Abstract
Impulsivity, a risk factor for substance abuse disorders, is modulated by the Val158 variant of the catechol‐O‐methyltransferase (COMT) gene. Rodent studies have shown that opioids enhance impulsivity. Furthermore, alcohol consumption leads to endogenous opioid release in the cortex and nucleus accumbens (NAc), and this opioid release is correlated with greater positive hedonic effect. Using the selective mu opioid receptor radioligand [11C] carfentanil, we find that, following alcohol consumption, individuals with the COMT Val158 allele have greater opioid release in the right NAc but less release in medial orbital frontal cortex (OFC). These data suggest that genetic regulation of dopamine levels can affect alcohol consumption in part by modulating endogenous opioid release in specific brain regions implicated in reward, which in turn promotes impulsive choice.
Keywords: alcohol, impulsivity, dopamine, positron emission tomography, mu opioid receptor
Introduction
Alcohol abuse is a common problem with major neurological and socioeconomic consequences. Impulsivity, a risk factor for alcohol abuse, is enhanced in individuals with the Val158 variant of the dopamine‐degrading enzyme, catechol‐O‐methyltransferase (COMT).1, 2, 3 This variant of COMT encodes an enzyme with elevated activity and, consequently, lower dopamine levels in the frontal cortex.4 We recently demonstrated that impulsivity can be reduced by inhibition of COMT using the brain penetrant enzyme inhibitor, tolcapone, which should increase frontal cortex dopamine levels.5
A relationship between impulsivity and fMRI BOLD activity in the OFC during decision making has been demonstrated in alcoholics, such that those most willing to wait for a delayed reward have greater OFC activity than those that act impulsively.1 Additionally, the COMT Val158Met genotype predicts activity in the prefrontal cortex (PFC) during performance of this decision making task; Val/Val homozygotes are both more impulsive and have greater dorsal PFC activity during task performance than either heterozygotes or Met/Met homozygotes.
The COMT Val158Met genotype affects opioid systems in the human brain, with Val/Val homozygotes displaying less mu opioid receptor expression, but greater Met‐enkephalin expression, in several brain regions, including the nucleus accumbens (NAc).6, 7 Additionally, the ability to wait for delayed rewards is accompanied by increased BOLD activity in the OFC and insula5 and decreased opioid binding in the NAc,8 and both ability to wait and BOLD activity in the OFC are enhanced by opioid blockade.9 A tendency to choose impulsively is also associated with increased BOLD signal in the NAc.10, 11 Furthermore, animal studies suggest that an opioid action within the ventral striatum can profoundly influence impulsive choice. For example, lesions of the NAc produce impulsivity in rodents,12 while opioid release in the NAc contributes to reward value13 and systemic opioid administration promotes impulsive choice.14 Together these findings raise the possibility that the differences in impulsivity that correlate with the COMT Val158Met genotype may be mediated through opioid release in PFC and/or NAc.
Here, we assessed endogenous opioid release by imaging mu opioid receptors with [11C] carfentanil before and after a drink of alcohol. We then correlated these changes with COMT genotype to determine if genetic variation regulates endogenous opioid release in brain regions implicated in intertemporal choice.
Materials and Methods
Subjects
Heavy social drinkers (n = 13) and matched healthy control subjects (n = 12) were recruited online as previously described.15 In brief, heavy drinking subjects consumed 10–16 drinks/week (women) or 14–20 drinks/week (men). Control subjects all consumed less than five drinks per week (women) or seven drinks per week (men). Subjects were required to fast for 4 hours and remain abstinent from alcohol for 3 days prior to scanning. The alcohol use disorders identification test (AUDIT) was used to assess hazardous drinking and the Barratt impulsivity scale (BIS) was used to quantify impulsivity. All participants gave written, informed consent and were paid for their participation.
Image acquisition
The mu opioid receptor selective agonist [11C] carfentanil was synthesized with 11CH3I at the Biomedical Isotope Facility, Lawrence Berkeley National Laboratory, as previously described.15 Dynamic PET data were collected for 90 minutes with a Siemens‐CTI ECAT EXACT (model 921) 47‐section scanner in 3D acquisition mode. An intravenous catheter was inserted for tracer injection and was kept in place for the duration of the experiment. Each scan entailed the injection of 10–15 mCi of high specific activity (average = 10577.38 Ci/mMole) radiotracer.
After the first scan (prealcohol), subjects were removed from the scanner, given 5 minutes to consume a standardized drink of alcohol, and were then immediately repositioned in the scanner for a second imaging sequence identical to the first.15 Temporal spacing of six radiotracer half‐lives between scans assured lack of contamination of the second scan (postalcohol) by the first. Due to the time required for alcohol to be fully metabolized, scans could not be randomized. Data were reconstructed using an ordered subset expectation maximization (OSEM) algorithm with weighted attenuation, an image size of 256 × 256, and 6 iterations with 16 subsets. A Gaussian filter with 6‐mm full width at half maximum was applied, with a scatter correction. Images were evaluated for patient motion and adequacy of statistical counts. To improve spatial localization of PET data, MRI images were collected for anatomical reference.
Data analysis
A Region of Interest (ROI) approach combined automated and manually defined ROIs, as previously described.15 In brief, PET data were preprocessed using SPM8 software. For each time point, frames corresponding to the first 20 minutes of CFN acquisition were realigned, averaged, and used to coregister to MRI data. The medial OFC ROI was derived using FreeSurfer (http://surfer.nmr.mgh.harvard.edu). NAc ROIs were hand drawn in native space following a previously described technique.16 Medial OFC (mOFC) and NAc ROIs were resliced into PET space and average binding potential values were created for use in subsequent analyses by employing a Logan graphical analysis approach17 and endogenous opioid release was defined as the difference between the prealcohol and postalcohol binding potentials. Regression analysis (with genotype as the independent variable) and t‐tests were conducted using both Microsoft Excel 2004 and Matlab 2008.
Results
The COMT Val158 allele showed a significant positive correlation with endogenous opioid release following alcohol consumption in the right NAc (r = 0.45, p = 0.02, n = 25; Figure 1 A) and a significant negative correlation in both the left mOFC (r = 0.44, p = 0.03, n = 25; Figure 1 B) and right mOFC (r = 0.48, p = 0.02, n = 25; Figure 1 C), brain regions that contribute to the rewarding effects of alcohol.15 Specifically, Val/Val subjects displayed greater opioid release in the right NAc (t = 3.25, p = 0.009) and less opioid release in both left (t = 2.34, p = 0.041) and right (t = 2.60, p = 0.027) medial OFC following alcohol consumption compared to Met/Met homozygotes. This pattern was independent of drinking history, as no correlation was found between COMT Val158 genotype and reported level of alcohol consumption. Additionally, although we found a significant positive correlation between hazardous drinking and impulsivity (r = 0.46, p = 0.02, n = 25; Figure 2), our sample was not sufficiently powered to confirm the previously reported relationship between COMT Val158 genotype and impulsivity.1
Figure 1.
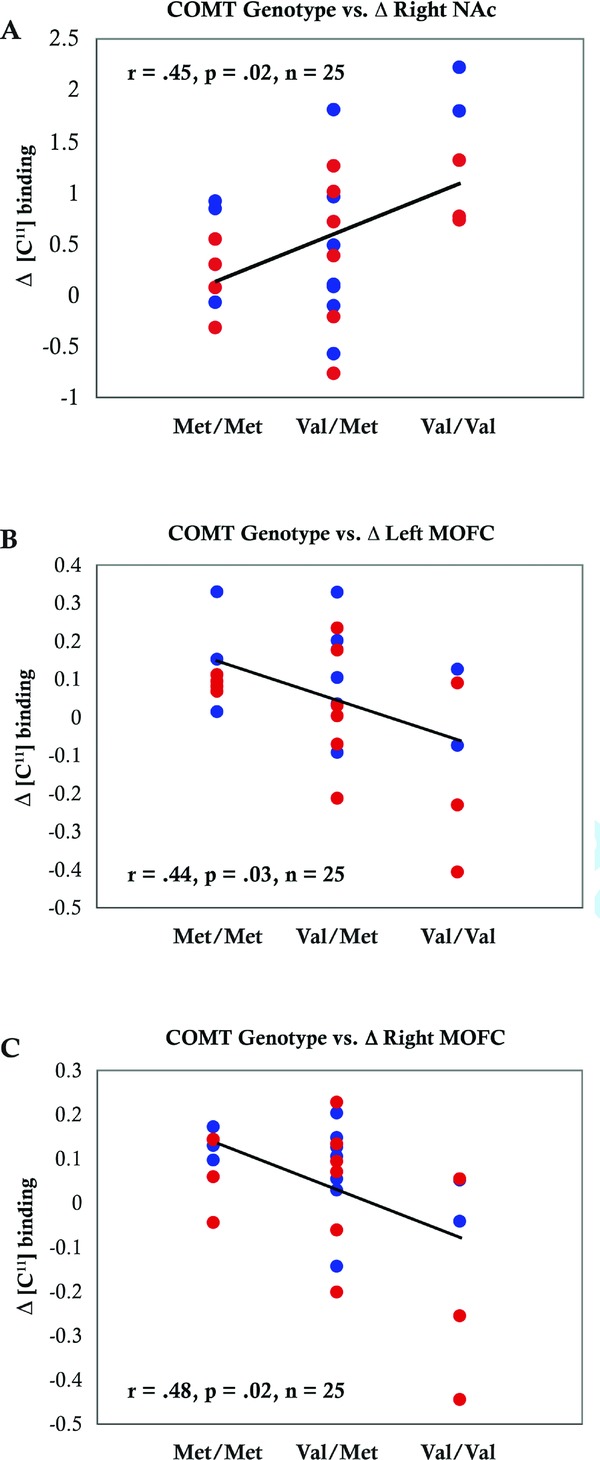
Relationship of the COMT Val158 genotype to endogenous opioid release following alcohol consumption. Endorphin release is significantly greater in the right NAc (A: t = 3.25, p = 0.009) and significantly lower in the left mOFC (B: t = 2.34, p = 0.041) and right mOFC (C: t = 2.60, p = 0.027) of individuals with the Val/Val genotype. Blue circles indicate control subjects, red circles indicate heavy social drinkers.
Figure 2.
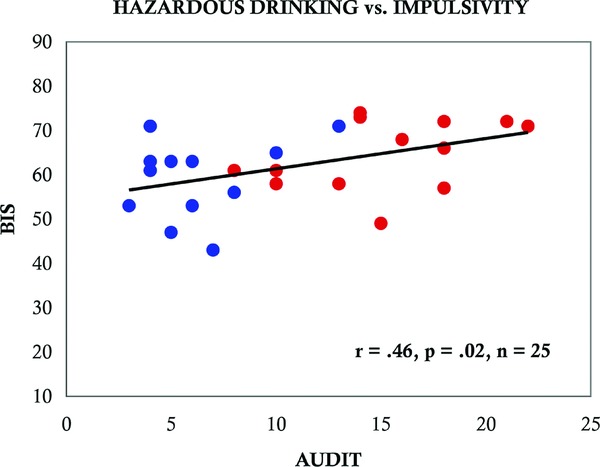
A significant positive correlation was identified between impulsivity (Barratt impulsivity scale; BIS) and problem alcohol use (alcohol use disorders identification test; AUDIT); more impulsive individuals had increased hazardous alcohol use (r = 0.46, p = 0.02, n = 25). Blue circles indicate control subjects, red circles indicate heavy social drinkers.
There was no significant effect of group, no significant gender difference in distribution of genotype, and no sex difference with respect to endogenous opioid release and [11C] carfentanil binding.
Contrary to recently published data,6, 7 we found no difference in basal number or distribution of mu opioid binding sites across COMT genotypes in the brain regions examined, suggesting that within this subject cohort the COMT Val158 genotype primarily regulates endogenous ligand release, independent of receptor expression.
Discussion
The COMT Val158 polymorphism regulates cortical DA levels, such that individuals with the Val/Val genotype have higher enzymatic activity and thus presumably lower PFC dopamine4 and increased impulsivity.9 Our data raise the interesting possibility that the impulsivity phenotype seen in individuals with the Val/Val genotype is mediated through dopamine regulation of endogenous opioid release in the NAc and PFC; two brain regions important for reward valuation and intertemporal choice. This interpretation is also consistent with our previous finding that, following alcohol consumption, opioid release in the NAc is correlated with a greater positive hedonic effect. The present data also suggest that drinking alcohol would be experienced as more rewarding and lead to greater alcohol consumption in individuals with the Val/Val genotype because these subjects have greater endogenous opioid release in the NAc in response to alcohol consumption. Additionally, the current data are consistent with previous reports that the Val/Val158 variant of COMT is a risk factor for alcohol abuse and dependence in certain genetically homogeneous populations.2, 3
Results from a previous study, in which endogenous opioid function was blocked during performance of a monetary decision‐making task, suggested that frontal dopamine levels are inversely related to the frequency of making impulsive choices.18 This idea is directly supported by the recent observation that tolcapone, an inhibitor of COMT that increases dopamine levels in the frontal cortex,4 decreases impulsive choice.5 Together with the current data, these findings raise the intriguing possibility that, by potentiating frontal dopamine, tolcapone might effectively decrease alcohol consumption and impulsivity in individuals with the Val/Val genotype.
Recent findings indicate that a COMT inhibitor most effectively reduces impulsivity in highly impulsive subjects, but may have opposite effects in nonimpulsive subjects.5 Similarly, a COMT inhibitor improves executive function in Val/Val homozygotes, while impairing cognitive function in Met/Met homozygotes.19 These results are in keeping with an inverted U‐shaped curve for dopamine function, in which the effects of a dopaminergic agent depend on baseline dopamine levels. Subjects with optimal dopamine tone may actually experience impaired task performance when dopamine levels are potentiated, while those with basal dopamine hypofunction will experience improvement when dopamine levels are increased. In finding that subjects with greater endogenous opioid release in the NAc following alcohol consumption have lower basal dopamine tone, our data suggest that a COMT inhibitor might decrease not only impulsivity but alcohol reward as well.
There is a robust projection from the OFC to the NAc20 that is impaired in abstinent alcoholics.21 Additionally, heroin abusers display dysfunctional OFC activation during a decision‐making task22 and in rodent studies microinjection of mu opioids into the NAc increases consumption of both alcohol23 and palatable food.24 Taken together with the current data, these results suggest that dysfunction of the opioid pathway at the level of the OFC and NAc negatively impacts decision making by increasing impulsivity, and may affect propensity to consume alcohol in individuals with the Val/Val genotype.
It is interesting that the Val/Val variant at COMT Val158 is also associated with greater NAc endogenous opioid release in response to prolonged muscular pain6 and that robust COMT Val158 genotype‐related differences in brain activation have been found following exposure to painful thermal stimuli.25 Individuals with the Val/Val variant report lower pain intensity levels, suggesting that endogenously released opioids exert an analgesic effect. These data are consistent with animal studies showing that NAc opioids can suppress pain responses.26
Conclusions
Individuals with the COMT Val158 Val/Val genotype show greater opioid release in the right NAc and less in the medial OFC following alcohol consumption, suggesting that cortical dopamine levels can influence alcohol reward in part through altering endogenous opioid release in reward related brain regions. The results further suggest that COMT inhibition will effectively decrease alcohol consumption and impulsivity in individuals with the Val/Val158 genotype. Additional studies are required to replicate the current findings and to determine if a COMT inhibitor will attenuate alcohol consumption.
Conflict of Interest
We have no financial interests or conflicts of interest to declare.
Acknowledgments
The authors thank A. Coker, C. Teague, and I. Yen for their technical contributions to this manuscript. The authors also thank S. Jivan at UBC/TRIUMF PET Centre for the generous donation of desmethylcarfentanil. This study was supported by Department of Defense W81XWH‐07–1–0431 and by California State Funds for Research on Drug and Alcohol Abuse.
References
- 1. Boettiger C, Mitchell J, Tavares V Robertson M Joslyn G D'Esposito M, Fields H. Immediate reward bias in humans: fronto‐parietal networks and a role for the catechol‐O‐methyltransferase 158(Val/Val) genotype. J Neurosci. 2007; 27: 14383–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. Sex differences in the influence of COMT Val158Met on alcoholism and smoking in plains American Indians. Alcohol Clin Exp Res. 2006; 30: 399–406. [DOI] [PubMed] [Google Scholar]
- 3. Serý O, Didden W Mikes V Pitelová R, Znojil V Zvolský P. The association between high‐activity COMT allele and alcoholism. Neuro Endocrinol Lett. 2006; 27: 231–235. [PubMed] [Google Scholar]
- 4. Chen J, Lipska BK, Halim N Ma QD, Matsumoto M Melhem S Kolachana BS, Hyde TM, Herman MM, Apud J et al. Functional analysis of genetic variation in catechol‐O‐methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004; 75: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kayser AS, Allen DC, Navarro‐Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012; 32: 9402–9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K Xu Y Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu‐opioid neurotransmitter responses to a pain stressor. Science 2003; 299: 1240–1243. [DOI] [PubMed] [Google Scholar]
- 7. Kowarik MC, Einhäuser J Jochim B Büttner A, Tölle TR, Riemenschneider M Platzer S Berthele A. Impact of the COMT Val(108/158)Met polymorphism on the mu‐opioid receptor system in the human brain: mu‐opioid receptor, met‐enkephalin and beta‐endorphin expression. Neurosci Lett. 2012; 506: 214–219. [DOI] [PubMed] [Google Scholar]
- 8. Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009; 66: 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boettiger CA, Kelley EA, Mitchell JM, D'Esposito, M , Fields HL. Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision‐making network. Pharmacol Biochem Behav. 2009; 93: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science 2004; 306: 503–507. [DOI] [PubMed] [Google Scholar]
- 11. Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H Manuck SB.. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006; 26, 13213–13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 2001; 292: 2499–2501. [DOI] [PubMed] [Google Scholar]
- 13. Taha SA, Norsted E Lee LS, Lang PD, Lee BS, Woolley JD, Fields HL. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006; 24, 1220–1226. [DOI] [PubMed] [Google Scholar]
- 14. Kieres AK, Hausknecht KA, Farrar AM, Acheson A de Wit H Richards JB. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl). 2004; 173: 167–174. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell JM, O'Neil JP, Janabi M Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012; 4, 116ra116. [DOI] [PubMed] [Google Scholar]
- 16. Mawlawi O, Martinez D Slifstein M Broft A Chatterjee R Hwang DR, Huang Y Simpson N Ngo K Van Heertum R Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001; 21: 1034–1057. [DOI] [PubMed] [Google Scholar]
- 17. Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996; 16: 834–840. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell JM, Tavares VC, Fields HL, D'Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007; 32: 439–449. [DOI] [PubMed] [Google Scholar]
- 19. Giakoumaki SG, Roussos P, Bitsios, P . Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology. 2008; 33: 3058–3068. [DOI] [PubMed] [Google Scholar]
- 20. Haber SN, Kunishio K, Mizobuchi M, Lynd‐Balta, E . The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995; 15: 4851–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volkow ND, Wang GJ, Telang F Fowler JS, Logan J Jayne M Ma Y Pradhan K Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007; 27: 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ersche KD, Fletcher PC, Roiser JP, Fryer TD, London M Robbins TW, Sahakian BJ. Differences in orbitofrontal activation during decision‐making between methadone‐maintained opiate users, heroin users and healthy volunteers. Psychopharmacology (Berl). 2006; 188: 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barson JR, Carr AJ, Soun JE, Sobhani NC, Leibowitz SF, Hoebel BG. Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav. 2009; 98: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woolley JD, Lee BS, Taha SA, Fields HL. Nucleus accumbens opioid signaling conditions short‐term flavor preferences. Neuroscience 2007; 146: 19–30. [DOI] [PubMed] [Google Scholar]
- 25. Loggia ML, Jensen K Gollub RL, Wasan AD, Edwards RR, Kong J. The catechol‐O‐methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PLoS One. 2011; 6: e27764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt BL, Tambeli CH, Barletta J Luo L Green P Levine JD, Gear RW. Altered nucleus accumbens circuitry mediates pain‐induced antinociception in morphine‐tolerant rats. J Neurosci. 2002; 22: 6773–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]


