Abstract
Anastomotic leaks are a dreaded surgical complication following colorectal operations. Creation of a temporary proximal diverting ileostomy is used in high‐risk anastomoses, however, additional surgical risk is accumulated with its creation and reversal. Endoluminal vacuum therapy has been shown to seal anastomotic defects in the prophylactic setting in a pig model and we hypothesized it could be utilized in a delayed fashion to rescue subjects with an active anastomotic leak. Yorkshire pigs underwent rectal resection, intentional leak confirmed by fluoroscopy, and endoluminal vacuum therapy device placement to low continuous suction. Following treatment, a contrast enema and necropsy was performed for gross and histopathology. Pigs underwent 2 (or 5) days of free intraperitoneal leak prior to device placement and 5 (or 7) subsequent days of endoluminal vacuum therapy. Six of seven early‐treated pigs sealed their anastomotic defect, while two of the four treated pigs in this extended group sealed the defect. Endoluminal vacuum therapy is feasible and well tolerated in a pig model, and it has been shown to seal a significant number of freely leaking anastomoses in the early period (86%). This technology warrants further study as it may provide a noninvasive means to treatment of anastomotic leaks.
Keywords: colorectal disease, surgery, complications, colon cancer, infection
Introduction
Rectal cancer is a common cause of cancer‐related mortality, and carries significant operative morbidity. Anastomotic leaks are the most dreaded surgical complication, and lead to creation of a temporary proximal diversion during anterior rectal resection and primary anastomosis. Anastomotic leaks may result in abscesses, enterocutaneous fistulae, severe sepsis and peritonitis, increasing reoperation, length of stay, recurrence, and mortality.1, 2, 3 Potential treatment of anastomotic leak includes laparotomy, proximal diversion, or a Hartmann's procedure with or without revision of the anastomosis.2, 4, 5
Negative pressure therapy, or vacuum‐assisted closure (VAC) has been used on superficial wounds, and increases healing by secondary intention.6 In doing so, it also increases local blood perfusion, accelerates granulation tissue formation, decreases tissue bacterial loads and inflammation, and increases nutrient blood flow.6, 7, 8 The Endo‐SPONGE (B. Braun Medical, Melsungen, Germany), a commercially available device in Europe, has been shown to be successful in closing perianastomotic and presacral abscess cavities after anastomotic leakage.9, 10, 11, 12, 13, 14 This previously described technique places a sponge into the perirectal space near the leak rather than within the anastomotic lumen.
We have previously shown that placement of a VAC sponge into the rectal lumen, in the presence of an intentional rectal defect, will heal the defect rapidly in a majority of cases, with minimal to no damage to the mucosa.15 This limited, initial study evaluated VAC placement into a freshly created, uncontaminated defect without significant leakage of enteric contents. To better mimic the clinical setting of undetected leak, our study evaluates an endoluminal VAC device placed within the bowel lumen several days after creation of an anastomotic defect and subsequent leak.
Objective/Hypothesis
This series is a follow‐up to the pilot study published by Shada and colleagues.15 Having initially shown endoluminal vacuum therapy could seal an anastomotic defect when placed at the time of the original operation (leak prophylaxis); we hypothesized that the same endoluminal vacuum therapy could be utilized in a delayed fashion to rescue subjects with an active anastomotic leak.
Methods
The University of Virginia's Institutional Animal Care and Use Committee approved this study (Protocol # 3849). As outlined in the Animal Welfare Act (CFR 9 as amended) and the Guide for the Care and Use of Laboratory Animals, 8th edition, 2010, the protocol ensured the appropriate care, use, and humane treatment of the animals being used for the duration of the research study.
Study population
We utilized a total of 13 female Yorkshire pigs (60 to 80 pounds) (Baugher Farms, Stanardsville, VA, USA). Upon arrival to our center, pigs were kept in standard husbandry conditions, fed a Teklad 7037 miniswine diet (Harlan Industries, Indianapolis, IN, USA), and acclimated for 72 hours. The preoperative preparation has been described previously.15 To ensure an adequate bowel preparation for the subsequent rectal operation, food was withheld 48 hours prior to surgery and pigs were provided ad libitum access to drinking water containing sucrose at 50 g/L. Pigs were also provided 24 fluid ounces of magnesium citrate in their drinking water to finalize the bowel preparation.
Operative technique
All pigs underwent induction of anesthesia with intramuscular (IM) xylazine (2 mg/kg) and telazol (6 mg/kg), followed by IM glycopyrolate (0.01 mg/kg), long‐acting tetracycline (LA‐200; Pfizer, New York, NY, USA; 9 mg/kg), and enroflozacin (Baytril 100, Bayer, Shawnee Mission, KS, USA; 7.5 mg/kg). The pigs were intubated and maintained on 2.5% isoflurane with continuous cardiopulmonary monitoring and warmed lactated ringer's solution (LRS) was provided intravenously (IV) at 5 mL/kg/hour. Last, 0.3 mg of buprenorphine was given IV and a 75 μg/hour transdermal fentanyl patch (75 mcg/hr, Watson Laboratories, Corona, CA, USA) was placed on the skin.
Operative sites including the neck, back, and abdomen were clipped, prepped, and draped in a sterile fashion. An open central venous catheter was placed into the internal jugular (IJ) vein utilizing a 7‐French silastic catheter, and tunneled subcutaneously to exit caudal and posterior to the ipsilateral scapula (Figure 1).
Figure 1.
Internal jugular central venous catheter, tunneled subcutaneously.
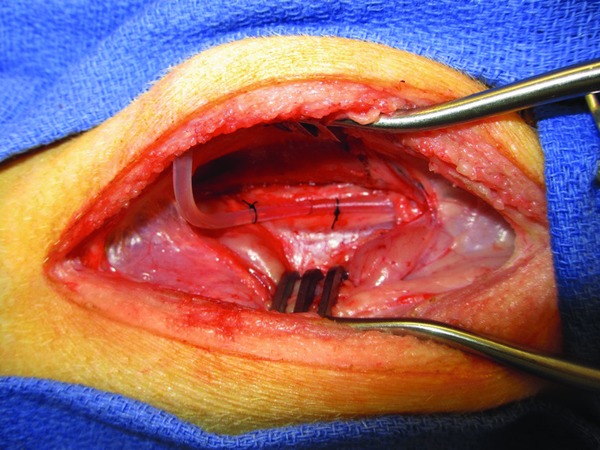
The operation was performed via a lower abdominal midline incision. The rectum was identified, mobilized, and a short‐segment rectal resection was performed in standard fashion. The distal colon was reanastomosed to the remaining rectum with an Endopath endoscopic curved intraluminal circular stapler (Ethicon Endo‐Surgery Inc., Cincinnati, OH, USA). A deliberate 2 cm incision was created across the anastomotic staple line to simulate an anastomotic dehiscence and allow subsequent leak (Figure 2). Midway into the study, two marking staples were added, placed just proximal and distal to the anastomotic defect, to allow easier identification of the defect fluoroscopically.
Figure 2.
Rectal anastomotic defect (indicator staples not shown).
Following rectal resection and anastomotic defect creation, the pigs were placed in restraints and kept in an elevated run. The restraint mechanism consisted of a jacket system with a swivel tether connecting to the top of the run. Additionally, a harness with chain tethers was added to decrease spinning that may kink the IV lines and subsequent vacuum tubing, as well as to avoid lines becoming tangled in the pig's legs. The chain tethers allowed enough freedom of movement to stand up, lie down, and make normal postural adjustments. Pigs were monitored twice daily by veterinary or research staff by an assessment for peritonitis, including fever, tachycardia, tachypnea, and abdominal pain aided by sedation with IV diazepam and acetylpromazine. Pigs exhibiting signs of peritonitis were removed from the study and euthanized.
Anastomotic leak and device placement
All pigs were maintained nil per os with continuous total parenteral nutrition and LRS via IJ central line for 2 days (or 5 days based on study group) to allow the anastomotic defect to intentionally leak. We studied two groups of pigs, a short leak of 2 days with the anastomotic defect and an extended leak of 5 days. On postoperative day 2 (8 pigs) or day 5 (5 pigs), the pigs underwent a fluoroscopic lower gastrointestinal contrast study utilizing iohexol (Omnipaque 350; GE Healthcare, Waukesha, WI, USA) and an OEC 8800 C‐arm fluoroscope. All pigs with an anastomotic leak on respective study day underwent subsequent device placement. The device was created prior to fluoroscopy for immediate placement after confirmation of the intraabdominal leak.
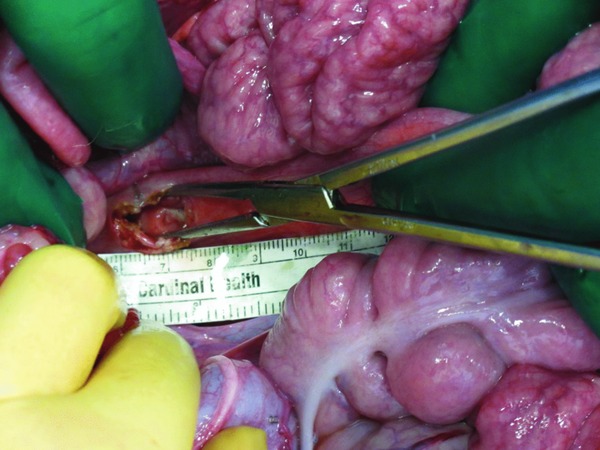
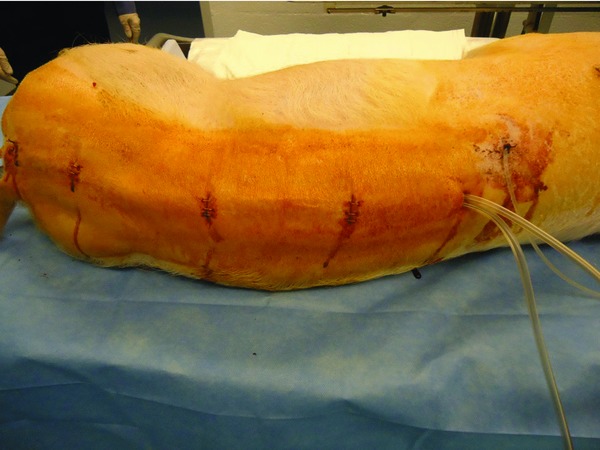
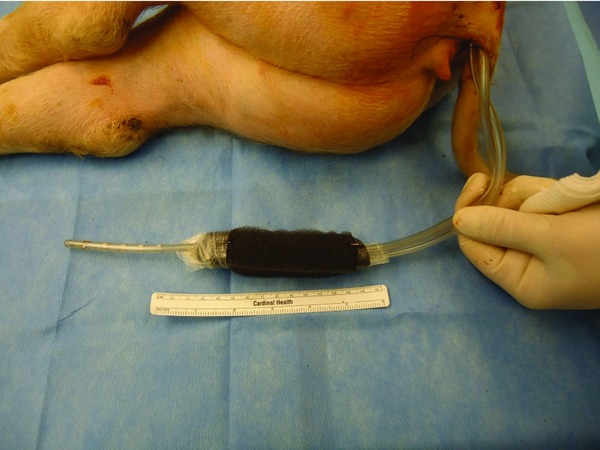
Devices were constructed as previously described, utilizing a dual‐chamber sump negative pressure system.15 GranuFoam (KCI, San Antonio, TX, USA) was wrapped circumferentially around two, 16‐French sump tubes (Bard Medical, Covington, GA, USA). These were tunneled subcutaneously from the subscapular area to the anus after a short segment was tunneled into and through the rectum (Figures 3 and 4). Following confirmation of anastomotic leak by fluoroscopy, the device was inserted in a retrograde fashion into the rectum using a large Kelly clamp, positioning the device with the black sponge centrally overlying the defect. Correct placement was established by visualization of the staples on the sponge splitting the anastomotic staple line or additional staples previously placed on either end of the defect (Figure 5).
Figure 3.
Central line (cephalad) and double negative pressure suction lines (caudal), tunneled subcutaneously.
Figure 4.
Suction tubing exiting rectum from subcutaneous tunneling. Indicator staples on either end of foam demarcates device end.
Figure 5.
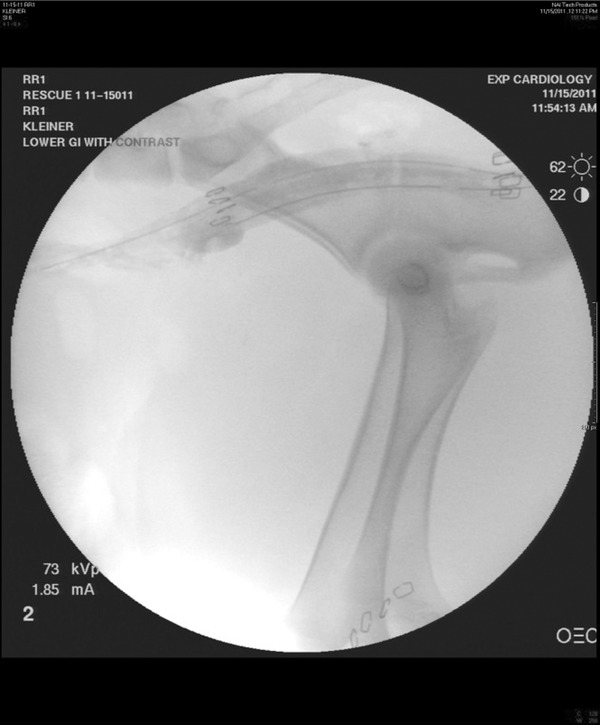
Device insertion and final placement of sponge through defect indicated by staples.
Postdevice placement
Following device placement, all pigs were returned to the run. The pigs in the short leak group (2 days) underwent 5 days of endoluminal vacuum therapy, while those in the extended leak group (5 days) underwent 7 days of vacuum therapy. These groups were not intended to be compared to each other but to establish feasibility of rescue in the immediate and delayed leaking anastomosis. At the end of the planned endoluminal vacuum therapy, all pigs had transanal device removal and subsequent fluoroscopy to determine whether the defect had sealed. Following final fluoroscopy, all pigs were euthanized and underwent gross necropsy.
Histopathology
Rectal anastomotic sites were excised and fixed in formalin. The specimens were dissected, and sections of the enterotomy site, uninvolved rectum, and any atypical appearing areas were taken. The sections were paraffin embedded, cut at 5 μm intervals with a standard microtome, and stained with hematoxylin and eosin (H&E). Slides were evaluated for mucosal integrity, overall inflammatory response, presence of serositis, mucosal necrosis, and/or fibrinous adhesions. Inflammatory response was evaluated adjacent to the enterotomy site, and graded as mild, moderate, or severe, with mild representing increased mucosal inflammation, moderate representing increased inflammation with crypt loss, and severe meaning complete absence of crypts. Serositis was scored as mild if minimal serosal inflammation was present, and moderate if inflammation and fibrinous adhesions were seen. Adhesions to any adjacent abdominal organs were recorded.
Results
Of the 13 pigs used, eight pigs underwent a 2‐day leak prior to rescue by endoluminal vacuum device placement, while five pigs underwent an extended rescue after a 5‐day period of leak prior to device placement. As can be seen in Table 1, one pig did not have an anastomotic leak on the day of rescue and therefore was euthanized due to a failed leak model. One pig in the extended series became too septic to continue in the study as evidenced by fevers, tachycardia, tachypnea, and decreased activity and was therefore euthanized as per humane treatment protocol on the day of initial fluoroscopy. Of the remaining 11 pigs that received device placement, 6 of 7 sealed their anastomosis in the short leak, early rescue group (2 days of leak prior to device placement) and only 2 of 4 sealed in the extended leak, extended rescue group (5 days of leak prior to device placement; Figure 6).
Table 1.
Rescue rectal series timing and outcomes
| Rescue rectal pig | Days of leak following operation | Leak on rescue day by fluoroscopy | Days of endoluminal vacuum therapy | Leak on final day by fluoroscopy | Result |
|---|---|---|---|---|---|
| RR 1 | 2 | Yes | 5 | No | Sealed |
| RR 2 | 2 | Yes | 5 | No | Sealed |
| RR 3 | 2 | Yes | 5 | Yes | Continued leak |
| RR 4 | 2 | No* | – | – | – |
| RR 5 | 2 | Yes | 5 | No | Sealed |
| RR 6 | 2 | Yes | 5 | No | Sealed |
| RR 7 | 2 | Yes | 5 | No | Sealed |
| RR 8 | 2 | Yes | 5 | No | Sealed |
| Extended Rescue Rectal Pig | |||||
| ERR 1 | 5 | Yes | 7 | Yes | Continued leak |
| ERR 2 | 5 | Yes | 7 | No | Sealed |
| ERR 3 | 5 | Yes | 7 | No | Sealed |
| ERR 4 | 5 | Yes† | – | – | – |
| ERR 5 | 5 | Yes | 7 | Yes | Continued leak |
*Pig was euthanized due to a failure of initial leak.
†Pig euthanized due to sepsis and humane protocol.
Figure 6.
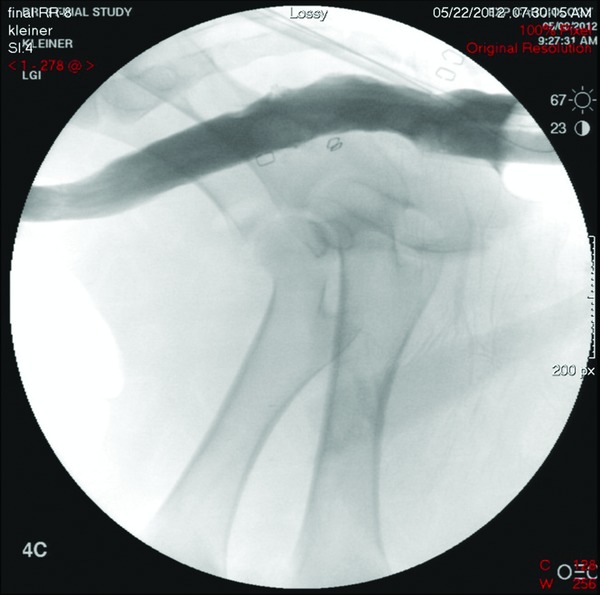
Final fluoroscopic rectal study following device removal without evidence of leak.
Gross pathology
All pigs were euthanized with Euthasol (pentobarbital sodium and phenytoin sodium solution; Virbac Animal Health, Fort Worth, TX, USA) on the final day, had the endoluminal negative pressure system removed and underwent a postmortem final fluoroscopy and necropsy. Upon entry into the peritoneal cavity, the majority had minimal adhesions, no leaks or abscess, and no pig had gross peritonitis. The three pigs with ultimate leak on final fluoroscopy had small contained abscesses. Figure 7 reveals a well‐healed anastomotic defect after 5 days of endoluminal vacuum therapy.
Figure 7.
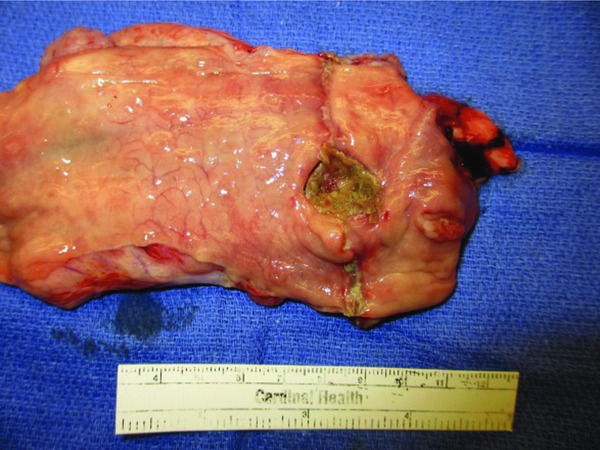
Gross pathology of the rectal anastomotic defect after opening the rectum longitudinally.
Histology
A representative histology specimen from both the vacuum and the previous study's control group are shown in Figure 8. All enterotomy sites had standard microscopic reparative changes including: increased acute and chronic inflammation, and granulation tissue with early scar formation. There were increased rates of mucosal inflammation and crypt dropout within the extended rescue series (75% moderate vs. 71% mild) compared to the rectal rescue group. Serositis tended to be less prominent in this group (75% mild vs. 43% mild), but an increased rate of bowel ischemia was seen adjacent to the enterotomy site (27% vs. 100%; Table 2 ) Fibrinous adhesions with involvement of the fallopian tubes were seen in both groups.
Figure 8.
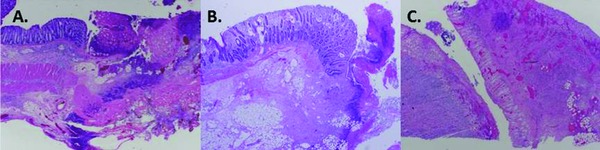
H&E, 100 × magnification. (A) Control pig with prominent transmural ischemic changes at enterotomy site (arrow). (B) Rectal rescue pig #2 showing mild‐to‐moderate inflammation, without ischemia. (C) Extended rectal rescue pig #5 showing transmural inflammation and ischemic changes.
Table 2.
Summary of histologic findings
| Group | Inflammation | Serositis | Adhesions | Ischemia/necrosis |
|---|---|---|---|---|
| Control* (n = 4) | 25% mild 75% moderate | 25% mild 75% moderate | Bladder, ovary, fibrinous (75%) | 100% |
| Rescue Rectal (n = 7) | 71% mild, 14% moderate, 14% severe | 43% mild 57% moderate | Fallopian tube, fibrinous (86%) | 14% |
| Extended Rectal Rescue (n = 4) | 0% mild, 75% moderate, 25% severe | 75% mild 25% moderate | Fallopian tube, fibrinous (25%) | 75% |
*Control data from Shada et al. (Shada, 2013).
Discussion
This study evaluated the use of endoluminal negative pressure therapy in a delayed, or “rescue,” fashion. We previously published a pilot study using the endoluminal negative pressure therapy in a prospective, prophylactic fashion.15 This previous series included a rectal resection leaving a large anastomotic defect and then immediate, intraoperative placement of the endoluminal device. This method proved to be successful in defect closure in 90% of the pigs. One limitation of this previous study was that the defects created at the time of rectal resection had immediate placement of the endoluminal device and never permitted time to leak or have gross enteric spillage. This limitation led us to wonder if this device only works in the prophylactic fashion, in a relatively nonsoiled environment. We sought to determine if the same technology would be feasible and effective for defect closure when the defect was permitted to have an active leak with delayed placement of the device, multiple days following defect creation.
We designed this study to allow the anastomotic defect to leak for 2 (and subsequently 5) days prior to device placement, more accurately mimicking a human anastomotic leak. In the early rescue group, having 5 days of endoluminal vacuum therapy allowed successful closure of the defect in 6/7 pigs (86%) after an active intraabdominal leak for 2 days. In the extended rescue group, having been allowed to leak for 5 total days, slightly fewer had successful closure with endoluminal vacuum therapy; 2/4 pigs (50%). These results are clearly promising with a large percentage of early leaks being effectively treated with a delayed endoluminal device and negative pressure therapy. This supports the possibility of endoluminal vacuum therapy as a treatment option for postoperative anastomotic leaks in the early period, potentially preventing reoperation or need for proximal diversion. The lower efficacy in the extended rectal pigs who were allowed to leak up to 5 days indicates late identification or grossly feculent leaks are likely best treated with operative management.
This lower success rate of closure in the extended leak group leads to a discussion of the pig model. In a human population, anastomotic leaks occur in a delayed fashion, a number of days following surgery as the anastomosis breaks down. Many surgeons perform a provocative intraoperative test by occluding the proximal lumen, insufflating the bowel under water, or instilling a dye agent to identify an immediate leak, allowing revision or repair.16 Upon completion of the case, the anastomosis therefore is not frankly leaking, but would develop over the subsequent days. The pig model, in contradistinction, has a large, persistent anastomotic leak immediately following surgery. The extended leak group would be comparable to identifying a leak in a human in the postoperative period but delaying treatment for another few days.
The gross and histopathologic findings are also promising. There were no pigs with peritonitis following device placement, despite 2 or 5 days of anastomotic leaks. This is in contradistinction to our initial study in which control pigs had gross, feculent peritonitis. This suggests the endoluminal vacuum therapy is able to fully control feculent spillage as well as sealing of the anastomotic defect. As seen in the initial study, the anastomotic defect with vacuum therapy was often adherent to surrounding pelvic structures, commonly the bladder or adjacent gynecologic structures. These adhesions were primarily to the serosal surface of adjacent structures and we did not identify any fistulae or breakdown of the adjacent structures. This indicates that this amount of vacuum therapy is sufficient to create a serosal patch but not so vigorous to create fistulae. A longer course of vacuum therapy in a trial setting is indicated to further investigate this potential complication prior to human clinical study.
One limitation of an animal model is the artificial nature of the clinical problem, in our study, the anastomotic defect. The surgically created anastomotic leak in this pig model was mechanically created without initial tension or infection. The definition and diagnosis of anastomotic leaks vary widely among colorectal surgeons and the clinical entity is a spectrum ranging from a simple, small pericolonic abscess to frank feculent peritonitis with severe enteric contrast extravasation.17, 18 While the etiology may differ, we believe our pig anastomotic defect was on average larger in size than a human leak, more grossly infected due to the large orifice, and required frank contrast extravasation during fluoroscopy to continue in the study. The defect was also created using coagulation diathermy causing a penumbra of ischemia, visualized on histopathology. All factors indicate a model with a clinically equivalent, if not worse, anastomotic “leak.”
Finally, there are also additional potential areas of use. Various other clinical scenarios where small, contained perforations may benefit from transanal, endoluminal negative pressure therapy. Diverticular disease is abundant with many internists, gastroenterologists, and surgeons managing small perforations and abscesses. Negative pressure therapy has been previously described in diverticular abscesses, however, their technique inserts the sponge into the abscess cavity itself. The current device on the market is the Endo‐SPONGE (B. Braun Medical, Melsungen, Germany), which is available and in use in Europe. This has been shown to be successful in closing abscess cavities from anastomotic leakage without the need for reoperation and has been utilized in presacral and paraanastomotic spaces.9, 10, 11, 12, 13, 14 Unfortunately, their current technique requires continued device exchange every 3 to 4 days and no management of proximal stool. We propose our technique of endoluminal sponge placement, as we described in this study, would allow closure of the anastomotic defect without proximal diversion.
Our device does require some continued modification as a single rectal specimen on gross pathology identified a small, 5‐mm deep ulceration in the mucosa, proximal to the location of vacuum sponge with the potential for perforation. We are confident that this small mucosal defect was due to the proximal end of the sump tube resting against the mucosa in a fixed location. As previously described, the end of numerous enteric tubes including nasogastric, nasoduodenal, and jejunal extension tubes may cause profound pressure necrosis against bowel mucosa and may result in ulceration and ultimately perforation.19, 20 As this technology is still in the development phase, we intend to alter the type and arrangement of the proximal aspect of the device allowing sump capabilities. A softer tip, pigtail, or protection piece may need to be added to prevent this potential complication. Of all other specimens, the remainder of the mucosa was without insult.
Conclusion
Endoluminal vacuum therapy is feasible and well tolerated in a pig model. It has been shown to prevent anastomotic defects, but also to seal a significant number of freely leaking anastomoses in the early period (86%). This technology warrants further study as it may provide a noninvasive means to treatment of anastomotic leaks without the need for proximal diversion, preventing the additional morbidity and mortality associated with a secondary operation.
Source of Funding
Dr. Rosenberger was funded under NIH 5–T32–AI–078875–02, PI: RG. Sawyer.
Conflict of Interest
Drs. Rosenberger and Shada received financial salary and academic travel support from senior author, DEK. Drs. Ritter, Mauro, Mentrikoski, and Feldman have no financial disclosures or conflict of interest related to this study. Dr. Kleiner is the inventor of the application of this technology and may, in the future, have some potential for financial benefit. Currently, there is no sponsoring company or any financial agreements.
References
- 1. Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006; 49: 1719–1125. [DOI] [PubMed] [Google Scholar]
- 2. Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999; 189: 554–559. [DOI] [PubMed] [Google Scholar]
- 3. Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004; 240: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hedrick TL, Sawyer RG, Foley EF, Friel CM. Anastomotic leak and the loop ileostomy: friend or foe? Dis Colon Rectum. 2006; 49: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 5. Gastinger I, Marusch F, Steinert R, Wolff S, Koeckerling F, Lippert H. Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg. 2005; 92: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 6. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997; 38: 553–562. [DOI] [PubMed] [Google Scholar]
- 7. Urschel JD, Scott PG, Williams HT. The effect of mechanical stress on soft and hard tissue repair; a review. Br J Plast Surg. 1988; 41: 182–186. [DOI] [PubMed] [Google Scholar]
- 8. Norbury K, Kieswetter K. Vacuum‐assisted closure therapy attenuates the inflammatory response in a porcine acute wound healing model. Wounds. 2007; 19:97. [PubMed] [Google Scholar]
- 9. Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum‐assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008; 22: 1818–1825. [DOI] [PubMed] [Google Scholar]
- 10. Mees ST, Palmes D, Mennigen R, Senninger N, Haier J, Bruewer M. Endo‐vacuum assisted closure treatment for rectal anastomotic insufficiency. Dis Colon Rectum. 2008; 51: 404–410. [DOI] [PubMed] [Google Scholar]
- 11. Bemelman WA. Vacuum assisted closure in coloproctology. Tech Coloproctol. 2009; 13: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durai R, Ng PC. Perirectal abscess following procedure for prolapsed haemorrhoids successfully managed with a combination of VAC sponge and Redivac systems. Tech Coloproctol. 2009; 13: 307–309. [DOI] [PubMed] [Google Scholar]
- 13. Van Koperen PJ, Van Berge Henegouwen MI, Slors JF, Bemelman WA. Endo‐sponge treatment of anastomotic leakage after ileo‐anal pouch anastomosis: report of two cases. Colorectal Dis. 2008; 10: 943–944. [DOI] [PubMed] [Google Scholar]
- 14. Arezzo A, Miegge A, Garbarini A, Morino M. Endoluminal vacuum therapy for anastomotic leaks after rectal surgery. Tech Coloproctol. 2010; 14: 279–281. [DOI] [PubMed] [Google Scholar]
- 15. Shada AL, Rosenberger LH, Mentrikoski MJ, Silva MA, Feldman SH, Kleiner DE. Endoluminal negative pressure therapy for prevention of rectal anastomotic leaks: a pilot study in a pig model. Surg Infect. 2013; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon S, Morris A, Billingham R, Fankhouse J, Horvath K, Johnson M, McNevin S, Simons A, Symons R, Steele S, et al. Surgical Care and Outcomes Assessment Program (SCOAP) Collaborative. Routine leak testing in colorectal surgery in the Surgical Care and Outcomes Assessment Program. Arch Surg. 2012; 147: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams K, Papagrigoriadis S. Little consensus in either definition or diagnosis of a lower gastro‐intestinal anastomotic leak amongst colorectal surgeons. Int J Colorectal Dis. 2013; 28: 967–971. [DOI] [PubMed] [Google Scholar]
- 18. Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013; 148: 177–182. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberger LH, Newhook T, Mauro DM, Hennessy SA, Sawyer RG. Jejunal tube extensions via percutaneous endoscopic gastrostomy and delayed small‐bowel perforations: a case series. Gastrointestin Endosc. 2012; 75: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenberger LH, Mauro DM, Sawyer RG. Profound pressure necrosis: the culprit in delayed small‐bowel perforations from jejunal tube extensions. Gastrointest Endosc. 2012; 76: 697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]


