Abstract
γ-Secretase is an intramembrane cleaving protease involved in Alzheimer's disease. γ-Secretase occurs as a high molecular weight complex composed of presenilin (PS1/2), nicastrin (NCT), anterior pharynx-defective phenotype 1 and PS enhancer 2. Little is known about the cellular mechanisms of γ-secretase assembly. Here we demonstrate that the cytoplasmic tail of PS1 fulfills several functions required for complex formation, retention of unincorporated PS1 and γ-secretase activity. The very C-terminus interacts with the transmembrane domain of NCT and may penetrate into the membrane. Deletion of the last amino acid is sufficient to completely block γ-secretase assembly and release of PS1 from the endoplasmic reticulum (ER). This suggests that unincorporated PS1 is actively retained within the ER. We identified a hydrophobic stretch of amino acids within the cytoplasmic tail of PS1 distinct from the NCT-binding site, which is required to retain unincorporated PS1 within the ER. Deletion of the retention signal results in the release of PS1 from the ER and the assembly of a nonfunctional γ-secretase complex, suggesting that at least a part of the retention motif may also be required for the function of PS1.
Keywords: Alzheimer's disease, amyloid precursor protein, nicastrin, presenilin, γ-secretase
Introduction
γ-Secretase is an unusual aspartyl protease that cleaves a variety of substrates within the transmembrane (TM) domain (De Strooper, 2003). Two of the most relevant substrates are the cell-fate-specifying receptor Notch, and the Alzheimer's disease-associated amyloid precursor protein (APP) (Haass, 2004). γ-Secretase is composed of four proteins, the presenilins 1 or 2 (PS1, 2), nicastrin (NCT), anterior pharynx-defective phenotype 1 (APH1) and PS enhancer 2 (PEN2) (De Strooper, 2003; Haass, 2004). These proteins assemble into a high molecular weight (HMW) complex (Haass, 2004). Co-expression of all four proteins restores γ-secretase activity in yeast, an organism lacking any endogenous γ-secretase activity (Edbauer et al, 2003). Presumably, γ-secretase assembles within the endoplasmic reticulum (ER) and is transported through the Golgi, where NCT is complex glycosylated and transported to the plasma membrane and the endosomal/lysosomal system (De Strooper, 2003).
PS1 is a polytopic membrane protein of around 50 kDa, whose topology is not fully established. In the widely accepted model, the 10 hydrophobic domains are thought to span the membrane eight times (Li and Greenwald, 1998), with N- and C-termini being cytosolic (Doan et al, 1996; Li and Greenwald, 1998). Alternatively, a ‘seven membrane-spanning and one membrane-embedded' topological model has been proposed, where the C-terminus is located in the lumen (Nakai et al, 1999). Biological activation of PS1 involves endoproteolysis, resulting in the tightly attached N-terminal fragment (NTF) and C-terminal fragment (CTF) (reviewed in De Strooper, 2003). Identification of the binding sites for NTF/CTF interaction and the interaction between PS fragments and the other complex components are currently topics of intense investigations. A direct interaction between APH1 and NCT was demonstrated (for a review, see Periz and Fortini, 2004). In addition, Iwatsubo and colleagues demonstrated that the integrity of the C-termini of PS1 and 2 is critical for complex assembly and γ-secretase function (Tomita et al, 1999; 2001; Takasugi et al, 2002).
We here studied the role of the PS1 C-terminus (PSCT) in assembly and transport of γ-secretase. We found that PSCT contains a binding site for the γ-secretase complex component NCT, and a distinct ER-retention/retrieval motif. Moreover, mapping the PS1-binding site on NCT to its TM implies a topology for PS1, where PSCT is diving into or penetrating completely the membrane.
Results
The C-terminus of PS1 binds nicastrin
PS1 probably has multiple interacting domains with other γ-secretase complex components. Hence, it may be difficult to analyse mutant variants, as the effect of the deletion or mutation might be masked by other domains still present on the molecule. We therefore decided to study PSCT (coding for the last 37 amino acids following TM8) outside of its context in the γ-secretase complex and attached it to a γ-secretase unrelated reporter, CD4 (Figure 1A and B). To analyse putative interactions of fusion protein CD4PSCT with γ-secretase complex components, we stably transfected HEK293 cells expressing Swedish mutant APP (Swe) and performed a co-immunoprecipitation (IP) analysis using CD4 antibody. In Swe cells and in Swe cells expressing CD4, no NCT was co-IPed (Figure 1C). However, in cells expressing CD4PSCT, endogenous immature NCT together with traces of mature NCT were co-IPed with a CD4 antibody (Figure 1C). To further define the interaction domain responsible for Nct binding, we deleted PSCT N- or C-terminally (Figure 1B). Deletion of the highly conserved PALP motif at the N-terminus of PSCT (Tomita et al, 1999) still allowed binding to NCT (Figure 1C). In contrast, deletion of 1, 3, or 5 amino acids from the C-terminus abrogates NCT binding, suggesting that the NCT-binding site is at the very C-terminus of PSCT (Figure 1C).
Figure 1.
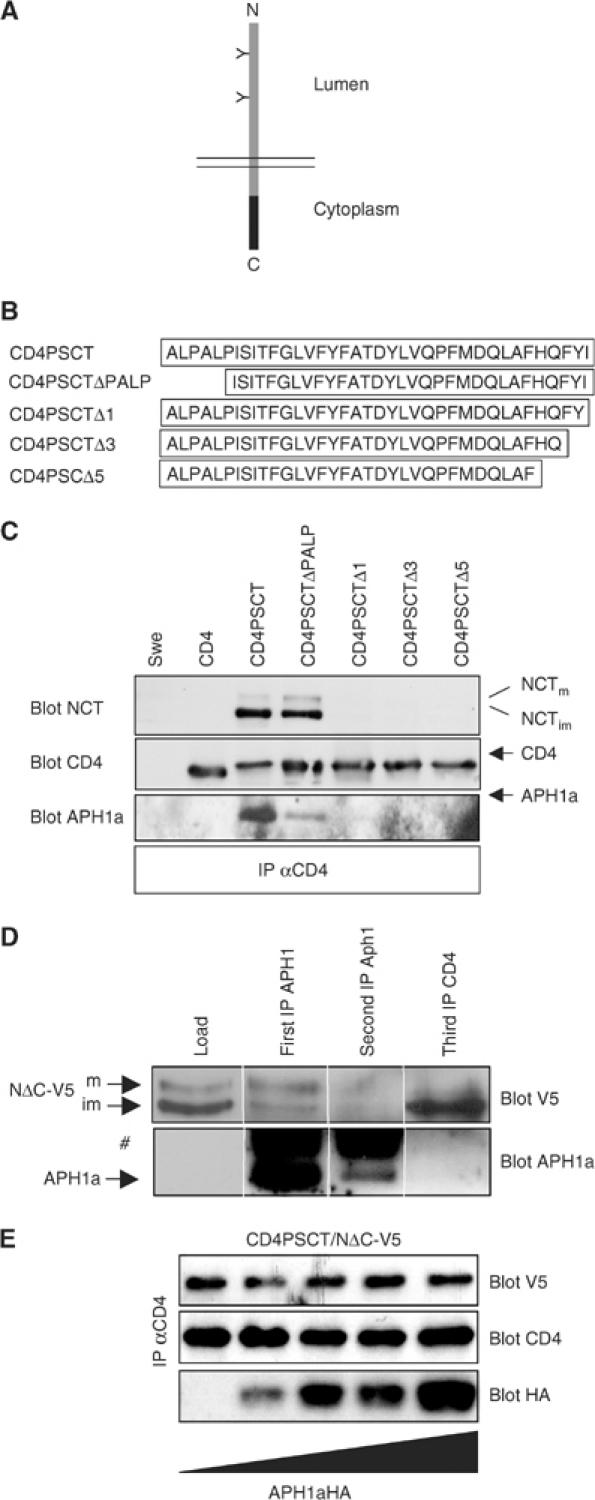
PSCT binds NCT. (A) Scheme of the reporter CD4, a type I TM protein (grey bar). Black bar, PS1 sequences to be tested for functional domains. (B) Scheme of PSCT and deletion variants. (C) Swe cells or Swe cells stably expressing CD4 variants were lysed in 2% CHAPSO/citrate and IPed with CD4 antibody. After SDS–PAGE and western blotting, membranes were probed with antibodies as indicated. NCTm, mature NCT; NCTim, immature NCT. Only CD4PSCT and CD4PSCTΔPALP bound immature NCT and some mature NCT. (D) Swe cells stably expressing CD4PSCT and NΔC-V5 were lysed in 2% CHAPSO/citrate and IPed with APH1a antibodies. The supernatant was subjected to a second APH1a IP, followed by a third IP, using CD4 antibody. After SDS–PAGE and western blotting, membranes were probed with antibodies as indicated. #, IgG. Note that CD4 was IPed with a monoclonal antibody, therefore no IgGs are visible. (E) Swe cells stably expressing CD4PSCT and NΔC-V5 were transiently transfected with increasing amounts of APH1aL-HA, as symbolized by the triangle. After 48 h, cell lysates were IPed with antibody against CD4 and blotted with antibodies as indicated. Binding of endogenous NCT or NΔC-V5 is independent of APH1a expression levels. NΔC-V5 is a NCT variant able to bind PSCT (see Figure 4).
APH1 might form a subcomplex with NCT (Periz and Fortini, 2004; Shirotani et al, 2004). Indeed, when blotted with an antibody against APH1a, small amounts of endogenous APH1a are detected upon long exposure, which co-precipitate with endogenous NCT and CD4PSCT or CD4PSCTΔPALP (Figure 1C). To analyse whether APH1a is directly involved in binding of PSCT to NCT, we depleted endogenous APH1a from cells stably overexpressing CD4PSCT and NΔC-V5, an NCT variant also able to bind CD4PSCT (see Figure 4). If APH1a is essential for the binding of NCT to CD4PSCT, depletion of APH1a should also deplete CD4PSCT bound to NΔC-V5. IP with APH1a antibody co-IPed preferentially mature NΔC-V5, indicating γ-secretase complex association (Figure 1D). A second IP with APH1a antibody IPed only minor amounts of APH1a and no detectable amounts of NΔC-V5, indicating nearly full depletion. This supernatant was then subjected to an IP with CD4 antibodies. Robust amounts of NΔC-V5 were co-IPed, while APH1a could not be detected, indicating that APH1a is not directly involved in binding of PSCT to NCT. Likewise, if APH1a is essential for the binding of NCT to PSCT, the endogenous pool available for binding should be limited and overexpression of APH1a would be expected to increase the binding of NCT to PSCT. We therefore expressed increasing amounts of APH1a in cells stably expressing CD4PSCT and NΔC-V5. IP with CD4 antibodies revealed that the amount of overexpressed NΔC-V5 bound to PSCT is independent of the amount of transfected APH1a, suggesting that APH1a is not involved in this binding (Figure 1E). These data strongly suggest that the binding of NCT to PSCT is direct and not APH1a dependent. We never found PEN2 co-precipitating with CD4PSCT and NCT in Swe cells. In addition, when expressed in PS1/2−/− mouse embryonic fibroblasts (MEFs), CD4PSCT also binds NCT (data not shown). In these cells, PEN2 is strongly downregulated (Steiner et al, 2002). These data further support the conclusion that the binding of the PSCT to NCT is direct and not mediated via other complex components.
Figure 4.
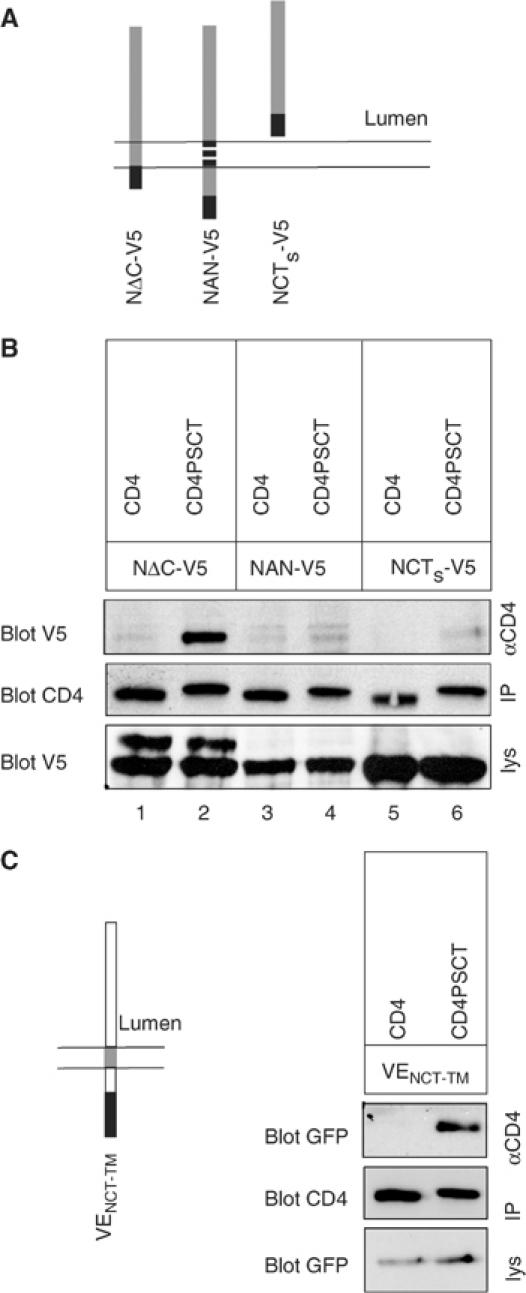
NCT binds to PSCT via its TM domain. (A) Scheme of NCT variants used for determination of the binding site. NΔC-V5 lacks the cytoplasmic tail of NCT, in NAN-V5 the TM is replaced by that of ADAM10, and NCTs-V5 lacks TM and the cytoplasmic tail of NCT. Black bar, V5 epitope; hatched box, ADAM10 TM. (B) Lysates of Swe stably expressing CD4 variants as indicated and NΔC-V5, NAN-V5, or NCTs-V5 were IPed using monoclonal CD4 antibodies. After western blotting, membranes were probed with V5 antibodies (top) or polyclonal CD4 antibodies (middle). To assess total levels of NCT V5 variants, cell lysates were probed using V5 antibody (lower panel, lys). Only NΔC-V5 binds CD4PSCT (lane 2). (C) The NCT TM binds PSCT also in the context of VSVG-EGFP, another type I protein. In VENCT-TM the TM of VSVG-EGFP (VE) was replaced by the NCT TM (grey bar on the scheme, black bar represents the EGFP moiety). Lysates of Swe stably expressing CD4 variants and VENCT-TM were IPed using a monoclonal CD4 antibody. After western blotting, membranes were probed with antibodies as indicated. To assess total levels of VENCT-TM, cell lysates were probed using GFP antibodies (lower panel). VENCT-TM only binds CDPSCT, not CD4.
To further prove the in vivo relevance of our findings, we made use of our previously established GFP-tagged PS1, PS-EGFP (PE). PE was shown in all aspects tested to be functionally equivalent to PS1 wild type (PS1wt; Kaether et al, 2002). To verify NCT binding to PSCT, we analysed the interaction using PE variants. Based on the data from the CD4 variants, PE(PALP/AAAA) (with the PALP motif mutated to four alanines) should bind NCT. On the other hand, a PE construct with a C-terminal deletion (PEΔ7) should not bind NCT. Swe cells stably expressing PE variants were lysed in CHAPSO, IPed with a GFP antibody and probed for the γ-secretase complex components NCT, APH1a and PEN2 (Figure 2A). PE binds to the other members of the γ-secretase complex NCT, APH1a and PEN2 (Figure 2A). As expected from the data in Figure 1, mutation of the PALP motif in PE does not abolish binding to NCT (Figure 2A). In addition, the other complex components APH1a and PEN2 are also bound, suggesting that PE(PALP/AAAA) is incorporated into a γ-secretase complex (Figure 2A). In contrast, PEΔ7, where the putative NCT-binding site is deleted, does not bind NCT and APH1a and only trace amounts of PEN2. This suggests that deletions at the C-terminus of PE abolish NCT binding and subsequent complex formation (Figure 2A, see also Figure 2C).
Figure 2.
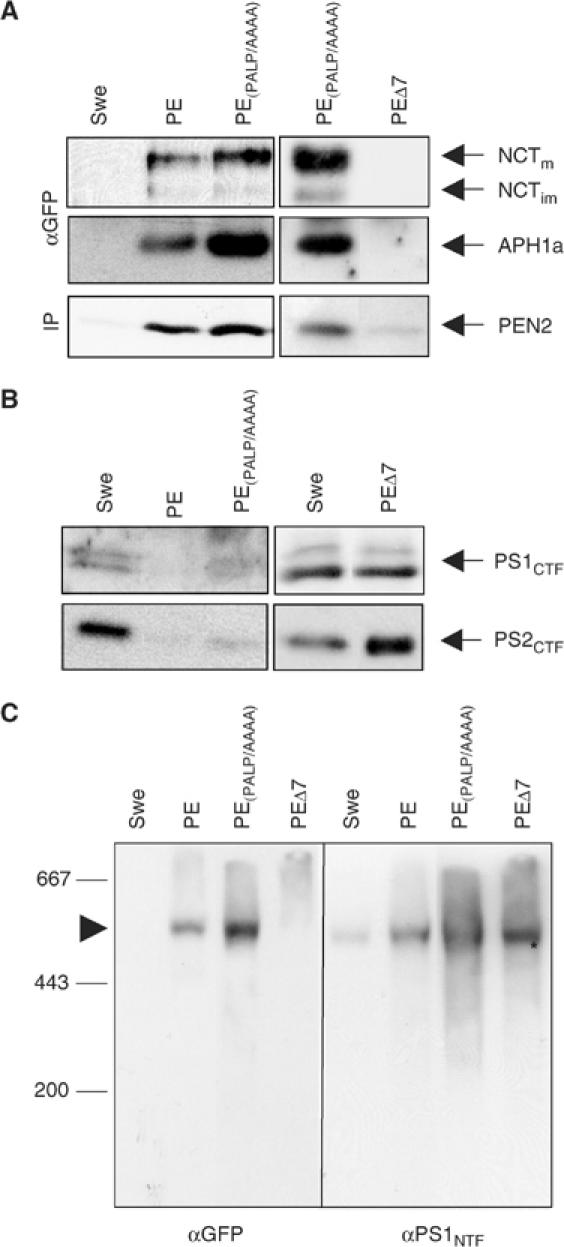
PSCT is critically involved in HMW γ-secretase complex formation. (A) PE and PE(PALP/AAAA), but not PEΔ7, bound NCT, PEN2 and APH1a. CHAPSO/citrate lysates of Swe and Swe cells stably expressing PE variants were IPed with an antibody against GFP and subjected to SDS–PAGE, followed by western blotting with antibodies as indicated. No γ-secretase complex components can be co-precipitated in Swe cells, showing the specificity of the GFP antibody and the co-precipitation. (B) PE and PE(PALP/AAAA), but not PEΔ7, replace endogenous PS1 and 2. Lysates of Swe and Swe cells stably expressing PE variants were probed using antibodies against PS1CTF or PS2CTF. (C) PE and PE(PALP/AAAA), but not PEΔ7, assemble into an HMW complex. Lysates of Swe and Swe cells stably expressing PE variants were prepared for blue-native gel electrophoresis, blotted and probed with antibodies against GFP (left) or PS1NTF (right). The endogenous γ-secretase complex (Swe on left and right, arrowhead) is visualized only with the PS1NTF antibody, showing the specificity of the GFP antibody. The GFP antibody only visualizes a HMW complex in PE- and PE(PALP/AAAA)-expressing cells. No HMW complex can be detected by GFP antibodies in PEΔ7 cells, indicating that this construct does not form a HMW complex. Instead, in these cells, the endogenous γ-secretase complex is still present, as demonstrated by PS1NTF staining (asterisk).
A phenomenon of expression of functional exogenous PS1 is the replacement of endogenous PS1 and PS2 (reviewed in De Strooper, 2003). Such replacement is an indicator for complex assembly of the exogenous PS. Cells expressing PE variants were analysed for PS1 or 2 replacement. Endogenous PS1 and 2 are replaced in cells expressing PE and PE(PALP/AAAA), but not in cells expressing PEΔ7 (Figure 2B). This shows that the distal-most part of PSCT is required for the replacement of endogenous PS by exogenous PS variants and hence required for complex formation.
The IP and replacement studies suggested that PE and PE(PALP/AAAA), but not PEΔ7, are incorporated into a HMW complex. To further prove this, we performed a blue-native gel analysis of cell lines expressing PE variants. Blots from blue-native gels were probed with antibodies against PS1NTF. This visualized the endogenous 500–600 kDa HMW γ-secretase complex in Swe cells as described (Edbauer et al, 2002; Figure 2C, αPS1NTF). Using GFP antibodies, no signal was detected at the position of the HMW complex in Swe cells, indicating the specificity of the GFP antibody (Figure 2C, αGFP). In cells expressing PE or PE(PALP/AAAA), the GFP antibody detected a HMW band at the position of the γ-secretase complex (Figure 2C, αGFP), demonstrating that both are incorporated into an HMW complex. The GFP-positive HMW complex in PE- and PE(PALP/AAAA)-expressing cells could also be stained with antibodies against NCT and APH1a, corroborating the co-IP data from Figure 2A (data not shown). PEΔ7 is not incorporated into a HMW complex (Figure 2C, left). Instead, in these cells, the endogenous complex that is not replaced (see Figure 2B) can be detected after probing for PS1NTF (Figure 2C, right, asterisk). Taken together, these data indicate that PE and PE without the PALP motif replace endogenous PS1/2, bind NCT and all known γ-secretase complex components, and are incorporated in a HMW complex. The PALP motif is therefore not involved in NCT binding and not required for HMW complex formation. Consistent with the CD4 data, a C-terminal deletion of the PSCT abolishes NCT binding, which in turn results in an inability of these PE variants to form a HMW complex.
Having shown that the PALP motif is not required for HMW complex formation, we wanted to know whether it is required for γ-secretase activity. To this end, we investigated the accumulation of APPCTF, a major substrate of γ-secretase (Figure 3A, bottom). As shown before (Kaether et al, 2002), APPCTF levels in PE-expressing cells are similar to levels in cells expressing endogenous PS (Figure 3A, compare APPCTF in Swe and PE), demonstrating the functionality of PE. In contrast, expression of PE(PALP/AAAA) led to the accumulation of APPCTF (Figure 3A, bottom), indicating a loss of γ-secretase activity. As expected, expression of PEΔ7 does not impair γ-secretase activity, because it is not replacing endogenous PS nor is it incorporated into a HMW complex.
Figure 3.

PE variants with mutations in the C-terminus are proteolytically inactive. (A) Lysates of Swe and Swe stably expressing the indicated PE variant were IPed using anti PS1CTF antibody 3027 (top) or directly loaded for APPCTF detection. After blotting, membranes were probed with antibodies against PS1CTF (top) and anti-APPCTF antibody (bottom). PE was largely cleaved endoproteolytically (CTF), whereas PE(PALP/AAAA) and PEΔ7 accumulated mainly as holoprotein (holo) and only faint bands of CTF can be detected. PE(PALP/AAAA), but not PEΔ7, led to APPCTF accumulation. (B) Scheme of NotchΔE processing. NotchΔE is cleaved constitutively by γ-secretase. The resulting intracellular fragment NICD is translocated to the nucleus. (C) PE variants with C-terminal deletions do not restore γ-secretase activity. MEF PS1/2−/− cells were transiently transfected with NotchΔE (∅) and with NotchΔE plus PE or PE variant as indicated. The following day, cells were fixed and processed for IF using anti-myc antibody against NΔE/NICD. Note that only PE restores γ-secretase activity, as indicated by nuclear translocation of the cleavage product NICD (arrows). All PE variants were expressed equally (data not shown).
Next, we investigated whether PE variants would be endoproteolytically processed into NTF and CTF, as does PS1wt. Lysates of Swe cells and Swe cells expressing PE variants were analysed by combined IP/blotting for PS1 (Figure 3A, top). The 80 kDa PE is processed into a 50 kDa CTF (PS1 CTF plus GFP) and a 30 kDa NTF as described (Kaether et al, 2002). In PE(PALP/AAAA) and PEΔ7, the ratio of holoprotein/CTF is reversed as compared to PE, demonstrating that endoproteolysis is strongly impaired in PE variants with manipulated C-termini (Figure 3A, top).
To obtain additional evidence for γ-secretase function of PE variants, we tested the ability of these variants to reconstitute γ-secretase in MEF PS1/2−/− cells and made use of NotchΔE, a reporter protein for γ-secretase activity (Schroeter et al, 1998). NotchΔE is a constitutively active Notch derivative that is cleaved by functional γ-secretase. The resulting Notch intracellular fragment (NICD) is translocated to the nucleus (Figure 3B). In cells without active γ-secretase, NotchΔE accumulates at the PM. These two conditions are easily discernible and directly indicate γ-secretase activity. NotchΔE was transiently transfected in MEF PS1/2−/− either alone (∅) or together with PE variants, as indicated in Figure 3C. MEF PS1/2−/− cells lack γ-secretase activity, resulting in an accumulation of NotchΔE at the PM (∅, myc). In cells cotransfected with NotchΔE and PE, functional γ-secretase is reconstituted and NICD can be visualized in the nucleus (Figure 3C, PE, arrows). In contrast, PE(PALP/AAAA) does not reconstitute γ-secretase activity, despite its ability to assemble into a HMW complex (Figure 3C, PE(PALP/AAAA)). As expected, PEΔ7, which does not reconstitute a HMW complex and does not bind NCT, PEN2 and APH1a, is not able to reconstitute functional γ-secretase, as shown by the intense PM staining of NotchΔE (Figure 3C, PEΔ7).
Using an yeast-based γ-secretase reconstitution assay (Edbauer et al, 2003) as an independent experimental paradigm, we also found that PS with a C-terminal mutation is inactive (see Supplementary data).
Taken together, these findings demonstrate that PSCT contains a functional domain important for HMW complex formation (the NCT-binding site) and another distinct site for γ-secretase activity and PS endoproteolysis (the PALP motif).
PSCT binds to the TM domain of NCT
Having shown that the distal part of PSCT provides a binding site for NCT, we wanted to identify the corresponding binding domain in NCT. To this end, we made use of a series of V5-tagged NCT constructs recently established (Capell et al, 2003). In NΔC-V5 the cytosolic C-terminus of NCT was deleted, in NAN-V5 the TM domain is replaced with that of ADAM10 and NCTs-V5 consists of the lumenal domain of NCT only (Figure 4A). Consistent with previous data (Capell et al, 2003), a fraction of NΔC-V5 maturates, as indicated by the upper band in Figure 4B, whereas NAN-V5 and NCTs-V5 are present in immature form only (Capell et al, 2003). To test for interaction with the NCT variants, lysates of cells stably expressing NCT variants and either CD4 or CD4PSCT were IPed with a CD4 antibody and blotted with a V5 antibody (Figure 4B). Immature NΔC-V5 co-precipitates with CD4PSCT, demonstrating that the binding domain on NCT is not located on its cytosolic tail (Figure 4B). NAN-V5, in contrast, was not bound to CD4PSCT, suggesting that the NCT TM contains the PS-binding site (Figure 4B). The lumenal NCTs-V5, which lacks the TM and the cytosolic domain, was also not bound to CD4PSCT (Figure 4B, lane 6). CD4 alone was not able to precipitate any of the NCT variants above background levels (Figure 4B).
To further prove that the NCT TM is involved in binding to PSCT, we made use of an independent reporter protein, VSVG-EGFP (Toomre et al, 1999). The VSVG TM was replaced by the NCT TM and the construct, VENCT-TM, stably transfected in Swe cells expressing CD4 or CD4PSCT (Figure 4C). Co-IP analysis showed that VENCT-TM binds to CD4PSCT, indicating that indeed the NCT TM contains the binding site for PSCT (Figure 4C). As expected, CD4 without PSCT failed to precipitate VENCT-TM.
In conclusion, our data suggest that PSCT binds to the TM of NCT. In turn, this suggests that PSCT dives into or penetrates the membrane.
The C-terminus of PS1 contains an ER-retention/retrieval motif
We next wanted to determine if PSCT has other functions beyond γ-secretase complex assembly and activity. PS1 localizes to the ER when not assembled in the γ-secretase complex (see Discussion). The CD4 fusion proteins we used for identification of the NCT-binding site can also be used to define trafficking signals. CD4 is normally transported to the PM and has been used to define ER-retention (we use the term ER retention for ER retention, retrieval or a combination retention/retrieval) signals by adding candidate sequences to its C-terminus (Zerangue et al, 1999). CD4 constructs containing a sequence with a known retention motif (CD4C+36RKR) (Zerangue et al, 1999) or a mutated retention motif (CD4C+36AAA) (Zerangue et al, 1999) were transfected in COS cells and analysed by immunofluorescence (IF, Figure 5A). The cells expressing CD4C+36RKR showed a strong ER staining, whereas the cells expressing CD4C+36AAA with the mutated motif showed a predominant PM staining together with Golgi and endosomal staining (Figure 5A, top). To search for similar motifs in PS1, we fused cDNAs coding for the first 79 amino acids preceding TM1 of human PS1 (PSNT) to CD4 cDNA (CD4PSNT) or coding for PSCT to CD4 cDNA (CD4PSCT). Transient expression in COS cells followed by IF revealed that addition of PSCT leads to ER retention of the CD4 reporter, whereas PSNT has no effect on the localization of CD4 (Figure 5A, bottom). The retention of CD4PSCT seems to be expression level dependent, since in cells with high expression levels some fusion protein escapes retention and is transported to the PM (Figure 5A, bottom left).
Figure 5.
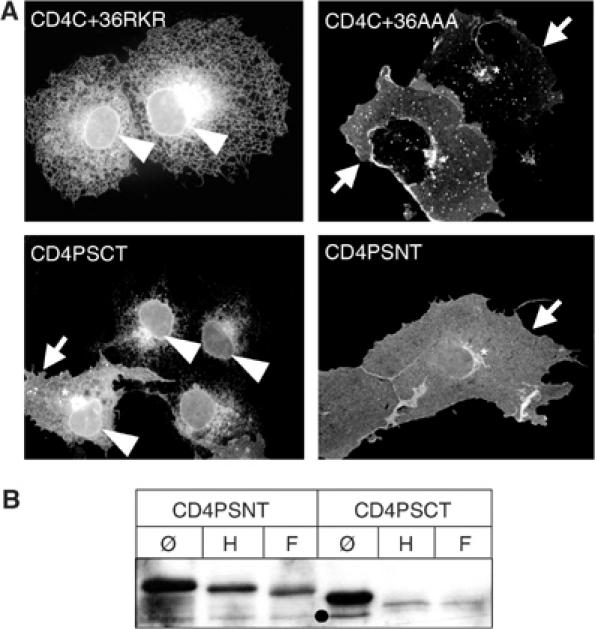
PSCT contains an ER-retention signal. (A) PSCT, but not PSNT, mediates ER retention of the CD4 reporter. COS cells were transiently transfected with CD4 constructs carrying a known retention signal (CD4CT+36RKR), a sequence where this signal is mutated (CD4CT+36AAA), or PSCT or PSNT. After fixation, cells were stained with monoclonal CD4 antibody. CD4CT+36RKR and CD4PSCT show ER and nuclear envelope staining (arrow head), CD4CT+36AAA and CD4PSNT show Golgi (asterisk) and PM (arrow) staining. Note one cell transfected with CD4PSCT showing Golgi and PM staining due to overexpression, in addition to the nuclear envelope staining (CD4PSCT, left cell, arrow head). (B) CD4PSCT, but not CD4PSNT, remained endoH sensitive, indicating an ER/early Golgi localization. Lysates of Swe cells stably expressing CD4PSNT or CD4PSCT were IPed with CD4 monoclonal antibody (∅) and subjected to endoH (H) or N-glyc. F (F) digestion. After SDS–PAGE and western blotting, bands were probed with CD4 antibody. Note that upon endoH digestion CD4PSNT migrates faster than the untreated sample, but clearly slower than the totally deglycosylated form, indicating partial endoH resistance. After endoH digestion, CD4PSCT migrates at the same position as that of the totally deglycosylated sample, indicating endoH sensitivity. Black dot, unspecific band or degradation product.
We further analysed the subcellular localization of the CD4 fusion proteins by stably expressing them in Swe cells. Cell lysates were subjected to endoglycosidase H (endoH) or N-glycosidase F (N-glyc. F) digestion (Figure 5B). EndoH cleaves high-mannose oligosaccharides that are present in the ER and the early Golgi only. EndoH sensitivity reflects an ER/early Golgi localization, endoH resistance a post-Golgi localization. N-glyc. F removes all N-linked sugars. Upon endoH digestion, CD4PSNT is shifted to faster mobility, but migrates clearly slower than the fully deglycosylated protein, indicating transport to late secretory compartments. Such behaviour is often observed when not all N-linked sugars are complex glycosylated (for example, in Edbauer et al, 2002). In the following, we refer to this species as being endoH resistant. In contrast, upon endoH digestion, CD4PSCT is shifted to the exact position of the fully deglycosylated protein, indicating that it did not reach late Golgi compartments (Figure 5B). Further evidence for an ER localization of CD4PSCT comes from IF analysis of COS cells transiently transfected with CD4PSCT and costained for CD4 and calreticulin, a marker for the ER. Both proteins intensively colocalized (Figure 6A), showing that CD4PSCT is indeed retained in the ER.
Figure 6.

The proximal part of PSCT is responsible for ER retention of a CD4 reporter. (A) Colocalization of CD4PSCT and calreticulin. COS cells transiently transfected with CD4PSCT were processed for IF with CD4 and calreticulin antibodies. The inset shows the merge of boxed area. Note the identical distribution of CD4PSCT and calreticulin. (B) Amino-acid sequences of the PSCT variants fused to CD4. (C) CD4PSCTΔPALP, −Δ18, −Δ21 and −Δ27 reach late Golgi compartments, indicated by endoH resistance. Lysates of Swe cells stably expressing CD4 or CD4 variants as indicated were IPed with CD4 monoclonal antibody (∅) and subjected to endoH (H) or N-glyc. F (F) digestion. After SDS–PAGE and western blotting, bands were visualized with polyclonal CD4 antibodies. CD4, −Δ18, −Δ21 and −Δ27 are endoH resistant, CD4PSCT and −Δ7 are endoH sensitive, CD4PSCTΔPALP is mainly endoH resistant and shows an additional endoH-sensitive band, CD4PSCTΔ15 is mainly endoH sensitive and shows in addition some endoH resistance. Lanes are from different gels and differ in exposure times. (D) CD4PSCTΔ1DY/AA is mainly endoH sensitive, only a longer exposure reveals a minor endoH-resistant fraction. EndoH-resistant bands are indicated by an asterisk, endoH-sensitive bands by an open circle. (E) IF of transiently transfected COS cells confirms the deglycosylation data. CD4 variants were transiently transfected in COS cells as indicated and stained with CD4 antibody.
The PALP motif is required for ER retention
We next wanted to identify the critical amino acids that are required for ER retention of PSCT. We constructed a series of deletion proteins where shortened PSCTs were fused to CD4 (schematized in Figure 6B). Subcellular localization was analysed in stably transfected Swe cells by deglycosylation experiments (Figure 6C). CD4 as a PM protein is endoH resistant, whereas addition of PSCT confers endoH sensitivity (compare also Figure 5B). Surprisingly, deletion of the PALP motif leads to the appearance of an endoH-resistant band and to a lower extent to an endoH-sensitive band (Figure 6C, CD4PSCTΔPALP). This suggests that the PALP motif is part of a larger ER-retention motif that is partially destroyed in the CD4PSCTΔPALP construct. Indeed, the PALP motif alone is not sufficient for retention, as suggested by the expression of a CD4 construct with PALP at its C-terminus, which is not retained (data not shown). Deleting the PALP motif has similar effects than mutating it to four alanines (data not shown). Deletion of seven amino acids from the C-terminus still allowed ER retention of the fusion protein, indicating that the retention motif is in the proximal part of PSCT (Figure 6C, CD4PSCTΔ7). Deletion of 15 amino acids leads to a fraction escaping the retention and becoming endoH resistant, whereas a significant fraction of CD4PSCTΔ15 is endoH sensitive, indicating that the ER-retention signal is at least partially intact. Deletion of 18 and more amino acids (−Δ18, −Δ21, −Δ27) confers endoH resistance, indicating destruction of the ER-retention motif (Figure 6C). PSCT contains a dihydrophilic motif (DY) surrounded by hydrophobic amino acids. Charged or polar amino acids in a TM domain have been shown to confer ER retention in a number of unassembled complex components (Bonifacino et al, 1991; Reth et al, 1991; Hennecke and Cosson, 1993; Sato et al, 2004). We therefore investigated whether the DY motif is involved in ER retention of CD4PSCT and analysed cells stably expressing CD4PSCTΔ1DY/AA by deglycosylation experiments (Figure 6D). CD4PSCTΔ1DY/AA is largely endoH sensitive, only a longer exposure reveals a very minor endoH-resistant fraction. This suggests that the DY motif, which is within the stretch of amino acids that are involved in ER retention (Figure 6C), is contributing to a minor extent to retention of CD4PSCT. The deletion of the last isoleucine (Δ1) has no effect (see below). Transient expression of the CD4 variants in COS cells followed by IF analysis confirmed the deglycosylation data. CD4PSCT and CD4PSCTΔ7 are retained in the ER, larger deletions at the C-terminus or deletion of the PALP motif lead to cell surface accumulation of the fusion protein, as does CD4 alone (Figure 6E).
ER retention of CD4PSCT is not NCT dependent
We showed above that PSCT binds NCT, and that this binding is dependent on the last isoleucine (Figures 1, 2, 3 and 4). To ensure that the ER retention of CD4PSCT is independent of its binding to NCT, which is ER-retained in its immature form (Edbauer et al, 2002; Leem et al, 2002), we repeated the deglycosylation experiments with CD4 variants lacking the NCT interaction domain (Figure 7A). CD4PSCTΔ1, shown to be deficient in NCT binding (Figure 1C), is efficiently retained in the ER, as suggested by its endoH sensitivity (Figure 7A). Further support for an NCT-independent ER retention of CD4PSCT came from downregulation of NCT. Swe cells stably expressing CD4PSCT were stably transfected with a NCT RNAi knockdown vector and the glycosylation status of CD4PSCT was monitored. EndoH sensitivity of CD4PSCT shows its efficient ER retention despite a strong downregulation of NCT (Figure 7B).
Figure 7.

ER retention of CD4PSCT is not NCT dependent. (A) Swe cells expressing CD4 variants as indicated and Swe cells expressing PSCT and NCT knockdown RNAi were IPed with CD4 monoclonal antibodies (∅) and subjected to endoH (H) or N-glyc. F (F) digestion. CD4PSCT remains endoH sensitive in the absence of NCT (CD4PSCT NCT RNAi) or when the NCT-binding site is deleted (CD4PSCTΔ1). (B) Efficient NCT knockdown was verified by blotting lysates against NCT or GSK3 as loading control.
These data demonstrate that the proximal 22 amino acids of PSCT, including the PALP motif, contain an ER-retention signal and are sufficient to retain a CD4 reporter protein in the ER. This retention is not due to the binding to NCT.
ER retention of PS is PALP-dependent
To further test the physiological relevance of our findings, we used PE, PE(PALP/AAAA) and PEΔ7 (see above) and determined their localization in stably transfected Swe cells. Confocal microscopy of living cells stably expressing PE revealed a prominent ER and some vesicular staining (Figure 8, top). In contrast, PE(PALP/AAAA) cells showed a strongly reduced ER staining and a prominent vesicular staining (Figure 8, middle), suggesting that PE(PALP/AAAA) left the ER and accumulated in a vesicular compartment. PEΔ7, shown not to be incorporated into the HMW complex (Figure 2) but still containing an intact ER-retention domain, showed a prominent ER staining with no vesicular staining (Figure 8, bottom). To characterize the vesicular compartment, we immunostained fixed cells expressing PE variants with antibodies against GFP and LAMP-1, a marker for late endosomes and lysosomes. Figure 9 shows that the vesicles of PE- and PE(PALP/AAAA)-expressing cells show a high degree of colocalization of PE variant and LAMP-1, suggesting that most of these vesicles are late endosomes and lysosomes. No LAMP-1-positive structures were found to colocalize with PEΔ7 (Figure 9). Identical results were obtained for LAMP-2, another late endosomal/lysosomal protein (data not shown). No colocalization of vesicular structures was found for EEA1, a marker for early endosomes (data not shown). Taken together, these experiments confirm the data from the CD4 variants by indicating that the PALP motif is part of an ER-retention signal located on the CT of PS1.
Figure 8.

The PALP motif is important for ER retention of PS-EGFP (PE). Swe cells stably expressing PE, PE(PALP/AAAA) or PEΔ7 as indicated were mounted for live-cell confocal microscopy. Single optical sections are shown cutting the cells at mid position or near the coverslip as schematized. PE cells show a reticular ER (arrowheads) and a nuclear envelope staining, whereas PE(PALP/AAAA) cells show a strongly reduced ER staining accompanied by a prominent vesicular staining (arrows). PEΔ7 cells, were the ER-retention motif is intact, show a prominent ER staining (arrowheads). The PM staining of PE described in Kaether et al (2002) is only observed upon longer exposure.
Figure 9.
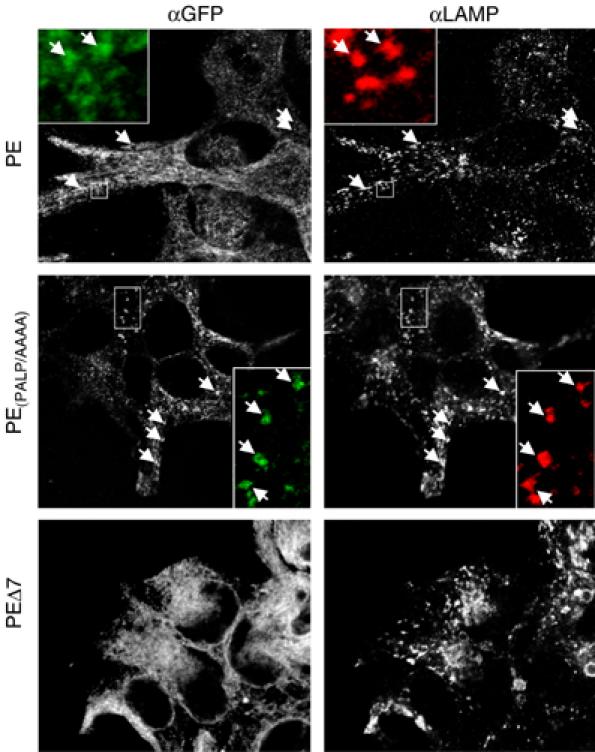
PE and PE(PALP/AAAA) are transported to the endosomal/lysosomal compartment. Swe cells stably expressing PE, PE(PALP/AAAA) or PEΔ7 as indicated were fixed and processed for IF using antibodies against GFP and LAMP-1 and imaged by confocal microscopy. Arrows indicate colocalized vesicular structures with GFP or LAMP-1. The insets show magnifications of the boxed areas. Note that in PEΔ7 no GFP-positive vesicular structures are visible. Single optical sections are shown.
Discussion
Our data suggest that PSCT, comprising the last 37 amino acids of PS1, is involved in three different functions. The very last amino acids are involved in NCT binding and thus in γ-secretase complex formation, whereas the proximal part is involved in γ-secretase activity and in ER retention (schematized in Figure 10A). Deletion of just one amino acid prevented PSCT/NCT interaction. Data from studies mainly with PS2, but also PS1, showed that the terminating isoleucine in PS1/2 is critical for complex assembly and γ-secretase activity, but no functional explanation was provided (Tomita et al, 1999). While revising this manuscript, Bergman et al (2004) showed that deletion of more than seven amino acids from the C-terminus of PS1 abolishes PS endoproteolysis and γ-secretase activity, in line with our results. They suggested that PSCT contains a binding site for NCT and/or APH1 (Bergman et al, 2004). Our findings are very similar and provide a functional explanation by demonstrating that the distal part of PSCT is essential because it binds NCT and this binding is required for complex formation and thus γ-secretase function.
Figure 10.

Proposed function for PSCT and putative topology of CD4PSCT. (A) Scheme of PSCT with the different motif identified here. The N-terminal part is needed for ER retention, the PALP motif for γ-secretase activity and the C-terminus for NCT binding. (B) Putative topology of CD4 (grey) and the C-terminally attached PSCT (black) that loops into the membrane and binds the TM of NCT (dark grey). l, lumen, c, cytosol.
The interaction between NCT and PSCT is probably direct and not mediated via one of the other complex components. It is unlikely that PS or PEN2 are involved in this binding, since CD4PSCT binds endogenous NCT in PS1/2 double knockout MEF cells (data not shown), where PS1/2 is absent and PEN2 is strongly downregulated (Steiner et al, 2002). We find minor amounts of APH1a co-precipitating with CD4PSCT and endogenous NCT (Figure 1C). Presumably, this reflects some APH1a bound to NCT, as has been suggested before (Periz and Fortini, 2004). This minor binding is unlikely to account for the strong binding of NCT to CD4PSCT. Moreover, when CD4PSCT and NΔC-V5 are overexpressed, they bind to each other (Figure 4). This makes it highly unlikely that the binding is mediated by APH1a, which was not overexpressed in this experiment. Along this line, depletion of APH1a or overexpression of APH1a did not change the binding of CD4PSCT to overexpressed NΔC-V5, supporting direct binding of PSCT to NCT (Figure 1D and E).
The identification of an NCT-binding domain in PSCT may provide new insights into the topology of PS1. Co-IP studies using CD4PSCT and NCT variants showed that NCT lacking the cytosolic domain was able to bind PSCT, whereas NCT with a heterologous TM domain or soluble NCT was unable to bind CD4PSCT. Moreover, when the NCT TM was transplanted to VSVG-EGFP, it allowed binding to CD4PSCT. This suggests that the PS-binding domain of NCT is located in its TM domain. Support for this binding comes from two recent studies where NCT variants were analysed for activity (Capell et al, 2003; Morais et al, 2003). In both studies, it was shown that the TM of NCT is required for HMW complex formation and γ-secretase function.
Binding of the C-terminal part of PSCT to the NCT TM implies that the PSCT dives into or penetrates the membrane, resulting in a topology of CD4PSCT as depicted in Figure 10B. We propose that this also reflects the orientation of the native PSCT for several reasons. First, deleting or mutating the PALP motif in CD4PSCT as well as in the fully functional PE (Kaether et al, 2002) has the same effect: in both cases the proteins are no longer retained in the ER. This suggests that the PALP motif in both cases is in the same intracellular environment. Second, both CD4PSCT as well as PE variants bind NCT dependent on the very distal part of PSCT, indicating that the orientation is the same in both cases. Third, additional evidence for such a topology comes from other studies. PSCT was shown to bind the N-terminal part of the TM of the type I protein telencephalin, suggesting a penetration of the hydrophobic PSCT into the membrane (Annaert et al, 2001). Whether PSCT assumes more than one topology is unclear at the moment, but a flip/flop mechanism upon NCT binding remains an intriguing possibility.
Quality control in the ER is crucial to prevent export of nonfunctioning or, worse, malfunctioning proteins. Cellular mechanisms ensure such quality control (Ellgaard and Helenius, 2003). We now show that the γ-secretase complex subunit PS1 contains an ER-retention signal in its C-terminus (schematized in Figure 10A). The sequence that we identified comprises 22 amino acids and is extremely well conserved between species (for an alignment, see Tomita et al, 2001). In PS1 from all examined species, apart from Caenorhabditis elegans and Arabidopsis thaliana, these amino acids are ALPALPISITFGL(V/I)FYF(A/S)TD(Y/N)L. The sequence does not resemble currently known cytoplasmic retention motifs (Nilsson et al, 1989; Zerangue et al, 1999). However, subunits of multi-protein complexes like CD8, the yeast iron transporter Fet3p, the acetylcholine receptor, as well as B- and T-cell receptors, possess retention signals in TM domains, which do not show a classical three or four amino-acid-based signal (Bonifacino et al, 1990; Reth et al, 1991; Hennecke and Cosson, 1993; Wang et al, 2002; Sato et al, 2004). Many of these signals involve polar or charged amino acids in a stretch of hydrophobic amino acids (Bonifacino et al, 1990; Reth et al, 1991; Hennecke and Cosson, 1993; Sato et al, 2004). These retention signals serve to prevent the unassembled subunits from getting exported out of the ER and are masked upon full assembly, a mechanism that we hypothesize applies also for γ-secretase.
PS holoprotein, which is believed not to be incorporated into a HMW complex, accumulates mainly in the ER (Zhang et al, 1998; Tomita et al, 2001), probably due to the ER-retention motif described here. The ER staining in PE-expressing cells thus corresponds to this unassembled PS holoprotein, whereas the vesicular staining represents PS assembled into a HMW complex (Figure 8; Kaether et al, 2002). Consequently, a nonfunctional ER-retention motif as in PE(PALP/AAAA) leads to ER export of unassembled PS and a reduced ER staining in PE(PALP/AAAA)-expressing cells. In contrast, PEΔ7 is detected exclusively in the ER, with no detectable vesicular staining, as expected for a PS containing the ER-retention domain, but being deficient in complex assembly. These data suggest that the export of the monomeric, unassembled PS1 is controlled and only the fully assembled complex is allowed to leave the ER, in analogy to the control mechanisms found in ion channels and cell surface receptors. This then allows the subsequent transport out of the ER. Based on this hypothesis, the other γ-secretase complex components should also carry retention signals. Indeed, NCT, which is not incorporated into a HMW complex, accumulates as an immature species, suggesting the presence of an ER-retention signal. This is especially pronounced in the absence of PS, where no complex formation takes place and NCT is fully retained in the ER (for a review, see Periz and Fortini, 2004). Furthermore, upon overexpression of NCT, where only a fraction of the overexpressed NCT participates in complex formation, the majority is retained as immature, ER-resident protein (Edbauer et al, 2002). Likewise, monomeric PEN2 is ER-localized due to a retention motif (J Scheuermann, C Kaether and C Haass, manuscript in preparation).
The PALP motif in the proximal part of PSCT is part of an ER-retention signal (Figures 6, 8 and 9) and is essential for γ-secretase activity (Figure 3), suggesting that it serves a dual role in PS biology. Previous data showed that the PALP motif is critically involved in complex formation and γ-secretase function (Tomita et al, 2001). We partially confirm these data by demonstrating that mutation of PALP to AAAA abolishes γ-secretase activity (Figure 3). In contrast to Tomita et al (2001), our PS variants with a mutated PALP motif replaced endogenous PS1 and 2 and assembled into a HMW complex (Figure 2), indicating that the PALP motif is not required for replacement and HMW complex formation. While this study was under revision, our data were confirmed by Wang et al (2004). How precisely mutation of the PALP motif abolishes γ-secretase activity remains to be established in further studies.
In summary, PS1 enters the HMW γ-secretase complex by binding with its C-terminus to the TM of NCT. The PALP motif contributes to γ-secretase activity, and a long hydrophobic stretch mediates ER retention of unassembled PS1.
Materials and methods
Antibodies and cell lines
APP, APPCTF, APH1aL, PEN2, PS1 and 2 were detected as described in Steiner et al (2002) and Shirotani et al (2004) and references therein. NotchΔE (Schroeter et al, 1998) and NICD were detected using monoclonal 9E10 antibody (Santa Cruz). NCT was detected using polyclonal anti-NCT C-terminal antibody (Sigma). GFP was detected using poly- or monoclonal anti-GFP antibody (Clontech) for IP and western blotting, respectively. Monoclonal H4A4 (Developmental Studies Hybridoma Bank) was used for LAMP-1 IF, polyclonal anti-calreticulin (Calbiochem) as ER marker. EDU-2 monoclonal antibody (Diatec, Oslo, Norway) was used for IF and IP of CD4, polyclonal antibody H-370 (Santa Cruz Biotechnology) was used for immunoblotting of CD4. GSK3 was detected using monoclonal GSK3β antibody (Santa Cruz Biotechnology).
HEK293 cells stably expressing Swe were described before (Citron et al, 1992). MEF PS1/2−/− cells are described in Herreman et al (2003).
cDNA constructs, transfections and screening of stably transfected cell lines
CD4C+36 and CD4C+36AAA (Zerangue et al, 1999) were kindly provided by LY Yan, San Francisco, CA. PS1-EGFP (PE) and the Swe cell line expressing it (PE17) were described (Kaether et al, 2002). CD4PSCT or PSNT fusion constructs were cloned using standard molecular cloning techniques and pCDNA3.1/Hygro (Invitrogen). PSNT refers to the first 79 amino acids from human PS1. PSCT refers to the last 37 amino acids from human PS1, as indicated in Figure 2. For introducing mutations, QuikChange site-directed Mutagenesis Kit (Stratagene) was used. cDNAs were stably transfected using Fugene (Roche) or lipofectamine (Invitrogen). Swe cells expressing CD4 variants were kept as pools of stably expressing cells, cells expressing PE variants and transfected MEF cells as single-cell clones. For analysis of the NCT interaction domain, CD4 variants in pCDNA3.1/Zeo were stably transfected in Swe cells expressing an NCT RNAi vector and RNAi-insensitive, V5-tagged NCT variants (Capell et al, 2003). VENCT-TM was cloned using VSVG3-EGFP kindly provided by Jamie White. VSVG TM was replaced by NCT TM using PCR technology. VENCT-TM in pCDNA3.1Zeo was stably transfected in Swe cells stably expressing CD4PSCT. For NCT downregulation, cells expressing CD4PSCT were stably transfected with an NCT RNAi vector (Capell et al, 2003) and single clones selected and analysed for NCT downregulation. Details of all cloning work are available on request.
Co-immunoprecipitation
Co-IP was performed from cell lysates extracted in 2% CHAPSO/150 mM sodium citrate (pH 6.5) and protease inhibitor mix. IPed proteins were separated on 7 or 8% SDS–PAGE gels or 10–20% Tris-Tricine gels (Invitrogen).
Deglycosylation of CD4 variants
Cell lines stably expressing various CD4 constructs were lysed in 50 mM Tris, pH 7.6, 150 mM NaCl, 2 mM EDTA, 1% NP-40 and protease inhibitor mix. CD4 constructs were IPed using monoclonal CD4 antibody and endoH, and N-glycosidase F digestion was performed according to the supplier's instructions (Roche). After trichloride acetic acid precipitation, proteins were separated on 10% SDS gels, transferred to PVDF membranes and detected using polyclonal CD4 antibody.
Microscopy
IF was performed using standard protocols (Wacker et al, 1997). Alexa 488-, 555- or 586-labelled secondary antibodies were used (Molecular Probes, Netherlands). Fixed cells were analysed on a Zeiss Axioskop2 microscope (Zeiss, Oberkochen, Germany) equipped with an × 63/1.25 objective and standard FITC and TRITC fluorescence filter sets, using an Axiocam HRm Camera and AxioVision software, or using a Zeiss 510Meta confocal system equipped with an × 100/1.3 objective. Living cells in Figure 8A were mounted for live-cell imaging as described (Kaether et al, 2002) and analysed by confocal microscopy. Images were assembled and processed using Adobe Photoshop.
Supplementary Material
Supplementary Data
Acknowledgments
This work was supported by the Hans und Ilse Breuer Stiftung and grants from the Deutsche Forschungsgemeinschaft (priority program ‘Cellular mechanisms of Alzheimer's Disease' to CK and CH and SFB 596 ‘Molecular Mechanisms of Neurodegeneration' to HS and CH) We thank B de Strooper and P Saftig for MEF PS1/2−/− cells, LY Yan for CD4 constructs, Jamie White for VSVG3-EGFP, R Nixon for PS1 N antibody and C Haffner and M Willem for critically reading the manuscript.
References
- Annaert WG, Esselens C, Baert V, Boeve C, Snellings G, Cupers P, Craessaerts K, De Strooper B (2001) Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron 32: 579–589 [DOI] [PubMed] [Google Scholar]
- Bergman A, Laudon H, Winblad B, Lundkvist J, Naslund J (2004) The extreme C terminus of presenilin 1 is essential for gamma-secretase complex assembly and activity. J Biol Chem 279: 45564–45572 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Cosson P, Klausner RD (1990) Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell 63: 503–513 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Cosson P, Shah N, Klausner RD (1991) Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J 10: 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell A, Kaether C, Edbauer D, Shirotani K, Merkl S, Steiner H, Haass C (2003) Nicastrin interacts with gamma-secretase complex components via the N-terminal part of its trans-membrane domain. J Biol Chem 278: 52519–52523 [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360: 672–674 [DOI] [PubMed] [Google Scholar]
- De Strooper B (2003) Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 38: 9–12 [DOI] [PubMed] [Google Scholar]
- Doan A, Thinakaran G, Borchelt DR, Slunt HH, Ratovitsky T, Podlisny M, Selkoe DJ, Seeger M, Gandy SE, Price DL, Sisodia SS (1996) Protein topology of presenilin 1. Neuron 17: 1023–1030 [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Haass C, Steiner H (2002) Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci USA 99: 8666–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C (2003) Reconstitution of gamma-secretase activity. Nat Cell Biol 5: 486–488 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191 [DOI] [PubMed] [Google Scholar]
- Haass C (2004) Take five-BACE and the gamma-secretase quartet conduct Alzheimer's amyloid beta-peptide generation. EMBO J 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke S, Cosson P (1993) Role of transmembrane domains in assembly and intracellular transport of the CD8 molecule. J Biol Chem 268: 26607–26612 [PubMed] [Google Scholar]
- Herreman A, Van Gassen G, Bentahir M, Nyabi O, Craessaerts K, Mueller U, Annaert W, De Strooper B (2003) gamma-Secretase activity requires the presenilin-dependent trafficking of nicastrin through the Golgi apparatus but not its complex glycosylation. J Cell Sci 116: 1127–1136 [DOI] [PubMed] [Google Scholar]
- Kaether C, Lammich S, Edbauer D, Ertl M, Rietdorf J, Capell A, Steiner H, Haass C (2002) Presenilin-1 affects trafficking and processing of betaAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol 158: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JY, Vijayan S, Han P, Cai D, Machura M, Lopes KO, Veselits ML, Xu H, Thinakaran G (2002) Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J Biol Chem 277: 19236–19240 [DOI] [PubMed] [Google Scholar]
- Li X, Greenwald I (1998) Additional evidence for an eight-transmembrane-domain topology for Caenorhabditis elegans and human presenilins. Proc Natl Acad Sci USA 95: 7109–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, Crystal AS, Pijak DS, Carlin D, Costa J, Lee VM, Doms RW (2003) The transmembrane domain region of nicastrin mediates direct interactions with APH-1 and the gamma-secretase complex. J Biol Chem 278: 43284–43291 [DOI] [PubMed] [Google Scholar]
- Nakai T, Yamasaki A, Sakaguchi M, Kosaka K, Mihara K, Amaya Y, Miura S (1999) Membrane topology of Alzheimer's disease-related presenilin 1. Evidence for the existence of a molecular species with a seven membrane-spanning and one membrane-embedded structure. J Biol Chem 274: 23647–23658 [DOI] [PubMed] [Google Scholar]
- Nilsson T, Jackson M, Peterson PA (1989) Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell 58: 707–718 [DOI] [PubMed] [Google Scholar]
- Periz G, Fortini ME (2004) Functional reconstitution of gamma-secretase through coordinated expression of presenilin, nicastrin, Aph-1, and Pen-2. J Neurosci Res 77: 309–322 [DOI] [PubMed] [Google Scholar]
- Reth M, Hombach J, Wienands J, Campbell KS, Chien N, Justement LB, Cambier JC (1991) The B-cell antigen receptor complex. Immunol Today 12: 196–201 [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A (2004) Endoplasmic reticulum quality control of unassembled iron transporter depends on Rer1p-mediated retrieval from the golgi. Mol Biol Cell 15: 1417–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386 [DOI] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Kostka M, Steiner H, Haass C (2004) Immature nicastrin stabilizes APH-1 independent of PEN-2 and presenilin: identification of nicastrin mutants that selectively interact with APH-1. J Neurochem 89: 1520–1527 [DOI] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C (2002) PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem 277: 39062–39065 [DOI] [PubMed] [Google Scholar]
- Takasugi N, Takahashi Y, Morohashi Y, Tomita T, Iwatsubo T (2002) The mechanism of gamma-secretase activities through high molecular weight complex formation of presenilins is conserved in Drosophila melanogaster and mammals. J Biol Chem 277: 50198–50205 [DOI] [PubMed] [Google Scholar]
- Tomita T, Takikawa R, Koyama A, Morohashi Y, Takasugi N, Saido TC, Maruyama K, Iwatsubo T (1999) C terminus of presenilin is required for overproduction of amyloidogenic Abeta42 through stabilization and endoproteolysis of presenilin. J Neurosci 19: 10627–10634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Watabiki T, Takikawa R, Morohashi Y, Takasugi N, Kopan R, De Strooper B, Iwatsubo T (2001) The first proline of PALP motif at the C terminus of presenilins is obligatory for stabilization, complex formation, and gamma-secretase activities of presenilins. J Biol Chem 276: 33273–33281 [DOI] [PubMed] [Google Scholar]
- Toomre D, Keller P, White J, Olivo JC, Simons K (1999) Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J Cell Sci 112: 21–33 [DOI] [PubMed] [Google Scholar]
- Wacker I, Kaether C, Kromer A, Migala A, Almers W, Gerdes HH (1997) Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. J Cell Sci 110: 1453–1463 [DOI] [PubMed] [Google Scholar]
- Wang J, Brunkan AL, Hecimovic S, Walker E, Goate A (2004) Conserved ‘PAL' sequence in presenilins is essential for -secretase activity, but not required for formation or stabilization of -secretase complexes. Neurobiol Dis 15: 654–666 [DOI] [PubMed] [Google Scholar]
- Wang JM, Zhang L, Yao Y, Viroonchatapan N, Rothe E, Wang ZZ (2002) A transmembrane motif governs the surface trafficking of nicotinic acetylcholine receptors. Nat Neurosci 5: 963–970 [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22: 537–548 [DOI] [PubMed] [Google Scholar]
- Zhang J, Kang DE, Xia W, Okochi M, Mori H, Selkoe DJ, Koo EH (1998) Subcellular distribution and turnover of presenilins in transfected cells. J Biol Chem 273: 12436–12442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data


