.
Discussion
Fusion vaccines generated from tumors cells and DCs represent a promising approach to cancer immunotherapy However, their wide scale application involves significant practical and technical challenges. Production of electrofusion vaccines typically requires the acquisition and processing of patient and/or donor material and must be tailored to each individual. To address these issues, alternative vaccine configurations utilizing allogeneic tissue or cell lines were explored in this study. First, it was determined that cells from a cultured tumor line and cells from primary tumor samples both represent adequate fusion partners for DCs as the resulting electrofusion vaccines induced equivalent levels of protection from tumor challenge in the M3 melanoma tumor model. Cultured allogeneic tumor cell lines expressing tumor‐associated antigens shared by a given tumor type could therefore be used to circumvent the practical issues surrounding surgical acquisition of tumor material from the patient. Indeed, the fusion product of allogeneic tumor cell lines and autologous or MHC‐matched DCs has been reported to effectively induce human CD8+ T cell responses in vitro 18 , 19 , 20 and induce tumor rejection in animals in vivo. 9 While this approach does not require the acquisition of tumor from the patient, it still requires the generation of autologous DCs from the patient or an MHC‐matched donor. Because direct MHC‐restricted antigen presentation by fusion hybrids has been postulated to account for the induction of antitumor immunity, testing of fully allogeneic hybrids, to our knowledge, has not been reported. Therefore, the M3 melanoma model was used to test the potential of a fully allogeneic vaccine compared to fusion vaccines where one or both fusion partners are syngeneic to the host. Interestingly, our results clearly showed that fully allogeneic vaccines were as efficacious as syngeneic or semi‐allogeneic vaccines and reproducibly provided equivalent levels of antitumor protection ( Figure 3). Moreover, a DC‐like cell line (JAWS II) was found to represent an adequate substitute for primary DCs in the fusion process ( Figure 4 ).
The latter result suggests that “off‐the shelf” fusion vaccines generated between well‐characterized tumor and DC cell lines may represent a viable, practical alternative to the current custom‐made, patient‐specific vaccines. It has been generally assumed that the tumor/DC hybrids themselves are responsible for directly presenting antigen to host T cells in an MHC‐restricted manner. Our findings clearly show that direct antigen presentation by the hybrids, although it may occur, is not an absolute requirement for induction of specific antitumor responses as evidenced by the activity of fully allogeneic vaccines. The most obvious explanation for this observation is the likely reprocessing of the vaccine by host antigen‐presenting cells. The existence of such a mechanism is supported by our results showing induction of a gp100‐specific response following immunization with fully allogeneic DCs expressing the gp100 antigen ( Figure 5). In a recent review, Shu et al. 21 have similarly suggested that, given the low percentage of documented hybrids in most fusion vaccines, antigen presentation by host cells is likely to be involved in the process.
It mustbe noted that ourselves and others have observed that the DC component of the fusion vaccine is required for efficacy and that the antitumor response induced is not simply due to immunization with inactivated tumor cells. 3 , 4 , 6 The role of the DC component in a fusion vaccine that is reprocessed by the host remains to be defined. As suggested by the results in Table 1, it is possible that, rather than acting primarily as antigen‐presenting cells, the DCs (fused and unfused) may also act by producing cytokines and chemokines that deliver a “danger signal” and promote the development of cell‐mediated immune responses. The exact mechanism of action requires further investigation but the possibility of producing well‐characterized fusion vaccines from allogeneic cell lines represents a significant advance in simplifying the manufacturing of electrofusion vaccines and expanding their applicability.
Conclusion
Our results indicate that fully allogeneic electrofusion vaccines can induce antitumor immunity equivalent to that of syngeneic or semi‐allogeneic fusion products. This finding introduces the possibility of using defined allogeneic tumor and DC lines to simplify vaccine manufacturing.
Acknowledgments
The authors wish to thank the Genzyme Department of Comparative Medicine for the animal care provided.
References
- 1. Kaplan JM. New cancer vaccine approaches. Drugs Today. 2004; 40: 913–929. [DOI] [PubMed] [Google Scholar]
- 2. Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. NotMed 1997; 3: 558–561. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T‐cell immunity against poorly immunogenic tumors by immunization with dendritic cell‐tumor fusion vaccines. J Immunol. 1998; 161: 5516–5524. [PubMed] [Google Scholar]
- 4. Lespagnard L, Mettens P, Verheyden A‐M, Tasiaux N, Thielemans K, Van Meirvenne S, Geldhof A, De Baetselier P, Urbain J, Oberdan L, Moser M. Dendritic cells fused with mastocytoma cells elicit therapeutic antitumor immunity. IntJ Cancer. 1998; 76: 250–258. [DOI] [PubMed] [Google Scholar]
- 5. Akasaki Y, Kikuchi T, Homma S, Abe T, Kufe D, Ohno T. Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J Immunother. 2001; 24 106–113. [PubMed] [Google Scholar]
- 6. Siders WM, Vergilis KL, Johnson C, Shields J, Kaplan JM. Induction of specific antitumor immunity in the mouse with the electrofusion product of tumor cells and dendritic cells. Mol Ther. 2003; 7 498–505. [DOI] [PubMed] [Google Scholar]
- 7. Orentas PJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001; 212: 4–13. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi T, Tanaka H, Tanaka J, Wang R, Averbook BJ, Cohen PA, Shu S. Immunogenicity and therapeutic efficacy of dendritic‐tumor hybrid cells generated by electrofusion. Clin Immunol. 2002; 104: 14–20. [DOI] [PubMed] [Google Scholar]
- 9. Lee WT, Shimizu K, Kuriyama H, Tanaka H, Kjaergaard J, Shu S. Tumor‐dendritic cell fusion as a basis for cancer immunotherapy. Otoloryngol Head Neck Surg. 2005; 132: 755–764. [DOI] [PubMed] [Google Scholar]
- 10. Yasuda T, Kamigaki T, Kawasaki K, Nakamura T, Yamamoto M, Kanemitsu K, Takase S, Kuroda D, Kim Y, Ajiki T, Kuroda Y. Superior anti‐tumor protection and therapeutic efficacy of vaccination with allogeneic and semiallogeneic dendritic cell/tumor cell fusion hybrids for murine colon adenocarcinoma. Cancer Immunol Immunother. 2007; 56: 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kugler A, Stuhler G, Walden P, Zuller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert R‐H. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell‐dendritic cell hybrids. Nature Med. 2000; 6: 332–336. [DOI] [PubMed] [Google Scholar]
- 12. Haenssle HA, Krause SW, Emmert S, Zutt M, Kretschmer L, Schmidberger H, Andreesen R, Soruri A. Hybrid cell vaccination in metastatic melanoma. Clinical and immunologic results of a Phase l/l study. J Immunother 2004; 27: 147–155. [DOI] [PubMed] [Google Scholar]
- 13. Avigan DE, Vasir B, George DJ, Oh WK, Atkins MB, McDermott DF, Kantoff PW, Figlin RA, Vasconcelles MJ, Xu Y, Kufe D, Bukowski RM. Phase l/ll study of vaccination with electrofused allogeneic dendritic cells/autologous tumor‐derived cells in patients with stage IV renal cell carcinoma. J Immunother. 2007; 30: 749–761. [DOI] [PubMed] [Google Scholar]
- 14. Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sharav T, Sparbier K, Sterry W, Walden P. Tumour‐dendritic hybrid cell vaccination for the treatment of patients with malignant melanoma immunological effects and clinical results. Vaccine. 2005; 23: 2367–2373. [DOI] [PubMed] [Google Scholar]
- 15. Marten A, Renoth S, Heinicke T, Albers P, Paufi A, Mey U, Caspari R, Flieger D, Hanfland P, Von Ruecker A, Eis‐Hubinger AM, Muller S, Schwaner I, Lohmann U, Heylmann G, Sauerbruch T, Schmidt‐Wolf IGH. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase l/ll trial in patients with metastatic renal cell carcinoma. Human Gene Ther. 2003; 14:483–494. [DOI] [PubMed] [Google Scholar]
- 16. Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, Atkins M, Mier J, McDermott D, Smith I Giallambardo N, Stone C, Schadt K, Dolgoff J, Tetreault J‐C, Villaroel M, Kufe D. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004; 10: 4699–4708. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan JM, Yu Q, Piraino ST, Pennington SE, Shankara S, Woodworth LA, Roberts BL. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector encoding endogenous tumor‐associated antigens. J Immunol. 1999; 163:699–707. [PubMed] [Google Scholar]
- 18. Parkhurst MR, DePan C, Riley JP, Rosenberg SA, Shu S. Hybrids of dendritic cells and tumor cells generated by electrofusion simultaneously present immunodominant epitopes from multiple human tumor‐associated antigens in the context of MHC Class I and Class II molecules. J Immunol. 2003;170:5317–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto S, Saito H, Tsujitani S, Ikeguchi M. Allogeneic gastric cancer cell‐dendritic cell hybrids induce tumor antigen (carcinoembryonic antigen) specific CD8+ T cells. Cancer Immunol Immunother. 2006; 55: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Ma B, Zhou Y, Zhang M, Qiu X, Sui Y, Zhang X, Ma B, Fan Q. Dendritic cells fused with allogeneic breast cancer cell line induce tumor antigen‐specific CTL responses against autologous breast cancer cells. Breast Cancer Res Treat. 2007; 105: 277–286. [DOI] [PubMed] [Google Scholar]
- 21. Shu S, Zheng R, Lee WT, Cohen PA. Immunogenicity of dendritic‐tumor fusion hybrids and their utility in cancer immunotherapy. Crit Rev Immunol. 2007; 27: 463–483. [DOI] [PubMed] [Google Scholar]


