Abstract
Minorities are underenrolled in clinical research trials, and one‐third of trials are underenrolled overall. The role of payment has not been studied at the national level as an explanation for enrollment patterns. Our objective was to examine the distribution of self‐reported previous research participation across different sociodemographic groups; to assess the public's perception of fair payment for a low‐risk medicine trial and the association between requested payment and sociodemographic characteristics; to estimate the amount of payment for a medication trial to achieve proportional representation of minorities and different socioeconomic groups. This was a cross‐sectional study with nationally representative data collected in 2011 by the C.S. Mott Children's Hospital National Poll on Children's Health. To determine the relationship between perceived fair payment and individual‐level characteristics, we used multivariable linear regression.
With 60% participation rate, in a sample of 2,150 respondents 11% (n = 221) of the sample had previously participated in medical research. Requested payment differed significantly by racial/ethnic group with Hispanics requesting more payment than non‐Hispanic whites (0.37 [95%CI 0.02, 0.72]) In contrast to payment at $49, $149, and $249, payment at $349 yielded proportional representation of racial/ethnic minority groups.
Hispanics requested higher payment for research participation, suggesting a possible explanation for their underenrollment.
Keywords: public, research, payment, income, race, Hispanic
Introduction
As the Belmont Report cautioned in 1979, the poor and minorities may be overrepresented in clinical research because their social status potentially leaves them vulnerable to manipulation.1 Today, with empiric evidence of underenrollment of minorities in clinical research, NIH guidelines require “appropriate representation” of minorities to achieve the goals of equitable distribution of benefits and burdens of research and identify if there are possible differences in treatment by race/ethnicity.2, 3 Increasing representation of minorities in clinical trials is intended to best serve their health needs by ensuring accurate data about response to interventions. While persistent underrepresentation of minorities has been well described,4, 5 reasons for their under‐representation are less clear.
One possible explanation is that minorities may expect different amounts of payment for participation in clinical research than others in society. No research has utilized a nationally representative sample of the public to determine the extent to which expectations of payment may affect participation of different racial/ethnic groups.6, 7, 8, 9, 10 Given that up to one‐third of studies do not meet enrollment goals,11 it is important to determine the potential role payment plays in that underenrollment.
Additionally, there are persistent theoretical concerns that the burdens of clinical research may be disproportionately experienced by the poor,1, 12 particularly because they will be unduly influenced to participate by even modest payments and overlook significant risk.13, 14 IRB members are sensitive to these concerns;15 46% of IRB participants acknowledge that their IRB panel sometimes questions the amount of proposed payment to participants.16 However, the distribution of research participation by income group is not reliably known because many clinical trials do not collect socioeconomic information on participants.
The first goal of this study was to examine how previous participation in research is distributed across different household income groups and racial/ethnic groups. The second goal of this study was to assess how the public perceives what is fair payment for a low‐risk clinical trial and whether there is an association between the amount of payment requested for research participation and sociodemographic characteristics of individuals. Using these findings, the third goal was to estimate the amount of payment that would be necessary for a hypothetical medication trial to achieve proportional representation of minorities and individuals from different socioeconomic groups.
Methods
Study sample
This is a cross‐sectional study with data collected in 2011 by the C.S. Mott Children's Hospital National Poll on Children's Health (NPCH), a Web‐based survey distributed by Knowledge Networks using a Web‐enabled KnowledgePanel.17 The KnowledgePanel is assembled using rigorous address‐based sampling and random‐digit dialing techniques and provides free computer hardware and Internet access for all contacted households that wish to participate. Participants received payment for participation in the survey. The KnowledgePanel has served as a sampling frame for multiple peer‐reviewed publications that have assessed public perceptions and opinions at the national level.18, 19, 20, 21, 22
This study sample included 2,150 adults nationally representative of the United States. Demographic information (age, sex, race/ethnicity, household income, and health conditions), previous research participation, distance to a research center and responses to hypothetical scenarios were provided by respondents. Race/ethnicity was categorized as non‐Hispanic white, non‐Hispanic black, other non‐Hispanic and Hispanic. Yearly household income was categorized as <$30,000; $30–60,000; $60–100,000; and >$100,000. A complex survey design was used to achieve a nationally representative sample of adults as determined by the 2010 Current Population Survey (CPS).
Survey instrument
The primary survey questions used for our analysis probed respondents' perceptions of a fair amount of payment paid as a “thank you” for participation in research (Appendix Figure A1). Respondents were presented a scenario describing participation as a volunteer in a low‐risk medication trial that would require daily medication for 2 months, then no medication during 10 months of follow‐up, and medical evaluation requiring two blood draws and one physical exam. Respondents were asked to indicate the “total payment you believe is fair for an adult to receive as a thankyou for participating;” they were told that all transportation costs would be reimbursed separate from the payment. There were no anchors provided and respondents could enter a number ranging from $0 to $10,000. We recognize that respondents could interpret the question as requesting their participation as either a healthy volunteer or if they had the disease being studied.
Additional survey questions probed the importance of different factors in respondents' willingness to participate in research as a healthy volunteer or if the respondent had the disease being studied. To minimize respondent burden, sets of questions were randomly presented so that not all questions were given to all respondents. Respondents were asked to indicate how important (very, somewhat, or not important) each of the following factors would be when considering whether they would participate in medical research: total amount of time required to participate; amount of pain/discomfort; risks of the research study; inconvenience or “hassle”; amount of payment or incentive for participating; benefits of research for one's own health; benefits of research for the health of others. In addition, a separate survey question asked that if respondents wanted to participate in medical research how far would they have to travel (categorized as 15 minutes or less; 15–30 minutes; 31–60 minutes; >60 minutes; unsure of where nearest medical research site is located). Finally, participants were asked, “have you ever participated in any type of medical research?”
Analyses of interest
The first analysis of interest included demographic information (race/ethnicity and yearly household income) associated with previous participation in research. The second analysis focused on the amount of payment perceived to be fair for a low‐risk medication trial, to investigate if there were differences across racial/ethnic and income groups. The third analysis centered on the amount of payment necessary for the hypothetical medication trial to achieve proportional representation of minorities and individuals from different socioeconomic groups.
Missing data
Multiple imputation using chained equations was used to impute missing data for how important different factors were in determining whether someone would be willing to participate in medical research (e.g., total amount of time required to participate; amount of pain/discomfort; risks of the research study; inconvenience or “hassle”; amount of payment or incentive for participating; benefits of research for one's own health; benefits of research for the health of others). Imputation was utilized for approximately 400 observations that were missing not at random due to our survey design intended to mitigate respondent burden. Imputations were calculated utilizing demographic information (sex, race/ethnicity, yearly household income) and responses to other questions including previous research participation and distance to research center.
Statistical analyses
First, to determine the distribution of research participation by race/ethnicity and income we used descriptive statistics to determine the percent of all respondents who participated in research over categories of race, ethnicity and income. We also used descriptive statistics to demonstrate the mean and median requested payment by racial/ethnic and income groups. Next, to determine the relationship between perceived fair payment and individual‐level characteristics we used multivariable linear regression with requested payment as the dependent variable. Because data for requested payment was skewed to the right, we log‐transformed the outcome. Independent variables included demographic characteristics (self‐reported age, sex, and race/ethnicity), annual household income, distance to travel to research center, and the importance of six different factors in the respondent's willingness to participate in research (importance of time, hassle, pain, risk, benefit to self, benefit to others). Subjects who refused participation in both healthy and patient‐based research (6% of the sample) were not included in the analysis.
The associations of race/ethnicity and income with requested payment were examined using regression coefficients. These coefficients represent the percent difference in requested payment for each category, because our outcome was log‐transformed.
Finally, to estimate the amount of payment required to achieve proportional representation of minorities and low‐income populations in our survey scenario, we varied the level of payment for research in $100 intervals and estimated the proportion of patients willing to participate at a given level of payment by summing the number of respondents believing a payment at that level or lower was fair payment until approximately 50% of respondents believed payment was fair. We then compared these racial/ethnic and household income distributions at each payment level to a nationally representative population of the United States using survey weighted proportions for those demographic characteristics as determined by the 2010 CPS. All analyses were performed using Stata, version 12.0 (StataCorp, College Station, TX, USA).
Results
Sample characteristics
The participation rate among those invited to answer the survey was 60%. The study sample included 2,150 participants, with nationally weighted demographic characteristics as shown in Table 1. Eleven percent (n = 221) of the sample had previously participated in medical research (Table 1).
Table 1.
Demographics of NPCH from 2011
| Frequency (unweighted proportion) | Weighted proportion % (95% CI) | |
|---|---|---|
| Sex | ||
| Male | 1,038 (48) | 48 (45–52) |
| Female | 1,112 (52) | 52 (48–55) |
| Age (in years) | ||
| 18—29 | 393 (18) | 19 (16–22) |
| 30—44 | 845 (39) | 28 (25–31) |
| 45—59 | 679 (32) | 29 (26–33) |
| >60 | 233 (11) | 24 (21–28) |
| Income (in dollars) | ||
| <$30K | 542 (25) | 34 (30–37) |
| $30–60K | 651 (30) | 31 (28–34) |
| $60–100K | 610 (28) | 23 (21–26) |
| >$100K | 347 (16) | 12 (10–14) |
| Race/ethnicity | ||
| Non‐Hispanic white | 1,587 (74) | 70 (67–73) |
| Non‐Hispanic black | 190 (9) | 11 (9–13) |
| Non‐Hispanic other | 146 (7) | 7 (5–9) |
| Hispanic | 227 (11) | 12 (10–15) |
| Previous research participation | 221 (10) | 11 (9–14) |
| Distance to travel for participation | ||
| 15 minutes or less | 327 (15) | 13 (11–16) |
| 15–30 minutes | 495 (23) | 20 (18–23) |
| 31–60 minutes | 314 (15) | 17 (14–20) |
| >60 minutes | 128 (6) | 7 (6–10) |
| Unsure of distance | 871 (41) | 42 (39–46) |
| Willingness to participate in clinical research | ||
| Yes | 1,070 (50) | 47 (43–50) |
| Unsure | 960 (45) | 47 (44–50) |
| No | 120 (6) | 6 (5–8) |
Distribution of research participation by race/ethnicity and income
In our sample, individuals in minority racial/ethnic groups were significantly less likely to have participated in research than were non‐Hispanic whites: 4% of Hispanics and 2% of non‐Hispanic blacks compared with 14% of whites (p = 0.001 and p < 0.001, respectively). There was comparatively less variation in prior participation by income (Table 2).
Table 2.
Proportion of previous research participants by race/ethnicity and income
| Weighted proportion of each group who reported prior participation n = 221% (95% CI) | |
|---|---|
| Race/ethnicity | |
| Non‐Hispanic white | 14 (11–17) |
| Non‐Hispanic black | 2 (0.8–6) |
| Non‐Hispanic other | 14 (6–30) |
| Hispanic | 4 (2–8) |
| Annual household income | |
| <$30,000 | 13 (9–18) |
| $30,000–$60,000 | 10 (7–15) |
| $60,001–$100,000 | 10 (7–15) |
| >$100,000 | 14 (9–21) |
Requested payment for research participation
Across the full sample, the median requested payment for adults' participation in the specified low‐risk research study was $300, with a mean of $1,160 (range $0–$10,000). Among those willing or unsure if they would participate in research the median requested payment was $300 with a mean of $1,119.
Median requested payments were approximately one‐third of the mean requested payments, but both median and means demonstrated similar trends that were also demonstrated in the multivariate linear regression. Requested payment differed significantly by annual household income. After adjusting for respondent characteristics, distance to research center and stated importance of factors affecting willingness to participate (Table 3), the $30,000–60,000 income group was requesting less payment than the lowest income group (–0.36 [95% CI –0.69, –0.03]). We found no significant differences in requested payment in higher income groups compared to the lowest income group. Unadjusted median and mean requested payment by income (Table 4) demonstrated a similar trend with lowest income group requesting median payment $400 with $30–60,000 requesting $300 and highest income group requesting $300.
Table 3.
Association between requested payment for research participation (log transformed), sociodemographic characteristics and the importance of different factors affecting willingness to participate in clinical research
| Coefficient | 95% Confidence interval | |
|---|---|---|
| Sex (reference: female) | ||
| Male | 0.10 | –0.15, +0.36 |
| Race/ethnicity (reference: non‐Hispanic white) | ||
| Non‐Hispanic Black | 0.16 | –0.33, +0.66 |
| Non‐ Hispanic other | 0.36 | –0.03, +0.74 |
| Hispanic | 0.37a | 0.02, +0.72 |
| Income (in dollars) (reference: <$30,000) | ||
| $30–60,000 | –0.36a | –0.69, ‐0.03 |
| $60–100,000 | –0.03 | –0.35, +0.29 |
| >$100,000 | –0.23 | –0.62, +0.19 |
| Previous research participation | –0.32 | –0.74, +0.09 |
| Distance to travel for participation (reference: < 15 min) | ||
| 15–30 minutes | –0.12 | –0.44, +0.20 |
| 31–60 minutes | –0.48a | –0.89, +0.06 |
| >60 minutes | 0.05 | –0.50, +0.59 |
| Unsure | –0.22 | –0.56, +0.11 |
| Importance of time (reference: very important) | ||
| Somewhat important | –0.14 | –0.52, +0.24 |
| Not important | –0.29 | –0.99, +0.42 |
| Importance of pain/discomfort (reference: very important) | ||
| Somewhat important | 0.48 | –0.31, +0.40 |
| Not important | –0.07 | –0.80, +0.65 |
| Importance of risk (reference: very important) | ||
| Somewhat important | –0.30 | –0.73, +0.13 |
| Not important | –0.36 | –1.40, +0.68 |
| Importance of payment (reference: very important) | ||
| Somewhat important | –0.04 | –0.43, +0.35 |
| Not important | –0.28 | –0.83, +0.30 |
| Importance of benefit to self (reference: very important) | ||
| Somewhat important | –0.27 | –0.66, +0.13 |
| Not important | 0.38 | –0.48, +1.23 |
| Importance of benefit to others (reference: very important) | ||
| Somewhat important | 0.08 | –0.25, +0.42 |
| Not important | 0.94a | 0.13, +1.86 |
Requested compensation figures were log transformed in analysis.
p ≤ 0.05.
Table 4.
Unadjusted median and mean requested payment in those unsure or willing to participate in research by income and race/ethnicity
| Median requested payment (interquartile range) | Mean requested payment (SD) | |
|---|---|---|
| Income | ||
| <$30,000 | 400 (900) | 1,252 (2,304) |
| $30–60,000 | 300 (500) | 1,064 (2,081) |
| $60–100,000 | 400 (900) | 1,077 (2,086) |
| >$100,000 | 300 (500) | 1,097 (2,274) |
| Race/ethnicity | ||
| Non‐Hispanic white | 300 (500) | 989 (2,008) |
| Non‐Hispanic black | 300 (1,100) | 1,537 (2,813) |
| Non‐Hispanic other | 500 (700) | 1,516 (2,689) |
| Hispanic | 500 (900) | 1,474 (2,241) |
Requested payment also different significantly by racial/ethnic group with Hispanics requesting more payment than non‐Hispanic whites (0.37 [95%CI 0.02, 0.72]) (Table 3). Unadjusted medians and means demonstrated the same trends with non‐Hispanic whites requesting $300 while Hispanics requested $500 (Table 4).
Finally, respondents who indicated that benefiting others was not important to them in determining whether they would participate in research were more likely to request higher payment for participation (coeff. 0.94; 95%CI 0.13, 1.86).
Estimated effects on sample composition of varying levels of payment
We also estimated the overall population proportion and the relative proportions of different racial/ethnic and income groups that would believe payment for participation is fair at different payment levels in increments of $100. These estimates permitted us to examine which level of payment would most closely approximate relative proportions of different racial/ethnic and income groups in the United States. For the different panels of Figures 1 and 2 representing proportions for different potential payment, the bars that are smaller and closer to the 0 baseline most closely are aligned with the representation of that group in the US population.
Figure 1.
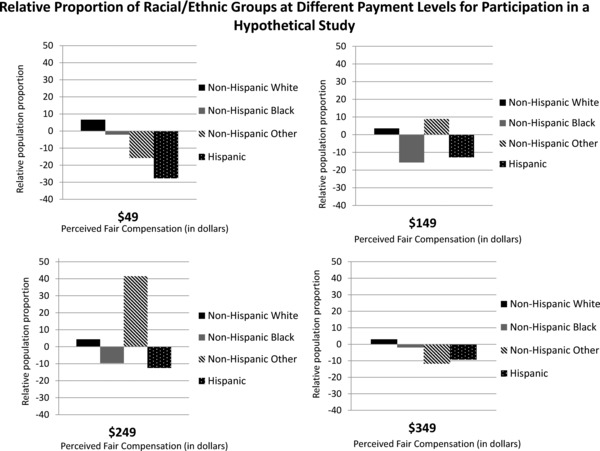
Relative proportion of racial/ethnic groups at different payment levels for participation in a hypothetical study.
Figure 2.
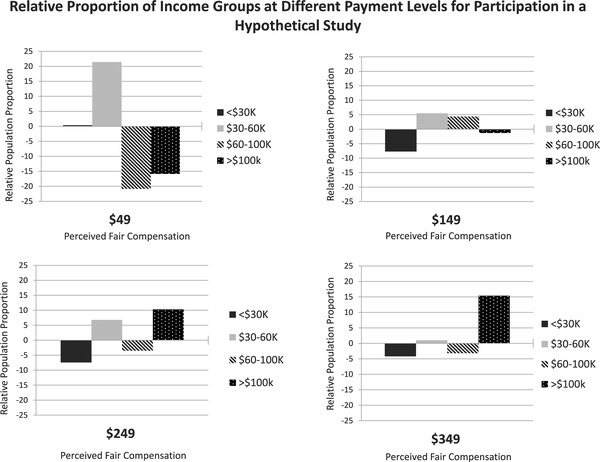
Relative proportion of income groups at different payment levels for participation in a hypothetical study.
Overall, increasing payment from $49 to $349 for the same low‐risk medical research would be expected to increase those believing payment was fair from 7% to 50%. For each cohort at the different payment levels, the relative proportion of an individual group shifts depending on what proportion of the overall whole they comprise.
For underrepresented racial/ethnic minority groups, payment at $349 yielded the closest overall match in perceived fairness when compared with population representation for adults (Figure 1). At lower levels of payment, non‐Hispanic whites were consistently overrepresented in believing payment was fair and Hispanics and non‐Hispanic blacks were typically under‐represented, compared with Census distributions of those racial/ethnic groups. At payment levels above $349, proportions of participants' perceptions of fairness remained matched to census distributions of racial/ethnic groups.
When examining relative proportional representation in research for different income groups related to perceived fairness of hypothetical payment (Figure 2), we again found that higher payment would be expected to yield not only higher likelihood of perceiving payment as fair, but closer population‐level proportional representation of each of the four income groups. However, the differences in perception of fairness versus Census distribution were not as large as for different racial/ethnic groups.
Discussion
This is the first study to examine a nationally representative sample to evaluate whether the amount of payment provided for clinical research may disproportionately affect recruitment of minorities and the lowest income group due to varied expectations about fair payment for participation in low‐risk clinical research. We found that racial/ethnic minorities in general, and Hispanics in particular, request higher payment than non‐Hispanic whites to participate in research with the same description of risk and benefit. While there were differences between the medians versus the means for whether non‐Hispanic blacks wanted more compensation than non‐Hispanic whites, the median requested income was the same for both blacks who were willing to participate and those who were unsure if they would participate, both of which were similar to requested payment by whites. This suggests that, contrary to conventional wisdom, members of racial/ethnic minority groups may not be more likely to participate than whites at lower levels of payment. Moreover, these findings may provide new insight about why racial/ethnic minorities (particularly Hispanics) are underrepresented in clinical trials. Furthermore, we found that low‐income subjects did not, in general, demand lower levels of payment for participation, offering evidence that they may not be unduly influenced by payment to participate in research.
This study addresses a key knowledge gap related to public participation in medical research, which is currently insufficient to meet enrollment goals for one‐third of studies.11 At present, there is no consensus regarding what constitutes appropriate or fair payment for clinical research participation, and most IRBs do not have written guidelines for determining how to pay participants.16, 23 A majority of IRB members are concerned that any payment may be coercive or unduly influential of potential research participants, with increasing concern for higher payments used as incentives or as payment for risk.15 While there is no research to date about how IRB members' perceptions may influence approval of protocols, it is conceivable that pervasive beliefs about undue influence may ultimately discourage investigators from offering payments to participants that would be perceived to be incentives or payment for risk by IRBs.
Members of IRBs demonstrate the broadest support for reimbursement of expenses or payment for time and inconvenience.15 However, most protocols do not indicate how much each clinic visit or hospitalization is compensated, and wide variation in payment exists for relatively comparable studies.23 For Phase III clinical trials, similar to the hypothetical scenario in this study, Grady found that the median payment for such studies was $275 with a mean of $361.23 For studies that enrolled both healthy volunteers and patients, the median payment was $100 with a mean of $199.23 These mean payment values fall substantively below payment requested by Hispanic and the lowest income group respondents in our sample, even when accounting for inflation.
Underenrollment and inequitable enrollment
Consequently, findings from this nationally representative sample raise the concern that underenrollment in clinical research may be explained in part by the public's perception that payment is less than fair. While there were a few respondents who are willing to participate without payment, the majority of respondents requested payment at levels higher than what Grady found in her national study for more invasive and time‐consuming studies of similar risk. The median requested payments were approximately one‐third the mean requested payments in our findings, and are closer to Grady's reported values, but in the Hispanic subgroup in particular the requests are still twice the median reported by Grady for trials that are likely more invasive than our hypothetical case. Our findings suggest that increasing subject payment may mitigate problems of underenrollment while potentially increasing trial costs. However, the increase in trial costs due to the costs of higher payment may be offset by shortening enrollment periods and thereby decreasing operating costs.
Concerns that the poor are overrepresented in clinical research and that they are more susceptible to undue influence from financial payments for participation14 are called into question by this study and warrant further investigation. While there are few data regarding participation of the poor in research because many protocols do not routinely collect socioeconomic data on participants, our study offers two key insights on this issue. First, in examining the income distribution of study respondents who stated they had previously participated in research, the lowest income group is not disproportionately represented. Second, our data demonstrate that payment requests do not decrease monotonically with income. In fact, when presented with exactly the same description of a scientific research opportunity, the lowest income subgroup did not request a significantly different payment than the highest income subgroup. While these findings do not demonstrate unequivocally that the lowest income group is not influenced by payment, they do suggest that the low levels of payment for clinical research currently offered across the nation do not preferentially lead the lowest income group to participate.
Regarding research payment and minority enrollment, Hispanics (who have participated significantly less than non‐Hispanic whites in clinical research) request a significantly higher payment than non‐Hispanic whites. While language barriers may contribute to the underenrollment of Hispanics in general in the United States,24 our survey was only administered in English and all Hispanic respondents considered themselves fluent in English, mitigating the possibility that language proficiency was driving the request for higher payment in this study. Alternatively, Hispanics' higher request for payment may correspond with concerns about their trust in the system—a barrier to participation believed to be held by African Americans.25 Importantly, non‐Hispanic black respondents in this study did not request significantly higher payment for participation than non‐Hispanic whites, suggesting that payment may not be the only factor driving blacks' underenrollment that mirrors that of Hispanics at the national level. Further research is warranted regarding other factors that may influence disparities in research participation for communities of color, including access to health care that is known to differ substantially by race/ethnicity26 and may affect how patients of color learn about research opportunities.
Our analysis of how proportional participation may vary with offered payment further illuminates the potential effects of IRB judgments about fair and noncoercive treatment for subjects. At payment levels below the median requested payment for the hypothetical trial described in this study, non‐Hispanic blacks and Hispanics are underrepresented with respect to their population composition. In contrast, proportional representation was more consistent for different income groups across the spectrum of payment levels. Given that the lowest and highest income groups believe similar payment levels are fair and racial/ethnic minorities believe higher payment is warranted to be fair, higher levels of payment may be a key to reducing minority underenrollment in clinical research and may not be unduly influential. Given the statistically significant association of increased requested payment with “importance of benefit to others,” there is an imperative to examine in greater detail how and why distinct racial/ethnic groups and income groups differ with regard to their consideration of participation in medical research. While not addressed in this paper, other analyses are ongoing regarding the nature and dynamics of differences by race/ethnicity with respect to other sociodemographic factors.
Limitations
Limitations to this study must be acknowledged. First, the hypothetical scenario addresses only one type of research study, which many not be generalizable to other types of clinical research circumstances. Particularly if participants were asked to participate as a healthy volunteer, higher risk studies may produce different requests for payment. Second, those who have participated or been recruited to participate in clinical research may have their requested payment influenced by previous offers for payment which anchored their expectations and lowered their requested amount. Third, perceptions about fair payment do not necessarily track with one's willingness to participate in research, however, the variation across different racial/ethnic and income groups tracks with enrollment and therefore should be considered as a factor in willingness to participate in research. Additionally, potential subjects may be willing to accept payment at levels lower than what they perceive to be fair.
Fourth, the survey provides respondents' stated preferences instead of observations of actual behavior when confronted with alternative payments. Further research that randomly varies payment would provide valuable data about whether individuals vary their willingness to participate in research, and whether this varies by racial/ethnic groups. Such research would be helpful in determining whether increased payment may reduce observed disparity in enrollment and whether and how it affects individuals' perceptions of risk. Finally, the sample comes from a panel of potential subjects who have agreed to respond to surveys, which could theoretically make the group self‐selected.
Conclusion
Our data is consistent with other studies that demonstrate that minorities have participated in medical research at lower rates than non‐Hispanic whites4, 5 but adds evidence that low‐income individuals are not disproportionately represented in medical research overall. Our finding that low‐income individuals request payment for participation similar to high‐income peers also mitigates the theoretical concern that the lowest income group is particularly susceptible to payment for participation. While proportional representation by socioeconomic status is achievable at payment levels similar to what is typically offered, our data indicate that higher payment may be necessary to increase Hispanics' participation in research and to achieve higher public participation in research overall. If the research community is committed to achieving adequate representation in clinical research to identify treatments that work in racial/ethnic minority populations, investigators will likely need to offer what these groups consider fair payments for participation.
Acknowledgments
This research project was supported by the Clinical Scholars program of the Robert Wood Johnson Foundation (JW, JB, MD) and an advanced fellowship through the Department of Veterans Affairs (JB). Data collection was funded by the Michigan Institute for Clinical and Health Research (NCRR UL1RR024986) and the University of Michigan Health System. We would like to thank Dr. Hwajung Choi, Ph.D. in the Robert Wood Johnson Foundation Clinical Scholars Program at the University of Michigan for her assistance with data analysis. Drs. Walter and Davis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figure A1.
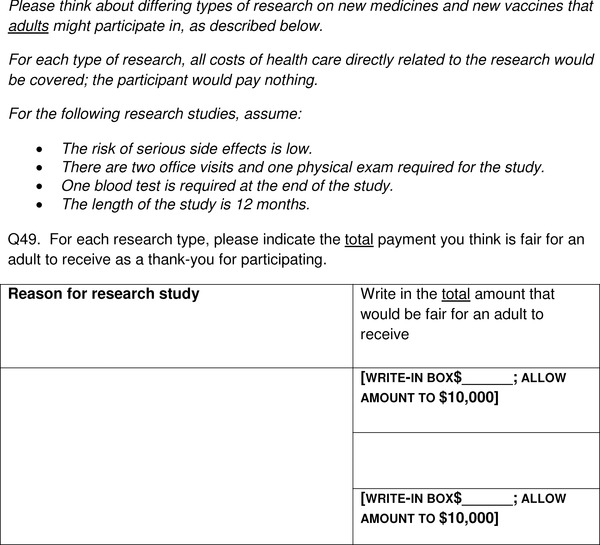
Survey item for requested payment for clinical research participation.
References
- 1. The Belmont Report: The Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC: US Government Printing Office; 1978; OS publication 78–0012. [Google Scholar]
- 2. NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. Vol. 59. Washington, DC: Federal Register; 1994: 14508–14513. [Google Scholar]
- 3. Corbie‐Smith G, Miller W, Fransohoff D. Interpretations of ‘appropriate’ minority inclusion in clinical research. Am J Med. 2004; 116(4): 249–252. [DOI] [PubMed] [Google Scholar]
- 4. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race‐, sex‐, and age‐based disparities. JAMA. 2004; 291(22): 2720–2726. [DOI] [PubMed] [Google Scholar]
- 5. Dignan M, Evans M, Kratt P, Pollack LA, Pisu M, Smith JL, Prayor‐Patterson H, Houston P, Watson C, Hullett S, Martin MY. Recruitment of low income, predominantly minority cancer survivors to a randomized trial of the I Can Cope cancer education program. J Health Care Poor Underserved 2011; 22(3): 912–924. [DOI] [PubMed] [Google Scholar]
- 6. Cryder C, London J, Volpp KG, Loewenstein G. Informative inducement: study payment as a signal risk. Soc Sci Med. 2010; 70(3): 455–464. [DOI] [PubMed] [Google Scholar]
- 7. Halpern S, Karlawish JH, Casarett D, Berlin JA, Asch DA. Empirical assessment of whether moderate payments are undue or unjust inducements for participation in clinical trials. Arch Inter Med. 2004; 164(7): 801–803. [DOI] [PubMed] [Google Scholar]
- 8. Czarny MJ, Kass NE, Flexner C, Carson KA, Myers RK, Fuchs EJ. Payment to healthy volunteers in clinical research: the research subject's perspective. Clin Pharmacol Therapeut 2010; 87(3): 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Festinger DS, Marlowe DB, Croft JR, Dugosh KL, Mastro NK, Lee PA, Dematteo DS, Patapis NS. Do research payments precipitate drug use or coerce participation? Drug Alcohol Depend 2005; 78(3): 275–281. [DOI] [PubMed] [Google Scholar]
- 10. Festinger DS, Marlowe DB, Dugosh KL, Croft JR, Arabia PL. Higher magnitude cash payments improve research follow‐up rates without increasing drug use or perceived coercion. Drug Alcohol Depend 2008; 96(1–2): 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, Entwistle V, Garcia J, Roberts I, Grant A, Grant A; STEPS group . Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess 2007; 11(48): iii, ix–105. [DOI] [PubMed] [Google Scholar]
- 12. Elliot C, Abadie R. Exploiting a research underclass in phase I clinical trials. New England J Med. 2008; 358: 2316–2317. [DOI] [PubMed] [Google Scholar]
- 13. Dickert N, Grady C. What's the price of a research subject? Approaches to payment for research participation. New England J Med. 1999; 341(3): 198–203. [DOI] [PubMed] [Google Scholar]
- 14. Macklin R. “Due” and “undue” inducements. IRB 1981; 3: 1–5. [PubMed] [Google Scholar]
- 15. Largent EA, Grady C, Miller FG, Wertheimer A. Money, coercion, and undue inducement: attitudes about payments to research participants. IRB. 2012; 34(1): 1–8. [PMC free article] [PubMed] [Google Scholar]
- 16. Ripley E, Macrina F, Markowitz M, Gennings C. Why do we pay? A national survey of investigators and IRB chairpersons. J Empirical Res Hum Res Ethics 2010; 5(3): 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dennis J. Summary of KnowledgePanel Design. 2010; Available at: www.knowledgenetworks.com/ganp/reviewer‐info.html. Accessed May 14, 2012.
- 18. Macy M, Clark S, Freed GL, Butchart AT, Singer DC, Sasson C, Meurer WJ, Davis MM. Carpooling and booster seats: a National Survey of Parents. Pediatrics 2012; 129(2): 290–298. [DOI] [PubMed] [Google Scholar]
- 19. Tarini B, Singer D, Clark S, Davis M. Parents' interest in predictive genetic testing for their children when a disease has no treatment. Pediatrics 2009; 124(3): e432. [DOI] [PubMed] [Google Scholar]
- 20. Tarini B, Singer D, Clark SJ, Davis MM. Parents' concern about their own and their children's genetic disease risk: potential effects of family history vs genetic test results. Arch Pediatr Adoles Med. 2008; 162(11): 1079–1083. [DOI] [PubMed] [Google Scholar]
- 21. Freed GL, Dunham K, Clark S. Perspective and preferences among the general public regarding physician selection and board certification. J Pediatr. 2010; 156(5): 841–845. [DOI] [PubMed] [Google Scholar]
- 22. Dempsey A, Singer D, Clark S. Adolescent preventive health care: what do parents want? J Pediatr. 2009; 155(5): 689–694. [DOI] [PubMed] [Google Scholar]
- 23. Grady C, Dickert N, Jawetz T, Gensler G, Emanuel E. An analysis of U.S. practices of paying research participants. Contemp Clin Trials. 2005; 26(3): 365–375. [DOI] [PubMed] [Google Scholar]
- 24. Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant‐friendly system. J Natl Cancer Inst. 1995; 87(23): 1747–1759. [DOI] [PubMed] [Google Scholar]
- 25. Ford M, Havstad S, Davis S. A randomized trial of recruitment methods for older African American men in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Clin Trials. 2004; 1(4): 343–351. [DOI] [PubMed] [Google Scholar]
- 26. Agency for Healthcare Research and Quality . 2010 National Healthcare Disparities Report 2010. AHRQ Publication 11–0005. Available at: http://www.ahrq.gov/qual/qrdr10.htm Accessed May 16, 2012.


