Abstract
Endometrial cancer (EC) is a hormone‐dependent, most frequent malignancy of the female genital tract, yet no molecular subtype classification based receptor status (estrogen receptor [ER], progesterone receptor [PR], human epidermal growth factor receptor 2 [HER2]) has been established so far. Assuming that molecular subtypes might differ fundamentally in EC, we analyzed expression levels of ER, PR, and HER2 with immunohistochemistry and aimed to determine clinical significance of four molecular subtypes: ER+/PR+/HER2+; ER+/PR+/HER2−, ER−/PR−/HER2+, and ER−/PR−/HER2−. The study included 400 formalin‐fixed paraffin‐embedded primary tumor EC samples which covered all stages of endometrial carcinoma, from IA to IVB. ER−/PR−/HER2+ subtype correlated with the poorest outcome, ER+/PR+/HER2− subtype was associated with the most favorable prognosis (p = 0.002). Molecular subtype division remained an independent prognostic factor in multivariate analysis, accompanying parameters such as diabetes, hypertension, stage, myometrial infiltration, and metastases, all of which yielded hazard ratios between 1.39 and 2.23. ER+/PR+/HER2+ and ER+/PR+/HER2− subtypes had low average TP53 and TOP2A expression levels when compared with ER−/PR−/HER2+ and ER−/PR−/HER2− (both p < 0.00001). Molecular subtypes in EC do show diversity in terms of prognosis, clinicopathological, and molecular characteristics. ER−/PR−/HER2+ subtype exhibit is exceptionally aggressive tumor characteristics. Subtype differentiation might aid prediction of treatment response in EC.
Keywords: molecular subtypes, estrogen receptor, progesterone receptor, HER2 receptor, endometrial cancer
Introduction
Endometrial cancer (EC) is a hormone‐dependent, most frequent malignancy of the female genital tract in the Western world, with approximately 90,000 new cases registered each year in the European Union.1 Despite the high prevalence of EC, no molecular subtype classification based on receptor status (estrogen receptor [ER], progesterone receptor [PR], human epidermal growth factor receptor 2 [HER2]) has been established thus far.
Molecular subtypes have been primarily proposed in breast cancer as the result of high‐throughput gene expression analysis, which yielded several substantially different groups: luminal A, luminal B (HER2−), luminal B (HER2+), HER2+ (nonluminal), triple negative (basal‐like).2 The elucidated subtypes differed in epidemiological risk factors, natural histories, and responses to systemic and local therapies.3 Especially, the latter was of utmost importance as the findings implied that clinicians who manage breast cancer patients should tailor the treatment according to molecular subtypes. As gene expression array information was not feasible in most of the cases, a simplified classification, based on immunohistochemistry (IHC), has been adapted.4 Consequently, luminal A subtype was characterized as ER and/or PR+, HER2−; luminal B (HER2−) as ER and/or PR+, HER2−; luminal B (HER2+) as ER and/or PR+, HER2 overexpressed or amplified; HER2+ (nonluminal) as ER and PR absent, HER2 overexpressed or amplified; and triple negative (basal‐like) as ER and PR absent, HER2−.3
Reports concerning receptor status classification in EC characterize triple‐negative phenotype only.5, 6 Assuming that molecular subtypes might differ fundamentally also in EC, we analyzed the expression levels of ER, PR, and HER2 with IHC and aimed to determine the clinical significance of four molecular subtypes: ER+ and/or PR+, HER2+ (ER+/PR+/HER2+); ER+ and/or PR+, HER2− (ER+/PR+/HER2−); ER− and PR−, HER2+ (ER−/PR−/HER2+); ER− and PR−, HER2− (ER−/PR−/HER2−). Proposed classification has been compared with clinicopathological characteristics, survival, and molecular data. Molecular characterization of the studied subtypes included protein expression analysis of mutated TP53 (tumor protein p53) and TOP2A (DNA topoisomerase II α 170 kDa), also measured by IHC.
Patients and Methods
Patients and tissues
The study included 400 formalin‐fixed paraffin‐embedded (FFPE) primary tumor samples retrospectively collected from a cohort of consecutive EC patients who were operated in the Department of Gynecology, Gynecological Oncology and Gynecological Endocrinology (Medical University of Gdansk) between 2000 and 2010. Samples included in the study were the total sum of eligible cases with available tissue material. Each patient was primarily treated by surgery, with the possible option of radiotherapy and/or chemotherapy administration. The inclusion criteria were operable EC (IVB stage patients underwent cytoreductive surgery) confirmed by histological examination and a signed consent form. The study was accepted by the Independent Ethics Committee of the Medical University of Gdansk (NKEBN/269/2009). Procedures involving human subjects were in accordance with the Helsinki Declaration.
The tumor samples included all stages of endometrial carcinoma, from benign IA to metastatic IVB cancer, as distinguished by FIGO in 2009 (International Federation of Gynecology and Obstetrics).7 All primary carcinomas of the uterine corpus were analyzed and divided into endometrioid and nonendometrioid subtypes. The latter included serous, clear cell, and mucinous adenocarcinomas. The patients’ characteristics are summarized in Table 1. The median age was 64 (range 26–89 years). Patients with a body mass index higher than 30 were classified as obese.8 A survival analysis was performed for 397 (99.3%) patients, 3 patients were lost to the follow‐up. After a median follow‐up of 72 months (range: 0–158), 113 (28.5%) patients had died. The last follow‐up data were collected in September 2013. The study was performed in accordance with the REMARK criteria.9
Table 1.
Clinicopathological data (N = 400)
| Variable | Number of cases (%) |
|---|---|
| Menopausal status | |
| Premenopausal | 28 (7.0%) |
| Perimenopausal | 26 (6.5%) |
| Postmenopausal | 345 (86.3%) |
| Missing data | 1 (0.3%) |
| Age | |
| ≤50 years | 42 (10.5%) |
| 50 years | 358 (89.5%) |
| Obesity | |
| Absent | 197 (49.3%) |
| Present | 202 (50.5%) |
| Missing data | 1 (0.3%) |
| Diabetes | |
| Absent | 300 (75.0%) |
| Present | 100 (25.0%) |
| Hypertension | |
| Absent | 140 (35.0%) |
| Present | 260 (65.0%) |
| Histology | |
| Endometroid | 293 (73.3%) |
| Nonendometroid | 105 (26.3%) |
| Missing data | 2 (0.5%) |
| Stage (FIGO*) | |
| IA–IB | 277 (69.3%) |
| II | 55 (13.8%) |
| IIIA–IIIC | 47 (11.8%) |
| IVA‐IVB | 16 (4.0%) |
| Missing data | 5 (1.3%) |
| Grade | |
| I | 191 (47.8%) |
| II | 148 (37.0%) |
| III | 49 (12.3%) |
| Missing data | 12 (3.0%) |
| Cervical invasion | |
| Absent | 300 (75.0%) |
| Present | 95 (23.8%) |
| Missing data | 5 (1.3%) |
| Myometrial infiltration | |
| ≤1/2 | 198 (49.5%) |
| 1/2 | 197 (49.3%) |
| Missing data | 5 (1.3%) |
| Metastases | |
| Absent | 262 (65.5%) |
| Present | 125 (31.3%) |
| Missing data | 13 (3.3%) |
| ER status | |
| Positive | 337 (84.3%) |
| Negative | 63 (15.8%) |
| PR status | |
| Positive | 323 (80.8%) |
| Negative | 77 (19.3%) |
| HER2 status | |
| Positive | 147 (36.8%) |
| Negative | 253 (63.3%) |
*FIGO = International Federation of Gynecology and Obstetrics.
IHC on tissue microarrays (TMA)
Samples were collected by surgical excision prior to any systemic treatment and were fixed in 10% (v/v) neutral buffered formalin for up to 24 hours, dehydrated in 70% ethanol, and embedded in paraffin. FFPE tissue blocks were stored at room temperature for up to 14 years. The percentage of tumor cells in each FFPE specimen was evaluated by hematoxylin and eosin staining reviewed by a certified pathologist. TMAs were constructed from FFPE surgical resection tumor specimens and control samples, as previously described.10 Four 1.5‐mm‐diameter cores from each tumor were obtained from the most representative areas using tissue‐arraying instrument (MTA‐I, Beecher Instruments, Sun Prairie, WI, USA), and then reembedded in microarray blocks. Punches of normal tissues were added to the each array to introduce built‐in internal controls to the system. Consecutive 4‐μm‐thick TMA sections were cut and placed on charged polylysine‐coated slides (Superfrost Plus, BDH, Menzel, Germany) for subsequent IHC analysis.
Protein expression was examined by IHC on TMA blocks using the following antibodies: ER—clone SP1 (Roche, Basel, Switzerland, dilution: ready to use, RTU), PR—clone 1E2 (Roche, dilution: RTU), HER2—clone 4B5 (Roche, dilution: RTU), TOP2A—clone Ki‐S1 (DAKO, Glostrup, Denmark, dilution: 1:200), TP53—clone BP‐53‐11 (Roche, dilution: RTU). The staining has been performed in accordance with manufacturers’ guidelines. Protein expression was evaluated by two pathologists blinded to clinical data (HM and JG). ER and PR evaluation of the nuclear staining was performed based on Allred score.11 HER2 receptor status was determined based on the criteria of HercepTest (DAKO) according to the manufacturer's guidelines. TOP2A expression was assessed based on the percentage of the stained nuclei (1: 0–5%, 2: 6–25%, 3: 26–50%, 4: 51–75%, 5: 76–100%). TP53 expression evaluation included staining intensity (0—negative, 1—weak, 2—intermediate, 3—strong) and the percentage of stained cells (0: negative, 1: up to 10%, 2: 11–25%, 3: 26–50%, 4: 51–75%, 5: 76–100%), which accounted for the score ranging from 0 to 8. Cutoff point determination of expression positivity, based on results' distribution, was performed with the use of Cutoff Finder Web Application12 and yielded values: ≥4 for ER and PR, and ≥2 for HER2. The assumed values were similar to those reported in the literature.5, 6
Statistical analysis
STATISTICA software (Statsoft Co., Tulsa, OK, USA, version 10) was used for all calculations. The tests that were used and their applications were as follows: testing normality of the data set—Shapiro–Wilk test; comparison of the tumor subtypes with clinicopathological data of the patients—crosstabs statistics with Pearson's chi‐square test; correlations between the tumor subtypes and assessed markers—Kruskal–Wallis test; ER, PR, and HER2 status in the context of clinicopathological data—crosstabs statistics with Pearson's chi‐square test; TOP2A and TP53 expression in the context of clinicopathological data—Mann–Whitney test. TOP2A and TP53 expression analysis were performed on continuous measurements in order to avoid information loss introduced by marker dichotomization.9 The Kaplan–Meier estimator was employed for survival analysis, and the generated curves were compared with the log‐rank test. The endpoint for the study was overall survival (OS). OS was defined as the time from sample collection to death from any cause or censoring. Censoring was defined as loss of follow‐up or alive at the end of follow‐up. Cox proportional hazards regression analysis was used to identify the independent predictors of OS. Statistical significance for all the aforementioned calculations was assumed when p ≤ 0.05. Univariate predictors significant with a value of p ≤ 0.10 were entered into a stepwise multivariate model to identify those with independent prognostic information. Missing data were not included into statistical analysis.
Results
Flow of samples
Constructed TMA blocks collectively included 406 patients. Information concerning the expression level of all three receptors simultaneously (ER, PR, HER2) was available for 400 (98.5%) cases. Within the group of 400 samples, 399 (99.8%) and 400 (100.0%) had their TOP2A and TP53 status assessed, respectively.
Correlation of molecular subtypes with clinical and pathological data
ER+/PR+/HER2+ subtype included 129 (32.3%) samples, ER+/PR+/HER2−: 224 (56.0%), ER−/PR−/HER2+: 18 (4.5%) and ER−/PR−/HER2−: 29 (7.3%). Clinicopathological characterization of the molecular subtypes is presented in Table 2. Patients classified as ER−/PR−/HER2+ or ER−/PR−/HER2− had higher stage of the disease, higher grade, histology type II, myometrial infiltration, cervical invasion, and metastases more frequently but were rarely obese. No statistically significant correlations between the subtypes and parameters such as age, menopausal status, or diabetes have been observed.
Table 2.
Comparison of molecular subtypes with clinicopathological data
| Variable | Molecular subtype | p Value | |||
|---|---|---|---|---|---|
| ER/PR+, HER2+ N = 129 (32.3%) | ER/PR+, HER2− N = 224 (56.0%) | ER/PR‐, HER2+ N = 18 (4.5%) | ER/PR‐, HER2− N = 29 (7.3%) | ||
| Menopausal status | |||||
| Premenopausal | 11 (39.3%) | 14 (50.0%) | 1 (3.6%) | 2 (7.1%) | 0.88 |
| Peri‐ and postmenopausal | 118 (31.8%) | 210 (56.6%) | 16 (4.3%) | 27 (7.3%) | |
| Age | |||||
| ≤50 years | 14 (33.3%) | 25 (59.5%) | 1 (2.4%) | 2 (4.8%) | 0.80 |
| >50 years | 115 (32.2%) | 199 (55.6%) | 17 (4.8%) | 27 (7.5%) | |
| Obesity | |||||
| Absent | 68 (34.5%) | 94 (47.7%) | 14 (7.1%) | 21 (10.7%) | 0.0006 |
| Present | 61 (30.2%) | 129 (63.9%) | 4 (2.0%) | 8 (4.0%) | |
| Diabetes | |||||
| Absent | 102 (34.0%) | 159 (53.0%) | 15 (5.0%) | 24 (8.0%) | 0.20 |
| Present | 27 (27.0%) | 65 (65.0%) | 3 (3.0%) | 5 (5.0%) | |
| Hypertension | |||||
| Absent | 50 (35.7%) | 67 (47.9%) | 6 (4.3%) | 17 (12.1%) | 0.01 |
| Present | 79 (30.4%) | 157 (60.4%) | 12 (4.6%) | 12 (4.6%) | |
| Histology* | |||||
| Type I | 82 (29.9%) | 179 (65.3%) | 6 (2.2%) | 7 (2.6%) | <0.000001 |
| Type II | 46 (37.1%) | 45 (36.3%) | 12 (9.7%) | 21 (16.9%) | |
| Stage (FIGO*) | |||||
| IA–IB, II | 104 (31.3%) | 198 (59.6%) | 9 (2.7%) | 21 (6.3%) | 0.00006 |
| IIIA–IIIC, IVA–IVB | 23 (36.5%) | 24 (38.1%) | 9 (14.3%) | 7 (11.1%) | |
| Grade | |||||
| 1, 2 | 112 (33.1%) | 201 (59.5%) | 11 (3.3%) | 14 (4.1%) | <0.000001 |
| 3 | 13 (26.5%) | 18 (36.7%) | 7 (14.3%) | 11 (22.5%) | |
| Cervical invasion | |||||
| Absent | 98 (32.7%) | 176 (58.7%) | 8 (2.7%) | 18 (6.0%) | 0.004 |
| Present | 29 (30.5%) | 46 (48.4%) | 10 (10.5%) | 10 (10.5%) | |
| Myometrial infiltration | |||||
| ≤1/2 | 62 (31.3%) | 122 (61.6%) | 6 (3.0%) | 8 (4.0%) | 0.02 |
| >1/2 | 65 (33.0%) | 100 (50.8%) | 12 (6.1%) | 20 (10.2%) | |
| Metastases | |||||
| Absent | 87 (33.2%) | 157 (59.9%) | 3 (1.2%) | 15 (5.7%) | 0.00001 |
| Present | 39 (31.2%) | 58 (46.4%) | 15 (12.0%) | 13 (10.4%) | |
*For statistical analysis “type II” included grade 3 tumors in addition to nonendometroid carcinomas. FIGO = International Federation of Gynecology and Obstetrics.
Survival analysis
ER−/PR−/HER2+ subtype correlated with the poorest outcome, ER+/PR+/HER2− subtype was associated with the most favorable prognosis (p = 0.002), as presented in Figure 1 . Univariate analysis performed for ER−/PR−/HER2+ subtype versus ER+/PR+/HER2− yielded hazard ratio of 3.49 (95% CI, 1.87–6.54, p = 0.00009). All of the studied parameters, excluding obesity, carried negative prognostic information in univariate analysis. Molecular subtype division remained an independent prognostic factor for overall survival in multivariate analysis, accompanying parameters such as diabetes, hypertension, stage, myometrial infiltration, and metastases, all of which yielded hazard ratios between 1.39 and 2.23. The results of univariate and multivariate analysis of all the studied parameters are presented in Table 3.
Figure 1.
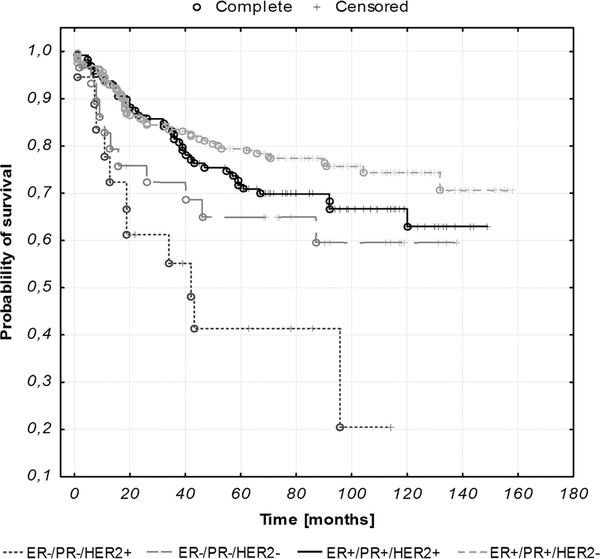
Kaplan–Meier curves illustrating overall survival of EC patients stratified against molecular subtypes (p = 0.002).
Table 3.
Univariate and multivariate analysis of clinicopathological and subtype data as prognostic factors in EC
| Analyzed parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p Value | |
| Menopausal status (postmenopausal vs. peri‐ and premenopausal) | 1.84 | 1.04–3.25 | 0.04 | Not significant | ||
| Age (>50 vs. ≤50 years) | 4.04 | 1.49–10.95 | 0.006 | Not significant | ||
| Obesity (present vs. absent) | 1.18 | 0.81–1.70 | 0.39 | |||
| Diabetes (present vs. absent) | 1.77 | 1.21–2.60 | 0.004 | 1.74 | 1.16–2.60 | 0.007 |
| Hypertension (present vs .absent) | 1.98 | 1.29–3.06 | 0.002 | 1.89 | 1.21–2.93 | 0.005 |
| Histology* (type II vs. type I) | 1.71 | 1.18–2.48 | 0.005 | not significant | ||
| Stage (3, 4 vs. 1, 2) | 1.97 | 1.62–2.39 | <0.0000001 | 1.39 | 1.09–1.78 | 0.008 |
| Grade (3 vs. 1,2) | 1.37 | 1.09–1.74 | 0.008 | not significant | ||
| Cervical invasion (present vs. absent) | 2.52 | 1.72–3.69 | 0.000002 | not significant | ||
| Myometrial infiltration (>1/2 vs. ≤1/2) | 2.40 | 1.62–3.56 | 0.00001 | 1.79 | 1.19–2.68 | 0.005 |
| Metastases (present vs. absent) | 3.74 | 2.57–5.46 | <0.0000001 | 2.23 | 1.39–3.58 | 0.0009 |
| Molecular subtype (ER/PR−, HER2+ vs. ER/PR+, HER2−) | 3.49 | 1.87–6.54 | 0.00009 | 2.07 | 1.07–4.02 | 0.03 |
*For statistical analysis “type II” included grade 3 tumors in addition to nonendometroid carcinomas.
Molecular characterization of the elucidated subtypes
ER+/PR+/HER2+ and ER+/PR+/HER2− subtypes had lower average TP53 and TOP2A expression levels when compared with ER−/PR−/HER2+ and ER−/PR−/HER2− (both p < 0.00001), as presented in Figure 2 .
Figure 2.
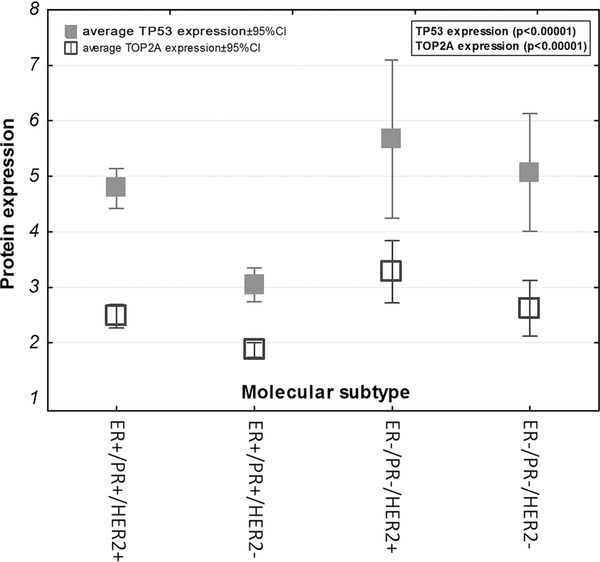
TP53 and TOP2A expression level within the studied subtypes.
Clinical and pathological data in the context of the studied proteins
Correlations between ER, PR, HER2 status, and clinicopathological data are presented in Table 4. ER and PR loss and HER2 overexpression correlated with more aggressive tumor characteristics. TOP2A and TP53 expression has been presented in Figure 2. High levels of TOP2A and TP53 correlated with more aggressive tumor characteristics.
Table 4.
ER, PR, HER2 status in the context of clinicopathological data (crosstabs statistics with Pearson's Chi‐square test)
| Variable | ER status | PR status | HER2 status | |||
|---|---|---|---|---|---|---|
| Number of positive samples (%) | p value | Number of positive samples (%) | p value | Number of positive samples (%) | p value | |
| Menopausal status | ||||||
| Premenopausal | 24 (85.7%) | 0.86 | 21 (75.%) | 0.42 | 12 (42.9%) | 0.45 |
| Peri‐ and postmenopausal | 316 (84.5%) | 303 (81.2%) | 135 (35.8%) | |||
| Age | ||||||
| ≤50 years | 38 (90.5%) | 0.25 | 34 (81.0%) | 0.95 | 15 (35.7%) | 0.92 |
| >50 years | 302 (83.7%) | 290 (80.6%) | 133 (36.5%) | |||
| Obesity | ||||||
| Absent | 159 (79.9%) | 0.02 | 146 (73.7%) | 0.0007 | 82 (41.0%) | 0.07 |
| Present | 180 (88.7%) | 177 (87.2%) | 66 (32.2%) | |||
| Diabetes | ||||||
| Absent | 252 (83.2%) | 0.25 | 237 (78.5%) | 0.06 | 118 (38.6%) | 0.12 |
| Present | 88 (88.0%) | 87 (87.0%) | 30 (30.0%) | |||
| Hypertension | ||||||
| Absent | 116 (81.7%) | 0.28 | 105 (75.0%) | 0.04 | 57 (39.9%) | 0.29 |
| Present | 224 (85.8%) | 219 (83.6%) | 91 (34.6%) | |||
| Histology | ||||||
| Type I | 251 (91.6%) | <0.000001 | 243 (88.7%) | <0.000001 | 88 (32.0%) | 0.007 |
| Type II | 88 (69.3%) | 80 (63.5%) | 59 (45.7%) | |||
| Stage (FIGO*) | ||||||
| IA, IB, II | 295 (88.1%) | 0.00002 | 278 (83.7%) | 0.0004 | 114 (33.9%) | 0.02 |
| IIIA, IIB, IIIC, IVA, IVB | 42 (66.7%) | 42 (64.6%) | 32 (49.2%) | |||
| Grade | ||||||
| 1, 2 | 301 (88.8%) | <0.000001 | 287 (84.9%) | <0.000001 | 123 (36.2%) | 0.56 |
| 3 | 30 (58.8%) | 27 (54.0%) | 21 (40.4%) | |||
| Cervical invasion | ||||||
| Absent | 267 (88.4%) | 0.0002 | 250 (83.1%) | 0.03 | 107 (35.3%) | 0.42 |
| Present | 70 (72.9%) | 70 (72.9%) | 39 (39.8%) | |||
| Myometrial infiltration | ||||||
| ≤1/2 | 178 (89.0%) | 0.02 | 167 (84.3%) | 0.06 | 69 (34.3%) | 0.39 |
| >1/2 | 159 (80.3%) | 153 (76.9%) | 77 (38.5%) | |||
| Metastases | ||||||
| Absent | 241 (91.3%) | <0.000001 | 224 (85.5%) | 0.0002 | 91 (34.5%) | 0.15 |
| Present | 90 (71,4%) | 88 (69.3%) | 54 (41.9%) | |||
*FIGO = International Federation of Gynecology and Obstetrics.
Discussion
The distinction of molecular subtypes in breast cancer has introduced valuable information about underlying tumor biology and made advances have already begun to translate into treatment individualization. Molecular subtypes in EC, similarly to breast cancer, do differ fundamentally in terms of prognosis, clinicopathological, and molecular characteristics. The greatest distinction was observed between ER−/PR−/HER2+ subtype exhibiting exceptionally aggressive tumor characteristics and ER+/PR+/HER2− subtype being the most benign. ER−/PR−/HER2+ subtype was characterized by the shortest overall survival, often falling into the categories of histology type II, advanced stage or grade, and frequently showing signs of cervical invasion, myometrial infiltration, or metastases. ER−/PR−/HER2+ subtype remained an independent prognostic factor for overall survival in multivariate analysis. Furthermore, it had the highest TOP2A and mutated TP53 protein expression of all four subtypes. ER+/PR+/HER2− subtype was the exact opposite of ER−/PR−/HER2+. The difference in survival between ER−/PR−/HER2+ and ER+/PR+/HER2− subgroups was 37% (41% vs. 78%, respectively) what gives the power of 82% with two‐sided α of 5%. Thus, our study is powered enough to detect the claimed difference in survival.
The other subtypes, ER−/PR−/HER2− and ER+/PR+/HER2+, have fallen in the middle of the molecular distinction, showing intermediate clinicopathological, prognostic, and molecular characteristics, with ER−/PR−/HER2− subtype classified as the second least favorable and ER+/PR+/HER2+ subtype—the second most favorable.
Lack of ER and PR expression and high expression of HER2 are treated as indicators of poor survival and aggressive tumor behavior in EC.13, 14 This explains why ER−/PR−/HER2+ subtype seems to be the most unfavorable subtype, whereas ER+/PR+/HER2− subtype—the least. Works on molecular subtypes in EC focus on triple‐negative phenotype being the indicator of poor prognosis.5, 6 Our data also point to triple‐negative phenotype having rather short overall survival and unfavorable tumor characteristics (high grade, advanced stage, type II histology, myometrial invasion), however it is ER−/PR−/HER2+ subtype, which determines exceptionally poor prognosis.
Data obtained on molecular subtypes in EC are in high concordance with subtype characterization in breast cancer. In terms of occurrence frequency, basal‐like (mostly ER−/PR−/HER2−) and HER2‐positive (often ER−/PR−/HER2+) tumors constitute a minority of cases when compared with luminal A (mostly ER+/PR+/HER2−) and B (usually ER+/PR+/HER2+) tumors. Similarly to EC, of the four subtypes, luminal A breast tumors are characterized by good prognosis, with high survival and low recurrence rates. Also, women with luminal B tumors have fairly high survival rates, yet not as high as those with luminal A. Few luminal A tumors but many basal‐like tumors have TP53 mutations.15, 16, 17 Additionally, also similarly to EC, no correlation between the subtypes and patients' age is observed.18
One of the limitations of the study was relatively short follow‐up period. Another problem was small sample size in ER+/PR+/HER2− and ER−/PR−/HER2− subgroup. Additionally, Ki67 expression status, often taken into account in molecular subtype determination in breast cancer, was unavailable. Ergo, we decided to include TOP2A expression as a surrogate of a proliferation marker, as proposed in the literature.19
In our study, TOP2A expression was higher in ER−/PR−/HER2+ and ER−/PR−/HER2− subtypes when compared to ER+/PR+/HER2+ and ER+/PR+/HER2−. High TOP2A expression has also been observed in triple‐negative breast cancer.20 Our study showed that TOP2A expression correlated with more aggressive tumor characteristics. Similar results were reported for nasopharyngeal carcinoma.21 We also assessed TP53 expression, as this marker is commonly studied in the context of breast cancer molecular subtypes22, 23 and TP53 is frequently found overexpressed in uterine carcinomas.24 TP53 overexpression correlated with more aggressive tumor characteristics and its level was higher in ER−/PR−/HER2+ and ER−/PR−/HER2− subtypes. TP53 overexpression as a determinant of the poor course of the disease is well documented in the literature, also in EC where it is typical for histology type II tumors.25
Conclusion
In EC molecular subtypes based on ER, PR and HER2 status differ fundamentally in terms of prognosis, clinicopathological, and molecular characteristics. The proposed classification might serve as a clinically valid molecular marker and IHC could be a fast and simple method of its determination. Continued investigation of the elucidated groups, especially in the aspect of targeted therapies, is necessary as the increasing body of evidence supports the use of ER, PR, and HER2 as markers of treatment response. However, their assessment is not a routine practice in EC.26, 27
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The research has been financed by the Ministry of Science and Higher Education grant N407571538.
Author contributions: Conception and design of the study: A.J.Z., A.S., S.L.S., A.L.; acquisition of data: S.L.S., W.B., D.W.; analysis and interpretation of data: A.S., A.J.Z., S.L.S., H.M., J.G., A.L.; drafting the paper: A.S., S.L.S., A.J.Z.; revising the paper: H.M., J.G., A.L., W.B., D.W.; final approval of the manuscript: S.L.S., A.S., H.M., J.G., A.L., W.B., D.W., A.J.Z.
References
- 1. Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C, Group EGW. endometrial cancer: esmo clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2011; 22(Suppl. 6): vi35–vi39. [DOI] [PubMed] [Google Scholar]
- 2. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406: 747–752. [DOI] [PubMed] [Google Scholar]
- 3. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, members P. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011; 22: 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101: 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kothari R, Morrison C, Richardson D, Seward S, O'Malley D, Copeland L, Fowler J, Cohn DE. The prognostic significance of the triple negative phenotype in endometrial cancer. Gynecol Oncol. 2010; 118: 172–175. [DOI] [PubMed] [Google Scholar]
- 6. Jiang XF, Tang QL, Shen XM, Li HG, Chen LH, Wang XY, Luo X, Lin ZQ, Jiang GY. Tumor‐associated macrophages, epidermal growth factor receptor correlated with the triple negative phenotype in endometrial endometrioid adenocarcinoma. Pathol Res Pract. 2012; 208: 730–735. [DOI] [PubMed] [Google Scholar]
- 7. Pecorelli S. Revised figo staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105: 103–104. [DOI] [PubMed] [Google Scholar]
- 8. Consultation WE. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 9. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (remark): explanation and elaboration. PLoS Med. 2012; 9: e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Supernat A, Markiewicz A, Welnicka‐Jaskiewicz M, Seroczynska B, Skokowski J, Sejda A, Szade J, Czapiewski P, Biernat W, Zaczek A. Cd73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol. 2012; 20: 103–107. [DOI] [PubMed] [Google Scholar]
- 11. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11: 155–168. [PubMed] [Google Scholar]
- 12. Budczies J, Klauschen F, Sinn BV, Gy˝orffy B, Schmitt WD, Darb‐Esfahani S, Denkert C: Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012; 7: e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, Njolstad TS, Stefansson IM, Marcickiewicz J, Tingulstad S, et al. Group Ms. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer. 2013; 49: 3431–3441. [DOI] [PubMed] [Google Scholar]
- 14. Togami S, Sasajima Y, Oi T, Ishikawa M, Onda T, Ikeda S, Kato T, Tsuda H, Kasamatsu T. Clinicopathological and prognostic impact of human epidermal growth factor receptor type 2 (HER2) and hormone receptor expression in uterine papillary serous carcinoma. Cancer Sci. 2012; 103: 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006; 295: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 16. Network CGA . Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genom. 2006; 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metzger‐Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione‐Gertsch M, Coates AS, Goldhirsch A, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node‐negative disease: results from international breast cancer study group trials viii and ix. J Clin Oncol. 2013; 31: 3083–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stromar IK, Jakic‐Razumovic J. The value of immunohistochemical determination of topoisomerase iiα and ki67 as markers of cell proliferation and malignant transformation in colonic mucosa. Appl Immunohistochem Mol Morphol. 2014; 22: 524–529. [DOI] [PubMed] [Google Scholar]
- 20. Mrkli´c I, Pogoreli´c Z, ´Capkun V, Tomi´c S. Expression of topoisomerase ii‐α in triple negative breast cancer. Appl Immunohistochem Mol Morphol 2014; 22: 182–187. [DOI] [PubMed] [Google Scholar]
- 21. Lan J, Huang HY, Lee SW, Chen TJ, Tai HC, Hsu HP, Chang KY, Li CF. Top2a overexpression as a poor prognostic factor in patients with nasopharyngeal carcinoma. Tumour Biol. 2014; 35: 179–187. [DOI] [PubMed] [Google Scholar]
- 22. Morrison DH, Rahardja D, King E, Peng Y, Sarode VR. Tumour biomarker expression relative to age and molecular subtypes of invasive breast cancer. Br J Cancer. 2012; 107: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarode VR, Han JS, Morris DH, Peng Y, Rao R. A comparative analysis of biomarker expression and molecular subtypes of pure ductal carcinoma in situ and invasive breast carcinoma by image analysis: relationship of the subtypes with histologic grade, Ki67, p53 overexpression, and DNA ploidy. Int J Breast Cancer. 2011; 2011: 217060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urabe R, Hachisuga T, Kurita T, Kagami S, Kawagoe T, Matsuura Y, Shimajiri S. Prognostic significance of overexpression of p53 in uterine endometrioid adenocarcinomas with an analysis of nuclear grade. J Obstet Gynaecol Res. 2014; 40: 812–819. [DOI] [PubMed] [Google Scholar]
- 25. Matias‐Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013; 62: 111–123. [DOI] [PubMed] [Google Scholar]
- 26. Sho T, Hachisuga T, Nguyen TT, Urabe R, Kurita T, Kagami S, Kawagoe T, Matsuura Y, Shimajiri S. Expression of estrogen receptor‐α as a prognostic factor in patients with uterine serous carcinoma. Int J Gynecol Cancer. 2014; 24: 102–106. [DOI] [PubMed] [Google Scholar]
- 27. Buza N, Roque DM, Santin AD. Her2/neu in endometrial cancer: a promising therapeutic target with diagnostic challenges. Arch Pathol Lab Med. 2014; 138: 343–350. [DOI] [PubMed] [Google Scholar]


