Abstract
Background
Nuclear factor‐κB p65 (NF‐κB p65) may play a significant role as a biomarker in tumor progression and metastasis. However, the correlation between cellular localization of NF‐κB p65 expression and the prognosis of gastric cancer (GC) patients has not been studied. The present study was designed to investigate the location of NF‐κB p65 expression in GC, and evaluate its correlation with clinicopathological parameters of GC patients.
Methods
NF‐κB p65 expressions in GC tissue and corresponding nonmalignant tissue from gastrectomy of 115 stage I–III GC patients were detected by immunohistochemistry. In addition, correlations between the staining results and the clinicopathologic features and survival of the GC patients were analyzed.
Results
The percentage of NF‐κB p65 expression in GC tissue and the corresponding nonmalignant tissue was 73.9% and 46.80%, respectively. No significant correlation was found between NF‐κB p65 expression and the clinicopathologic parameters. Cox univariate analysis indicated that both nuclear staining and cytoplasmic staining of NF‐κB p65 expression correlated with the prognosis of GC patients (log‐rank, p = 0.0182; p = 0.0144, respectively).
Conclusion
High nuclear expression of NF‐κB p65 is an independent prognostic marker predicting a better survival, while high cytoplasmic staining indicates a worse prognosis of GC patients.
Keywords: gastric cancer, NF‐κB p65, cellular localization, prognosis
Introduction
Although the incidence of gastric cancer (GC) has gradually declined worldwide,1 GC remains one of the most common malignancies worldwide and a major cause of cancer mortality in Asia.2 The clinical progression of GC is rapid and the prognosis is usually poor due to adjacent tissue invasion and distant organ metastasis at a very early stage.3, 4, 5 Therefore, improving early diagnostic accuracy could increase the curative opportunity or prolong the survival of GC patients. However, the conventional serum markers for GC, such as carbohydrate antigen 19‐9 (CA19‐9) and carcinoembryonic antigen (CEA), lack sufficient sensitivity and specificity to facilitate early detection.6 Thus, finding an indicator for early diagnosis of GC is of vital importance to improve the therapeutic effect and prolong survival of GC patients.
Nuclear factor‐kappa B p65 (NF‐κB p65) is a ubiquitously existing transcription factor that affects the proliferation, invasion, angiogenesis, and metastasis of cancer cells by regulating the expression of numerous genes.7, 8, 9 In an inactive status, NF‐κB p65 family members exist as dimmers with the predominance of p65/p50 heterodimers and are sequestered in the cytoplasm.10 Activation of NF‐κB p65 pathway by a series of stimuli would induce its translocation to the nucleus, thus activating the expression of downstream genes.11, 12 Constitutive activation of NF‐κB p65 has been observed to positively correlate with tumor progression in various cancer types, including renal cancer, cervical cancer, and esophageal cancer.13 Activated NF‐κB p65 is therefore suggested as a potential therapeutic target for the treatment of these tumors.14
There is evidence that NF‐κB level in GC cells is higher than that in adjacent normal epithelial cells, and activation of NF‐κB in GC is related to lymphatic invasion.15, 16 It was reported that patients with high NF‐κB activation in carcinoma tissue did not survive as long as those with low NF‐κB activation, and p65, but not NF‐κB, is a prognostic indicator of GC.17 Although Kojima et al.18 reported the expression level of NF‐κB p65, its correlation with the clinicopathologic features (such as tumor progression) of GC patients, and its significance as a prognostic marker, the correlation between the location of NF‐κB p65 expression and the prognosis of GC patients has not been reported. The present study was intended to investigate the potential correlation between the location of NF‐κB p65 expression and the prognosis of GC patients by immunohistochemistry, in an attempt to further expound the role of NF‐κB p65 expression in predicting the prognosis of GC patients, and see whether it could be used as an option for early clinical diagnosis and treatment of GC.
Materials and Methods
Specimen source
A total of 115 GC patients who underwent radical surgery at our hospital between November 2007 and March 2009 were evaluated retrospectively. They included 78 (67.8%) male patients and 37 (32.2%) female patients, with a mean age of 60.1 years (range 36–90 years) and a median follow‐up period of 42 months (range 8–59 months). Histologically, they included 90 (78.3%) cases of poorly differentiated, 18 (15.7%) cases of moderately differentiated, and 7 (6%) cases of well differentiated adenocarcinoma. According to the seventh edition of TNM classification for GC as reported by Biondi et al.,19 TNM stage of the 115 patients was assessed as follows: stage I in 31 patients (27%), II in 21 patients (18.3%), and III in 63 patients (54.7%). Her‐2/neu overexpression was detected in 11 (10.5%) cases by postoperative fluorescence in situ hybridization (FISH). No patient received neoadjuvant treatment. No patient included in the study had evidence of distant metastasis at the time of diagnosis. Patients with a history of previous malignancy were excluded. The clinical characteristics of the patients are shown in Table 1.
Table 1.
Correlation among NF‐κB p65 staining and clinical characteristics
| Characteristics | No. | NF‐κB p65‐high (n = 65) | NF‐κB p65‐low (n = 50) | p |
|---|---|---|---|---|
| Gender | ||||
| Male | 78 | 45 | 33 | 0.713 |
| Female | 37 | 20 | 17 | |
| Age (y) | ||||
| <60 | 55 | 31 | 24 | 0.974 |
| ≥60 | 60 | 34 | 26 | |
| Tumor differentiation | ||||
| Well | 7 | 4 | 3 | 0.762 |
| Moderate | 18 | 11 | 7 | |
| Poor | 90 | 50 | 40 | |
| Tumor size (cm) | ||||
| <4.5 | 59 | 32 | 27 | 0.067 |
| ≥4.5 | 56 | 33 | 13 | |
| TNM stage | ||||
| I–II | 52 | 30 | 22 | 0.818 |
| III | 63 | 35 | 28 | |
| Her‐2 status | ||||
| Negative | 104 | 58 | 46 | 0.617 |
| Positive | 11 | 7 | 4 | |
| Primary/Recurrent | ||||
| Primary tumor | 88 | 52 | 36 | 0.316 |
| Recurrent tumor | 27 | 13 | 14 |
Specimens obtained from the 115 patients who underwent radical resection were fixed in neutral buffered formol saline and paraffin‐embedded for immunohistochemical observation by a pathologist to confirm the malignancy. The study protocol was approved by the ethics committee of the medical faculty, and informed consent was obtained from all patients.
Immunohistochemistry
Streptavidin‐peroxidase immunohistochemical technique was used to determine the level of NF‐κB p65. The monoclonal antibody (E379, ab32536, Abcam Inc.) to NF‐κB p65 was from Abcam (Cambridge, United Kingdom). Paraffin‐embedded tissue blocks were sliced into 3–4 mm sections and placed on antislides. The sections were dewaxed, hydrated, rinsed in phosphate‐buffered saline (PBS), blocked with 3% hydrogen peroxide for 10 minutes to deprive the endogenous peroxidase activity. After antigen retrieval with the use of a microwave, the sections were incubated with the antinucleolin MAb (diluted 1:100 in PBS) at 37°C for 70 minutes, washed 3 × 3 minutes in PBS, and incubated with 1 and 2 Reagent of PV9000 Mouse/Rabbit hypersensitivity two‐step immunohistochemical Kit (Beijing fir Jinqiao, China) for 50 minutes at 37°C in a humid chamber. The sections were washed again 3×3 minutes with PBS, followed by the addition of diaminobenzidine (DAB) as a chromogen. Antibodies were optimized using positive control tissue according to manufacturer's instructions. In negative controls, the primary antibody was replaced with PBS. The rest procedures were undertaken in parallel with other specimens. Each slide was scored in a blinded fashion by two pathologists according to the manufacturer's recommended criteria at ×100 and ×200 magnification. The overall percentage of cells on an immunostained section was determined according to the pattern of intracellular localization. The extent and pattern of the NF‐κB p65‐specific immunostaining within a tissue section was determined by the percentage of cells with a nucleus and cytoplasm staining. The immunostaining was read in a semiquantitative manner. Three visual fields were examined randomly and the rate of positive cells was classified as less than 5% (0 score), 6–25% (1 score), 26–50% (2 score), 51–75% (3 score), and more than 75% (4 score). The staining intensity was classified as four grades: no staining (0 score), slightly yellowish (1 score), brownish yellow (2 score), and dark brown (3 score). The multiplication of the two was graded as 0 (0 score), 1+ (1–4 score), 2+ (5–8 score), and 3+ (9–12 score). Intensity scores of 0 or 1+ were designated as low expression, and 2+, 3+ were designated as high expression.
Statistical analysis
Statistical analysis was performed using SAS 9.2 software system (SAS, Cary, NC, USA). Chi‐square statistics and Fisher's extract test were applied to assess correlations between NF‐κB p65 and clinicopathological parameters. Kaplan‐Meier survival analysis was performed for univariate analysis of the survival rate, and Cox regression analysis was performed for multivariate analysis. Univariate analysis was also performed to assess the prognostic value of NF‐kappa B p65 expression and other clinicopathological features. A p‐value less than 0.05 was considered statistically significant.
Results
NF‐κB p65 expression and clinicopathological characteristics
All the 78 male and 37 female patients in this series received radical resection for GC, including 88 primary and 27 recurrent GC patients. Pathologically, all the 115 cases were adenocarcinoma, of which 7 cases were well differentiated, 18 were moderately differentiated, and 90 cases were poorly differentiated (including 3 cases of signet‐ring cell carcinoma). For all specimens, 11 cases were confirmed as Her‐2/neu‐positive.
Immunohistochemical analysis
NF‐κB p65 immunohistochemical staining showed a nuclear staining pattern in both nonmalignant tissue (Figure 1, panel b) and GC tissue (Figure 1, panel a–b). The nuclear expression of NF‐κB p65 in GC tissue was significantly higher than that in nonmalignant tissue. Cytoplasmic staining was detected in 16.5% (19/115) cases of cancer tissue (Figure 1, panel d). High expression of NF‐κB p65 was detected in 60.87% (65/115) cases of cancer tissue, while no high expression of NF‐κB p65 was detected in the adjacent benign gastric tissue.
Figure 1.
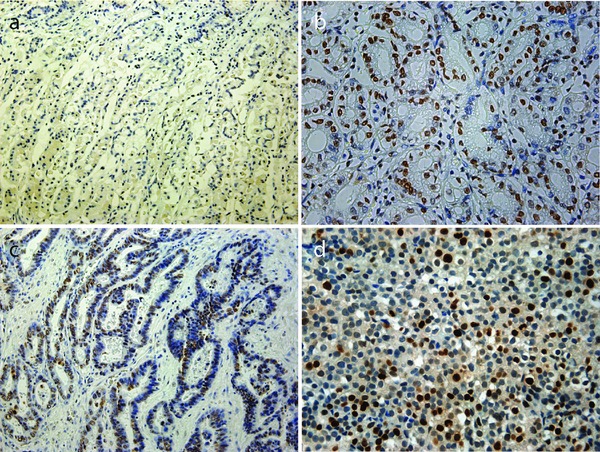
No expression of NF‐κB p65 in corresponding nonmalignant tissues (a) and low NF‐κB p65 expression in corresponding nonmalignant tissues (b). Low NF‐κB p65 expression in cancer tissues (c), high NF‐κB p65 expression in cancer tissues (d). Original magnification of (a) and (c), ×200; original magnification of (b) and (d), ×400.
Correlations between NF‐κB p65 expression and clinicopathologic parameters
Data representing the correlations between NF‐κB p65 levels and the clinicopathologic features of the 115 GC cases are summarized in Table 1. No significant correlation was found between NF‐κB p65 expression and the clinicopathologic parameters.
Correlations between the expression level of NF‐κB p65 and prognosis
Correlation between the high expression of nuclear staining or cytoplasmic staining of NF‐κB p65 and patient survival was assessed by Cox survival analysis. The result showed that NF‐κB p65 overexpression was identified as the only independent prognostic factor for better survival of GC patients after adjusting patient age, gender, tumor size, differentiation, TNM classification, and Her‐2/neu status (Table 2).
Table 2.
Univariate and multivariate analysis of overall survival in patients with GC
| No. | HR (95% CI) | p | |
|---|---|---|---|
| Univariate analysis | |||
| Gender | |||
| Male | 78 | 0.9164 | |
| Female | 37 | ||
| Age (y) | |||
| <60 | 55 | 0.4858 | |
| ≥60 | 60 | ||
| Tumor differentiation | |||
| Well | 7 | 0.2724 | |
| Moderate | 18 | ||
| Poor | 90 | ||
| Tumor size (cm) | |||
| <4.5 | 59 | 0.9465 | |
| ≥4.5 | 56 | ||
| TNM stage | |||
| I–II | 52 | 0.4460 | |
| III | 63 | ||
| Her‐2 status | |||
| Negative | 104 | 0.2585 | |
| Positive | 11 | ||
| Nucleolar staining of nucleolin | |||
| Low | 50 | 0.0182 | |
| High | 65 | ||
| Cytoplasmic staining of nucleolin | |||
| Low | 96 | 0.0144 | |
| High | 19 | ||
| Multivariate analysis | |||
| Nucleolar staining of nucleolin | |||
| Low | 50 | 0.4 | 0.0232 |
| High | 65 | 0.181–0.882 | |
| Cytoplasmic staining of nucleolin | |||
| Low | 96 | 2.714 | 0.0193 |
| High | 19 | 1.176–6.266 |
Patients with overexpression of nuclear staining had a longer survival time (log‐rank, p = 0.0182; Figure 2, panel a). Inversely, high expression of cytoplasmic staining was found to be associated with a significantly high recurrence rate and a shorter survival rate (log‐rank, p = 0.0144; Figure 2, panel b). Univariate survival analysis showed that high nuclear staining was correlated with a better prognosis, while high cytoplasmic staining was closely associated with a poor prognosis. In terms of prognosis of patients, high nuclear expression with low cytoplasmic expression indicated the best prognosis while low nuclear expression with high cytoplasmic expression indicated the poorest prognosis. Low nuclear expression with low cytoplasmic expression and high nuclear expression with high cytoplasmic expression ranked the second and third (log‐rank, p < 0.01; Figure 2, panel c).
Figure 2.
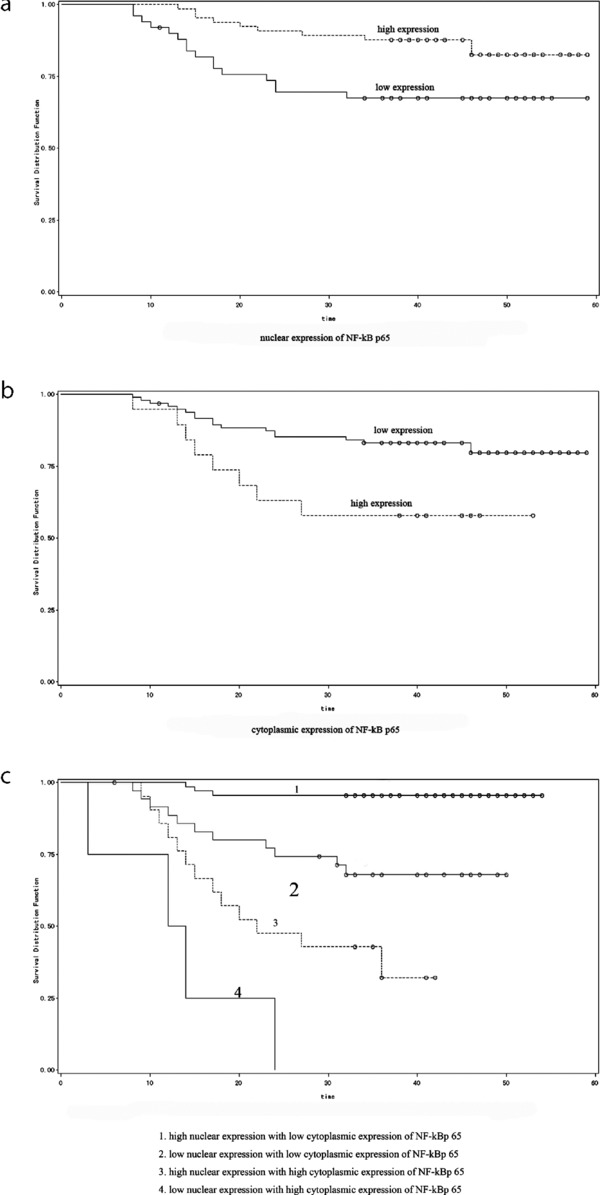
Survival analysis of NF‐κB p65 expression in GC patients. Overexpression of nucleolar staining showed longer survival time (a), high expression of cytoplasmic staining associated with shortened survival rate (b) and different expression sites and levels of NF‐κB p65 showed different prognosis (c).
Discussion
Although a large number of investigations strongly suggest that NF‐κB p65 is activated in gastric and other carcinomas and is an important transcriptional factor related to biological malignant characteristics such as anti‐apoptosis and invasiveness of carcinoma,20, 21, 22 the biological significance of different cellular localization of NF‐κB p65 in carcinoma tissues remains unclear. In the present study, we focused on the prognostic value of different expression sites of NF‐κB p65 in GC tissue. It was found that nuclear expression of NF‐κB p65 was significantly higher in 72.1% GC tissue specimens versus 16.5% for cytoplasmic staining. Cox univariate analysis indicated that both cytoplasmic and nuclear staining of NF‐κB p65 expression significantly correlated with the prognosis of GC patients.
Knowing that NF‐κB p65 activation is a new prognostic parameter in GC as reported by previous studies,23 the present study was intended to see whether NF‐κB p65 was an independent prognostic indicator. At present, the most important factor in predicting the prognosis of GC patients is TNM stage, which is determined by the primary tumor size, degree of metastasis to regional lymph nodes, and distant metastasis.24 These traditional clinicopathological parameters seem to be the most important risk factors for predicting overall survival of GC patients. However, no significant correlation was observed between TNM stage and NF‐κB p65 (Table 1 ), suggesting that NF‐κB p65 may be a prognostic indicator independent of TNM stage. In fact, our multivariate analysis showed that only NF‐κB p65 was a prognostic parameter and that NF‐κB p65 was independent of the traditional pathological parameters, including TNM stage.
In the past few years, several studies have shown the function of NF‐κB p65 as a prognostic biomarker in various types of cancer.23, 25 Unlike many other transcription factors involved in cancer biology, NF‐κB p65 plays a two‐side role in the process of carcinogenesis.26, 27 The tumor‐promoting role of NF‐κB p65 has been studied exhaustively. Although previous studies reported that NF‐κB p65 could induce G1 cell cycle arrest in human epithelial cells,28 the mechanism underlying the tumor‐suppressor role of NF‐κB p65 remains poorly understood. Unexpectedly, this study has, for the first time, shown that the clinical outcomes are different in GC cases with different cellular localizations of NF‐κB p65, which is consistent with the previous studies. It was found in our study that patients who showed high cytoplasmic staining of NF‐κB p65 in carcinoma tissue did not survive as long as those with high nuclear staining, indicating that a high level of nuclear expression of NF‐κB p65 is a prognostic marker for better survival, while high cytoplasmic staining was closely associated with a worse prognosis for GC patients, which may suggest that different cellular localizations of NF‐κB p65 may play a role in cancer progression.
In conclusion, our immunohistochemical results showed that nuclear expression of NF‐κB p65 was markedly increased in GC tissue as compared with that in nonmalignant tissue. A high level of nuclear expression of NF‐κB p65 was associated with a better prognosis and was an independent prognostic indicator for GC when overall survival was used as the endpoint. In addition, high cytoplasm staining indicates a worse prognosis, suggesting that the combination of cytoplasmic and nuclear detection could better predict the prognosis of GC. High cytoplasm expression with low nuclear expression may indicate a shorter survival, while low cytoplasm expression with high nuclear expression may indicate a better prognosis. Further investigation is needed to clarify the mechanism of NF‐κB as a new prognostic biomarker in GC.
Conflict of interest statement
We have no conflicts of interest to report.
Acknowledgments
This study was supported by Shandong Natural Science Foundation (Y2006C23); Shandong Excellent Young Scientist Research Award Fund Project (2006BSB14114; BS2010YY013); and Shandong Tackle Key Problems in Science and Technology (2010GSF10245).
References
- 1. Ali R, Barnes I, Cairns BJ, Finlayson AE, Bhala N, Mallath M, Beral V. Incidence of gastrointestinal cancers by ethnic group in England, 2001–2007. Gut. 2012. Oct 23 [Epub ahead of print]. [DOI] [PubMed]
- 2. Hu Y, Fang JY, Xiao SD. Is it possible to further reduce the incidence of gastric cancer in the new century? J Dig Dis. 2013; 14: 11–15. [DOI] [PubMed] [Google Scholar]
- 3. Tang Y, Dai Y, Huo J. Decreased expression of T‐cadherin is associated with gastric cancer prognosis. Hepatogastroenterology. 2012; 59: 1294–1298. [DOI] [PubMed] [Google Scholar]
- 4. Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010; 16: 260–268. [DOI] [PubMed] [Google Scholar]
- 5. Zhao HC, Qin R, Chen XX, Sheng X, Wu JF, Wang DB, Chen GH. Microvessel density is a prognostic marker of human gastric cancer. World J Gastroenterol. 2006; 12: 7598–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emara M, Cheung PY, Grabowski K, Sawicki G, Wozniak M. Serum levels of matrix metalloproteinase‐2 and ‐9 and conventional tumor markers (CEA and CA 19‐9) in patients with colorectal and gastric cancers. Clin Chem Lab Med. 2009; 47: 993–1000. [DOI] [PubMed] [Google Scholar]
- 7. Liu T, Liu D, Liu J, Song JT, Gao SL, Li H, Hu LH, Liu BR Effect of NF‐kappaB inhibitors on the chemotherapy‐induced apoptosis of the colon cancer cell line HT‐29. Exp Ther Med. 2012; 4: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, et al. Telomerase directly regulates NF‐kappaB‐dependent transcription. Nat Cell Biol. 2012; 14: 1270–1281. [DOI] [PubMed] [Google Scholar]
- 9. Zhu G, Yin F, Deng X. Effect of NF‐kappaB on inhibition of non‐small cell lung cancer cell cyclooxygenase‐2 by brucine. Zhongguo Zhong Yao Za Zhi. 2012; 37: 1269–1273. [PubMed] [Google Scholar]
- 10. Gagliardo R, Chanez P, Mathieu M, Bruno A, Costanzo G, Gougat C, Vachier I, Bousquet J, Bonsignore G, Vignola AM. Persistent activation of nuclear factor‐kappaB signaling pathway in severe uncontrolled asthma. Am J Respir Crit Care Med. 2003; 168: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 11. Ren Z, Cui J, Huo Z, Xue J, Cui H, Luo B, Jiang L, Yang R. Cordycepin suppresses TNF‐alpha‐induced NF‐kappaB activation by reducing p65 transcriptional activity, inhibiting IkappaBalpha phosphorylation, and blocking IKKgamma ubiquitination. Int Immunopharmacol. 2012; 14: 698–703. [DOI] [PubMed] [Google Scholar]
- 12. Wu SY, Leu YL, Chang YL, Wu TS, Kuo PC, Liao YR, Teng CM, Pan SL. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF‐kappaB and generating reactive oxygen species. PLoS ONE. 2012; 7: e40727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian F, Zhang C, Tian W, Jiang Y, Zhang X. Comparison of the effect of p65 siRNA and curcumin in promoting apoptosis in esophageal squamous cell carcinoma cells and in nude mice. Oncol Rep. 2012; 28: 232–240. [DOI] [PubMed] [Google Scholar]
- 14. Lafarge S, Hamzeh‐Cognasse H, Richard Y, Pozzetto B, Cogne M, Cognasse F, Garraud O. Complexes between nuclear factor‐kappaB p65 and signal transducer and activator of transcription 3 are key actors in inducing activation‐induced cytidine deaminase expression and immunoglobulin A production in CD40L plus interleukin‐10‐treated human blood B cells. Clin Exp Immunol. 2011; 166: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao HJ, Fang Y, Zhang X, Chen WJ, Zhou WP, Wang H, Wang LB, Wu JM. Tumor metastasis and the reciprocal regulation of heparanase gene expression by nuclear factor kappa B in human gastric carcinoma tissue. World J Gastroenterol. 2005; 11: 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M, et al. Nuclear factor‐kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001; 7: 4136–4142. [PubMed] [Google Scholar]
- 17. Guo XL, Ma NN, Zhou FG, Zhang L, Bu XX, Sun K, Song JR, Li R, Zhang BH, Wu MC, et al. Up‐regulation of hTERT expression by low‐dose cisplatin contributes to chemotherapy resistance in human hepatocellular cancer cells. Oncol Rep. 2009; 22: 549–556. [DOI] [PubMed] [Google Scholar]
- 18. Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, Katano M. Increased nuclear factor‐kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004; 24: 675–681. [PubMed] [Google Scholar]
- 19. Biondi A, Hyung WJ. Seventh edition of TNM classification for gastric cancer. J Clin Oncol. 2011; 29: 4338–4339; author reply 4340–4342. [DOI] [PubMed] [Google Scholar]
- 20. Starska K, Forma E, Lewy‐Trenda I, Stasikowska O, Brys M, Krajewska WM, Lukomski M. The expression of SOCS1 and TLR4‐NFkappaB pathway molecules in neoplastic cells as potential biomarker for the aggressive tumor phenotype in laryngeal carcinoma. Folia Histochem Cytobiol. 2009; 47: 401–410. [DOI] [PubMed] [Google Scholar]
- 21. Bendinelli P, Matteucci E, Maroni P, Desiderio MA. NF‐kappaB activation, dependent on acetylation/deacetylation, contributes to HIF‐1 activity and migration of bone metastatic breast carcinoma cells. Mol Cancer Res. 2009; 7: 1328–1341. [DOI] [PubMed] [Google Scholar]
- 22. Djordjevic G, Matusan‐Ilijas K, Sinozic E, Damante G, Fabbro D, Grahovac B, Lucin K, Jonjic N. Relationship between vascular endothelial growth factor and nuclear factor‐kappaB in renal cell tumors. Croat Med J. 2008; 49: 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamanaka N, Sasaki N, Tasaki A, Nakashima H, Kubo M, Morisaki T, Noshiro H, Yao T, Tsuneyoshi M, Tanaka M, et al. Nuclear factor‐kappaB p65 is a prognostic indicator in gastric carcinoma. Anticancer Res. 2004; 24: 1071–1075. [PubMed] [Google Scholar]
- 24. Asoglu O, Karanlik H, Parlak M, Kecer M, Muslumanoglu M, Igci A, Ozmen V, Gulluoglu M, Kapran Y. Metastatic lymph node ratio is an independent prognostic factor in gastric cancer. Hepatogastroenterology. 2009; 56: 908–913. [PubMed] [Google Scholar]
- 25. Baiocchi G, Begnami MD, Fukazawa EM, Oliveira RA, Faloppa CC, Kumagai LY, Badiglian‐Filho L, Pellizzon AC, Maia MA, Jacinto AA, et al. Prognostic value of nuclear factor kappa B expression in patients with advanced cervical cancer undergoing radiation therapy followed by hysterectomy. J Clin Pathol. 2012; 65: 614–618. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Wang K, Chen X, Meng H, Song M, Wang Y, Xu X, Bai Y. Transcriptional activation of microRNA‐34a by NF‐kappa B in human esophageal cancer cells. BMC Mol Biol. 2012; 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Miano MP, de Nigris F, Casalino L, Curcio F, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression. Oncogene. 1997; 15: 1987–1994. [DOI] [PubMed] [Google Scholar]
- 28. Subramaniam D, Giridharan P, Murmu N, Shankaranarayanan NP, May R, Houchen CW, Ramanujam RP, Balakrishnan A, Vishwakarma RA, Anant S. Activation of apoptosis by 1‐hydroxy‐5,7‐dimethoxy‐2‐naphthalene‐carboxaldehyde, a novel compound from Aegle marmelos. Cancer Res. 2008; 68: 8573–8581. [DOI] [PMC free article] [PubMed] [Google Scholar]


