Abstract
Aspirin has a range of antineoplastic properties linked to inhibition of cyclooxygenase enzymes in tumor cells, platelet inhibition and to inhibition of angiogenesis. We undertook a prospective study to determine the influence of a 45‐day course of aspirin therapy on circulating and intraplatelet levels of selected proangiogenic (vascular endothelial growth factor [VEGF]) and antiangiogenic (thrombospondin‐1 [TSP‐1]) proteins, and platelet protein release in women diagnosed with breast cancer who were receiving tamoxifen therapy. Initiation of aspirin therapy increases serum and intraplatelet levels of TSP‐1 without a corresponding increase in VEGF levels. Following aspirin therapy, VEGF levels decreased (relative to pretreatment levels) while TSP‐1 returned to pretreatment levels. Plasma TSP‐1 and VEGF levels did not change on aspirin therapy. Aspirin use also decreased thrombin receptor mediated release of TSP‐1 and VEGF from platelets. The selective impact on platelet angiogenic protein content and release supports one mechanism by which aspirin can modify the angiogenic balance in women receiving tamoxifen therapy. Aspirin therapy appears to favor an overall antiangiogenic balance in women with breast cancer who are receiving tamoxifen therapy.
Keywords: platelet activation, VEGF, TSP‐1 tamoxifen, breast cancer
Introduction
The Nurse's Healthy Study has reported an association between aspirin use and a decrease in distant recurrence and improved survival in women with breast cancer who had survived a minimum of 1 year following cancer diagnosis.1 This most recent report adds to a growing literature suggesting a potential benefit for aspirin use in the prevention of breast cancer.2, 3, 4, 5, 6, 7 Aspirin's clinical benefit in patients with cancer has been linked in part to inhibition of cyclooxygenase in tumor cells.8, 9 Laboratory evidence also suggests aspirin decreases tumor angiogenesis and vascular endothelial growth factor (VEGF) levels, a protein found largely in platelets and a potent stimulator of angiogenesis.10, 11, 12, 13
In addition to direct tumor and tissue effects, aspirin also moderates agonist stimulated platelet activation. The platelet serves as a reservoir for proangiogenic proteins, such as VEGF, and antiangiogenic proteins, such as thrombospondin‐1 (TSP‐1), which can be released from platelets following activation.14, 15 In the laboratory, platelet inhibition by aspirin has been demonstrated to reduce agonist stimulated VEGF and TSP‐1 release from the platelet alpha granule suggesting a plausible mechanism by which local angiogenic protein levels might be controlled in the tumor vasculature micro‐environment in aspirin users.16 In laboratory models, platelet inhibition by aspirin and thienopyridine derivatives has been demonstrated to decrease angiogenesis, further supporting the suppression of platelet activation as a viable mechanism of influencing tumor angiogenesis.17
We have previously demonstrated that the selective endocrine receptor modulators tamoxifen and aromatase inhibitors (AIs; anastrozole, letrozole, and exemestane) have differential effects on serum angiogenic protein levels.18 In that study, tamoxifen use was associated with an increase in serum VEGF levels; a result consistent with higher platelet derived VEGF levels in tamoxifen users as compared to nonusers.19 Therefore, women receiving tamoxifen therapy might be hypothesized to derive particular benefit from aspirin associated changes in circulating angiogenic proteins. Thus, we prospectively studied the impact of aspirin therapy on circulating levels of the proangiogenic protein, VEGF, and the antiangiogenic protein, TSP‐1, as well as platelet mediated angiogenic protein release.
Materials and Methods
Twelve women with a diagnosis of breast cancer (Stage I–IV) or DCIS who were current users of tamoxifen therapy for a minimum of 90 days were enrolled in this single center study. To minimize potential confounding effects of prior therapy, a predefined interval of a minimum of 30 days since the last chemotherapy, radiation therapy or surgery was required prior to study enrollment. Current users of aspirin, antiplatelet or anticoagulation therapy were excluded from enrollment. Intermittent aspirin or nonsteroidal antiinflammatory (NSAID) users who were willing to abstain from periodic use for the duration of the study were enrolled. All study participants were queried as to their intake of prescription and over the counter medications as well as dietary supplements at each study visit. Tylenol use was allowed during the study, and no restrictions on dietary supplements were imposed.
Patients with a history of prior gastrointestinal or central nervous system bleeding or a recent (within 12 months) history of any clinically significant bleeding were excluded from the study. Patients receiving investigational agents for the treatment of breast cancer, with the exception of a gonadotropic releasing hormone (GnRH) antagonist, were not included in the study.
Patients received study vials containing noncoated acetylsalicylic acid (aspirin) 325 mg tabs at initiation of the study and following informed consent. Participants were instructed to take one aspirin daily for a total of 45 days. Study medication compliance was obtained by verbal report and patient interview at follow up visits. All adverse events reported by the patient during the duration of the study were recorded.
The study was approved by the institutional review board of University of Vermont and written informed consent meeting all federal, state and institutional guidelines was obtained from all patients. A local, study independent, data safety monitor was established prior to clinical study initiation. This clinical study is registered at clinicaltrials.gov (NCT00727948).
Blood sample collection
Venous blood samples were collected prior to initiation of aspirin therapy, at 30 and 45 days on‐therapy, and 30 days postaspirin completion. Plasma samples were collected into vacutainer tubes supplemented with 0.5 mL of 3.2% sodium citrate (for plasma). Plasma samples were mixed for 30 seconds, separated by centrifugation (3,000 rcf for 10 minutes at room temperature [RT]) and stored at –80°C. Serum samples were incubated at RT for 45 minutes, centrifuged at 2,000 rcf for 15 minutes, and stored at –80°C. Standardized sample collection was used for all subjects to minimize platelet activation during phlebotomy.
Ex vivo platelet activation
Venous blood was collected into silicon‐coated vacutainers containing 0.5 mL of 3.2% buffered sodium citrate (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Immediately following venipuncture, the blood was treated with 25 μM (final) thrombin receptor activating peptide (TRAP; SFLLRNPNDKYEPF, also referred to as TRAP14; Bachem Americas, Torrance, CA, USA) in HEPES‐Tyrode's buffer (5 mM HEPES, 137 mM NaCl, 2.7 mM NaHCO3, 0.36 mM NaH2PO4, 2 mM CaCl2, and 5 mM dextrose, pH 7.4), mixed for 30 seconds at RT, and centrifuged at 2,000 rcf for 15 minutes (30,000 g‐minutes) at RT within 10 minutes of collection. Plasma was collected and stored at –80°C.
Enzyme immunoassay
VEGF and TSP‐1 levels were measured with a quantitative sandwich enzyme immunoassay (Quantikine human VEGF kit, Quantikine human Endostatin Kit, R&D Systems, Inc. Minneapolis, MN, USA) according to the manufacturer's instructions. All measurements were performed in duplicate and the average value reported for each patient at each time point.
Statistical analysis
Data are presented as mean ± SEM. Repeated measures analysis of variance was used to compare means among the 4 time points. A significant F‐test was followed by all possible pairwise t‐tests. The quadratic effect was used to examine the effect of aspirin on the release of angiogenic proteins from platelets prior to and posttherapy compared to during aspirin therapy. The analyses were conducted using SAS (Version 9.2, SAS Institute Inc., Cary, NC, USA). Statistical significance was based on α = 0.05. Reported intraplatelet levels of protein were calculated by subtracting plasma values from serum values at a given (each) time point.
Results
The characteristics of the women initiating aspirin therapy are seen in Table 1. The majority of women had DCIS or stage 1 invasive ductal carcinoma. Only one patient had metastatic disease and was being treated in the nonadjuvant setting. Smoking status was assessed but subsequent analysis found no differences between smokers and nonsmokers in any of the parameters measured. The average duration of prior tamoxifen use was 14 months (range 2–46 months). Eleven women completed the study with one patient lost to follow‐up at 30 days postaspirin treatment. No differences in platelet counts were observed over time (p = 0.98). Adverse events were reported in two patients and included dyspepsia and increased bruising. Both events were considered minor and did not preclude completion of the study. All women reported continued compliance with aspirin intake throughout the study although five women (58%) reported missing one to three doses of aspirin at some time during the 45 days of treatment. During the 30‐day postaspirin study period, no participants reported using NSAID therapy.
Table 1.
Characteristics of patients included in the study
| N | Percent | |
|---|---|---|
| Age | ||
| 40–50 | 3 | 25 |
| 50–60 | 7 | 58 |
| 60–70 | 2 | 17 |
| Sex | ||
| Female | 12 | 100 |
| Disease stage | ||
| DCIS | 5 | 42 |
| I | 4 | 33 |
| II | 2 | 17 |
| III | 0 | 0 |
| IV | 1 | 8 |
| Duration of tamoxifen therapy | ||
| <3 months | 1 | 8 |
| >3 months | 11 | 92 |
| Prior chemotherapy | 4 | 33 |
| Smoking | ||
| Former | 4 | 33 |
| Active | 0 | 0 |
| Never | 8 | 67 |
| Other malignancies | 0 | 0 |
The effect of aspirin on intraplatelet VEGF and TSP‐1 levels
Repeated measures analysis of variance detected significant differences over time in mean intraplatelet TSP‐1 and VEGF (p value = 0.01 and 0.02, respectively) when analyzed for the total study period (four time points). As seen in Figure 1, TSP‐1 but not VEGF levels increased following the start of aspirin therapy. TSP‐1 levels were significantly greater than pretreatment following 45 days of aspirin therapy. The initial increase in TSP‐1 levels represented a mean 1.3‐fold increase in intraplatelet levels.
Figure 1.
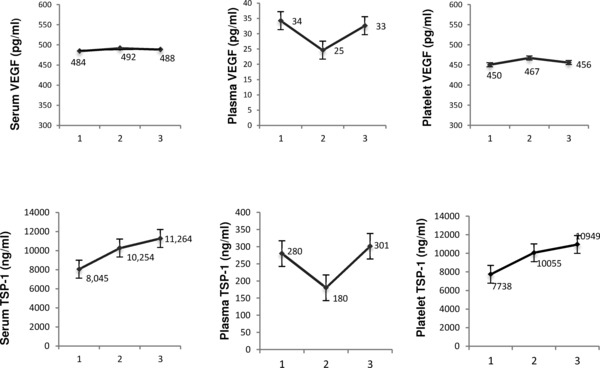
Angiogenic protein levels following the initiation of aspirin therapy. Participants received 325 mg aspirin therapy daily following an initial blood draw. Intraplatelet, serum, and plasma levels of VEGF and TSP‐1 were subsequently measured by solid phase ELISA at the following time points: (1) prior to aspirin therapy, (2) 30 days on aspirin therapy, (3) 45 days on aspirin therapy. Data labels indicate mean values with error bars indicating SEM.
In contrast, after completing a 45‐day course of aspirin therapy, 30‐day postaspirin levels of VEGF were significantly less than prior to treatment (Table 2). VEGF was 21% lower than the mean pretreatment level. Mean TSP‐1 at 30 days postaspirin therapy returned to pretreatment levels and was not significantly less than prior to treatment.
Table 2.
Levels of VEGF and TSP‐1 prior to and 30 days after completion of aspirin therapy. Aspirin (325 mg) was initiated in women receiving a stable dose of tamoxifen therapy. Following a total duration of 45 days of aspirin therapy, platelet and plasma levels of VEGF and TSP‐1 were measured using solid phase ELISA. Values represent mean
| VEGF (pg/mL) | TSP‐1 (ng/mL) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Intraplatelet | 450.3 (73.2) | 355.41 (61.7) | 7737.5 (969.3) | 6234.6 (986.3) |
| Plasma | 34.2 (4.8) | 29.2 (3.2) | 279.8 (53.0) | 243.6 (65.5) |
(±SEM). * Denotes a significant (p < 0.05) change in protein levels postaspirin therapy.
The effect of aspirin on serum VEGF and TSP‐1 levels
The impact of daily aspirin therapy on serum protein levels is seen in Figure 1. Repeated measures analysis of variance detected differences over time in the mean serum levels of TSP‐1 and VEGF (p value = 0.01 and 0.02, respectively). TSP‐1 increased during aspirin therapy and was significantly higher at 45 days when compared to pretherapy, with a 33.7% increase in serum TSP‐1 levels. In contrast, no significant changes in VEGF were found between mean pretreatment level and mean levels during aspirin therapy. Thirty days following completion of aspirin therapy, VEGF levels were significantly decreased by 21% (Table 2). Like intraplatelet levels, serum TSP‐1 levels following a course of aspirin therapy were not different from pretreatment levels.
Plasma VEGF and TSP‐1 levels after aspirin therapy initiation
Plasma VEGF and TSP‐1 levels were also assessed prior to, during and subsequent to daily aspirin therapy. We did not detect any significant difference over time in VEGF or TSP‐1 levels (p = 0.11 and 0.44, respectively). Figure 1 contrasts the plasma levels with serum and intraplatelet protein levels following aspirin therapy initiation.
Aspirin therapy decreases activation dependent angiogenic protein release
Because thrombin is considered a major driver of thrombosis and platelet activation in tumors, thrombin receptor stimulated release of angiogenic proteins was studied in an ex vivo platelet activation whole blood assay (Figure 2). Analysis of released TSP‐1 and VEGF revealed significant differences over time in mean TSP‐1 release (p = 0.01). A trend toward inhibition of VEGF release was also demonstrated (p = 0.07). Maximal inhibition of thrombin receptor‐mediated release was noted at 45 days on aspirin therapy. At 45 days on therapy, mean VEGF release was decreased by 11% while TSP‐1 was decreased by 14%.
Figure 2.
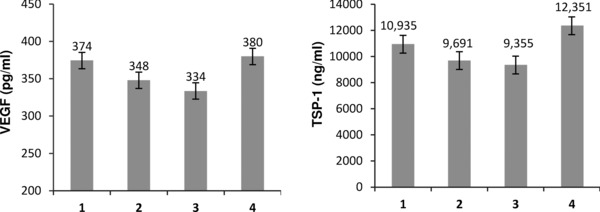
The impact of aspirin therapy on thrombin receptor‐mediated release of angiogenic proteins. Patients initiated 325 mg of aspirin therapy daily for duration of 45 days. An ex vivo assay of thrombin receptor agonist peptide (TRAP) mediated release was used to determine the impact of aspirin therapy on protein release. Angiogenic protein levels were measured by ELISA (1) prior to aspirin therapy; (2) 30 days on aspirin therapy; (3) 45 days on aspirin therapy; and (4) 30 days postaspirin therapy. Data labels represent mean protein values ±SEM.
Discussion
We prospectively demonstrated the impact of a short course of aspirin therapy on circulating angiogenic protein levels in women receiving tamoxifen therapy. Based on two pivotal proteins that contribute to the angiogenesis balance, we found aspirin therapy has time and protein specific effects on circulating protein levels. The majority of our study population was receiving tamoxifen in the nonmetastatic setting suggesting our results are likely of most relevance to that patient group. Our findings suggest that aspirin modulates circulating angiogenic proteins and may favor a systemic antiangiogenic balance.
For the antiangiogenic protein TSP‐1, the initiation of aspirin therapy resulted in significant increases in platelet and serum levels. TSP‐1 is an antiangiogenic protein stored within the platelet alpha granule and platelet (but not plasma) levels of TSP‐1 have been demonstrated to regulate early stages of tumor angiogenesis.20 Importantly, plasma levels of this protein were not found to change in our study. The increase in TSP‐1 seen in our study, however, was dependent on the presence of drug (aspirin) as posttreatment levels were not different from pretreatment levels. This observation suggests that aspirin treatment may shift the angiogenic balance by favoring an increase in the antiangiogenic protein TSP‐1. The mechanism that underpins this observation will need further assessment in subsequent studies.
In contrast to TSP‐1, VEGF values did not change while on aspirin therapy, however, platelet VEGF levels decreased following the completion of 45 days of aspirin. VEGF is a potent proangiogenic growth factor that has been associated with poor prognosis in patients with breast cancer and serum VEGF levels correlate with intratumoral microvessel density.21, 22, 23 The magnitude of the decrease in platelet VEGF that was seen in our study was approximately 20%. While we are aware of no conclusive data with regard to the degree of decrease needed in circulating VEGF to have a clinically significant impact, Banerjee and colleagues have found tamoxifen use was associated with a 30% increase in VEGF levels.24 Thus, the magnitude of our effect is at least consistent with other documented effects of drugs on VEGF levels in patients. The mechanisms that underpin our findings are not known. Decreased prostaglandin production (as seen with aspirin therapy) has been linked to decreased levels of VEGF.25 In addition, in rat models of mammary carcinogenesis, acetylsalicylic acid decreased both VEGF concentration and tumor diameter.11 In vitro studies of lung cancer, sarcoma, and colon cancer models showed similar results.12, 26
A lack of an early decrease in VEGF levels was surprising based on the above data. The reasons for the delay in response that was seen in our study are not known. The timing of inhibition of tissue production of VEGF relative to aspirin therapy initiation is not known. Longer duration of aspirin use and a larger sample size will need to be explored in subsequent prospective studies using aspirin in cancer patients.
Unique to our study is the assessment of the effect of aspirin on agonist‐induced platelet protein release. Several model systems have demonstrated the proangiogenic effects of platelets (reviewed by Bambace and Holmes27). Platelets can contribute to the balance of tumor‐associated angiogenesis through release of both stimulators and inhibitors of angiogenesis.15, 28, 29 We found the release of both angiogenic proteins studied was inhibited by aspirin therapy, however this result was only significant for the antiangiogenic protein, TSP‐1. Similarly, Coppinger reported in a mass spectrometry based analysis of platelet protein release, the inhibition of TRAP induced TSP‐1 release in healthy individuals.16 Additionally, aspirin has been shown to inhibit VEGF release from resting platelets as well as platelets exposed to ADP and MCF‐7 cells.30 In our study, the inhibition of release was modest as anticipated given the use of a direct thrombin receptor agonist (TRAP) that preferentially (but not exclusively) activates through the PAR1 pathway. The decrease in release of these angiogenic proteins suggests the need for additional platelet pathway specific investigations in patients with cancer.
Limitations of our study include the study of only a subset of potential protein contributors to angiogenesis, the short course of aspirin therapy and small sample size. We chose to initially study early effects of the drug to avoid confounding by changes in underlying disease state. A dose‐dependent effect of aspirin on angiogenic protein levels is also not known. We chose an aspirin dose of 325 mg daily based on observational data suggesting that 325 mg of aspirin might be necessary to achieve maximum chemopreventative effect.31 In breast cancer, the benefits of any particular aspirin regimen (dose or duration) are not well established. Additional studies of longer duration that include concurrent tissue assessment of angiogenesis will be needed to further extend our observations. Whether or not the changes we have seen in angiogenic protein levels will ultimately be the most important protein specific effects of aspirin relative to angiogenesis remains unknown at this time.
Our data suggests aspirin therapy impacts angiogenic protein levels and may modify the angiogenic balance in women treated with tamoxifen therapy. The increase in anti‐angiogenic protein levels (TSP‐1) while taking aspirin therapy without a concurrent increase in pro‐angiogenic VEGF levels suggests this impact may be, on balance, antiangiogenic. These observations are likely most clinically relevant in the primary and secondary prevention setting for women with breast cancer (including DCIS) receiving tamoxifen therapy. Given the small size of our study, additional studies are imperative to fully understand the impact of aspirin therapy on angiogenesis in patients with breast cancer. Our results should be viewed as only a first step in understanding the impact of aspirin therapy on the angiogenic balance and important angiogenic proteins. However, our data in combination with the observed decrease in cancer recurrence in aspirin users in observational clinical trials continues to support a role for investigating less expensive agents such as aspirin therapy in women with breast cancer.
Conflict of Interest
The authors have no conflict of interest to declare.
Sources of Funding
This work is supported by a grant from The Breast Cancer Research Foundation, New York, NY and the Charles H. Smith Memorial Fund (University of Vermont/Fletcher Allen Health Care).
References
- 1. Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010; 28: 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti‐inflammatory drugs: prospective results from the Women's Health Initiative. Cancer Res. 2003; 63: 6096–6101. [PubMed] [Google Scholar]
- 3. Takkouche B, Regueira‐Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti‐inflammatory drugs: a meta‐analysis. J Natl Cancer Inst. 2008; 100: 1439–1447. [DOI] [PubMed] [Google Scholar]
- 4. Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti‐inflammatory drug use and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001; 10: 1213–1217. [PubMed] [Google Scholar]
- 5. Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004; 291: 2433–2440. [DOI] [PubMed] [Google Scholar]
- 6. Garcia Rodriguez LA, Gonzalez‐Perez A. Risk of breast cancer among users of aspirin and other anti‐inflammatory drugs. Br J Cancer. 2004; 91: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazhar D, Ang R, Waxman J. COX inhibitors and breast cancer. Br J Cancer. 2006; 94: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu JF, Jamieson GG, Drew PA, Zhu GJ, Zhang SW, Zhu TN, Shan BE, Wang QZ. Aspirin induces apoptosis in oesophageal cancer cells by inhibiting the pathway of NF‐kappaB downstream regulation of cyclooxygenase‐2. ANZ J Surg. 2005; 75: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 9. Li P, Zhang ST, Yu ZL, Wu YD, Liu X, Xu CM, Cho CH. Effects of cyclooxygenase‐2 non‐selective and selective inhibitors on proliferation inhibition and apoptosis induction of esophageal squamous carcinoma cells. Dis Esophagus. 2009; 22: 21–31. [DOI] [PubMed] [Google Scholar]
- 10. Gately S. The contributions of cyclooxygenase‐2 to tumor angiogenesis. Cancer Metastasis Rev. 2000; 19: 19–27. [DOI] [PubMed] [Google Scholar]
- 11. Ghezzo F, Cesano L, Mognetti B, Pesce E, Pirro E, Corvetti G, Berta GN, Zingaro B, Di Carlo F. Salicylate inhibition of rat mammary carcinogenesis and angiogenesis in female rat compatible with misoprostol administration. Int J Oncol. 2005; 26: 697–702. [PubMed] [Google Scholar]
- 12. Shtivelband MI, Juneja HS, Lee S, Wu KK. Aspirin and salicylate inhibit colon cancer medium‐ and VEGF‐induced endothelial tube formation: correlation with suppression of cyclooxygenase‐2 expression. J Thromb Haemost. 2003; 1: 2225–2233. [DOI] [PubMed] [Google Scholar]
- 13. Yanni SE, Barnett JM, Clark ML, Penn JS. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Invest Ophthalmol Vis Sci. 2009; 50: 5479–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson JE, Zurakowski D, Italiano JE, Jr. , Michel LV, Fox L, Klement GL, Folkman J. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 2010; 85: 487–493. [DOI] [PubMed] [Google Scholar]
- 15. Italiano JE, Jr. , Richardson JL, Patel‐Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro‐ and antiangiogenic proteins are organized into separate platelet {alpha} granules and differentially released. Blood. 2008; 111: 1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coppinger JA, O'Connor R, Wynne K, Flanagan M, Sullivan M, Maguire PB, Fitzgerald DJ, Cagney G. Moderation of the platelet releasate response by aspirin. Blood. 2007; 109: 4786–4792. [DOI] [PubMed] [Google Scholar]
- 17. Mah‐Becherel MC, Ceraline J, Deplanque G, Chenard MP, Bergerat JP, Cazenave JP, Klein‐Soyer C. Anti‐angiogenic effects of the thienopyridine SR 25989 in vitro and in vivo in a murine pulmonary metastasis model. Br J Cancer. 2002; 86: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holmes CE, Huang JC, Pace TR, Howard AB, Muss HB. Tamoxifen and aromatase inhibitors differentially affect vascular endothelial growth factor and endostatin levels in women with breast cancer. Clin Cancer Res. 2008; 14: 3070–3076. [DOI] [PubMed] [Google Scholar]
- 19. Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000; 60: 2898–2905. [PubMed] [Google Scholar]
- 20. Zaslavsky A, Baek KH, Lynch RC, Short S, Grillo J, Folkman J, Italiano JE, Jr. , Ryeom S. Platelet‐derived thrombospondin‐1 is a critical negative regulator and potential biomarker of angiogenesis. Blood. 2010; 115: 4605–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foekens JA, Peters HA, Grebenchtchikov N, Look MP, Meijer‐van Gelder ME, Geurts‐Moespot A, van der Kwast TH, Sweep CG, Klijn JG. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001; 61: 5407–5414. [PubMed] [Google Scholar]
- 22. Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, Vinante O, Bonoldi E, Boracchi P, et al. Prognostic significance of vascular endothelial growth factor protein in node‐negative breast carcinoma. J Natl Cancer Inst. 1997; 89: 139–147. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto Y, Toi M, Kondo S, Matsumoto T, Suzuki H, Kitamura M, Tsuruta K, Taniguchi T, Okamoto A, Mori T, et al. Concentrations of vascular endothelial growth factor in the sera of normal controls and cancer patients. Clin Cancer Res. 1996; 2: 821–826. [PubMed] [Google Scholar]
- 24. Banerjee S, Pancholi S, A'Hern R, Ghazoui Z, Smith IE, Dowsett M, Martin LA. The effects of neoadjuvant anastrozole and tamoxifen on circulating vascular endothelial growth factor and soluble vascular endothelial growth factor receptor 1 in breast cancer. Clin Cancer Res. 2008; 14: 2656–2663. [DOI] [PubMed] [Google Scholar]
- 25. Yanni SE, Clark ML, Yang R, Bingaman DP, Penn JS. The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res Bull. 2010; 81: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshida S, Amano H, Hayashi I, Kitasato H, Kamata M, Inukai M, Yoshimura H, Majima M. COX‐2/VEGF‐dependent facilitation of tumor‐associated angiogenesis and tumor growth in vivo. Lab Invest. 2003; 83: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 27. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011; 9: 237–249. [DOI] [PubMed] [Google Scholar]
- 28. Bambace NM, Levis JE, Holmes CE. The effect of P2Y‐mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets. 2010; 21: 85–93. [DOI] [PubMed] [Google Scholar]
- 29. Chatterjee M, Huang Z, Zhang W, Jiang L, Hultenby K, Zhu L, Hu H, Nilsson GP, Li N. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood. 2011; 117: 3907–3911. [DOI] [PubMed] [Google Scholar]
- 30. Battinelli EM, Markens, BA , Italiano JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiological and pathological responses. Blood. 2011; 118: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long‐term use of aspirin and nonsteroidal anti‐inflammatory drugs and risk of colorectal cancer. JAMA. 2005; 294: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]


