Abstract
Kainate receptors (KARs) play important roles in the modulation of neurotransmission and plasticity, but the mechanisms that regulate their surface expression and endocytic sorting remain largely unknown. Here, we show that in cultured hippocampal neurons the surface expression of GluR6-containing KARs is dynamically regulated. Furthermore, internalized KARs are sorted into recycling or degradative pathways depending on the endocytotic stimulus. Kainate activation causes a Ca2+- and PKA-independent but PKC-dependent internalization of KARs that are targeted to lysosomes for degradation. In contrast, NMDAR activation evokes a Ca2+-, PKA- and PKC-dependent endocytosis of KARs to early endosomes with subsequent reinsertion back into the plasma membrane. These results demonstrate that GluR6-containing KARs are subject to activity-dependent endocytic sorting, a process that provides a mechanism for both rapid and chronic changes in the number of functional receptors.
Keywords: endocytosis, kainate receptors, receptor recycling, surface expression
Introduction
Kainate receptors (KARs) are highly expressed in many brain regions. At presynaptic terminals, they can modulate both excitatory and inhibitory neurotransmitter release (Chittajallu et al, 1999; Lerma et al, 2001). Postsynaptically, they mediate a slow component of synaptic transmission and are involved in plasticity (Lerma, 2003). Overall, low to moderate activation of KARs enhances whereas strong KAR activation reduces synaptic transmission (Schmitz et al, 2001). There are five KAR subunits: GluR5, GluR6 and GluR7 that can form functional homomeric receptors and KA1 and KA2 that can only form part of a functional receptor when coassembled with at least one of the GluR5–GluR7 subunits (Chittajallu et al, 1999; Lerma et al, 2001). Thus KARs, probably with different subunit compositions, can be specifically targeted to pre-, post- and extrasynaptic locations within the same cell. Functional KARs at thalamocortical synapses are subject to acute activity-dependent and longer term developmental regulation. During the critical period of experience-dependent plasticity at these synapses, the contribution of KARs is decreased. Similarly, long-term potentiation (LTP) at these synapses involves a rapid switch from kainate to AMPA receptor-mediated synaptic transmission, suggesting that KARs are regulated in response to synaptic plasticity during development (Kidd and Isaac, 1999). These results suggest that KARs and AMPARs are subject to independent and differential trafficking events.
In cultured hippocampal neurons, the locations and roles of KARs are less clearly defined. Despite punctate, synaptic-like staining with anti-KAR antibodies and the demonstration of functional KARs using application of agonists, no synaptic role for these receptors has been established since all fast excitatory transmission can be blocked by AMPAR-selective antagonists (Lerma et al, 1997). One possible explanation for these observations is that hippocampal neurons in culture have similar properties to CA1 pyramidal cells that do not express postsynaptic KARs. Recently, however, evidence for CA1 postsynaptic GluR6-containing KARs metabotropic receptors has been reported (Melyan et al, 2004).
By comparison with AMPAR trafficking and the processes controlling their surface and synaptic expression, little is known about KAR trafficking. However, an ER retention signal around Arg896 in the GluR5-2b subunit has been identified (Ren et al, 2003b) and a forward trafficking motif that promotes surface expression of homomeric and heteromeric KARs has been identified in a splice isoform of GluR6 (Jaskolski et al, 2004; Yan et al, 2004). The KA2 subunit, which likely forms heteromers with GluR6 in hippocampal neurons (Darstein et al, 2003), is retained in the ER by specific motifs until sterically hindered by assembly with other subunits and also contains a di-leucine endocytotic motif in the C-terminal of KA1 and KA2 that is highly selective for clathrin internalization pathways (Ren et al, 2003a).
GluR6 mRNA, together with KA1 and KA2 mRNAs, is highly expressed in most cultured embryonic hippocampal neurons that display characteristic native kainate responses (Ruano et al, 1995). Furthermore, the channel properties of receptors in cultured hippocampal neurons are similar to homomeric GluR6 receptors expressed in clonal cell lines (Lerma et al, 2001). In the hippocampal mossy fibre pathway, KARs mediate slow frequency-dependent postsynaptic responses (Castillo et al, 1997; Vignes and Collingridge, 1997), and subsequent studies with knockout mice have supported that GluR6 is required for these responses (Mulle et al, 1998). Presynaptic GluR6 has been implicated in the kainate-induced inhibition of GABA release on CA1 pyramidal cells (Mulle et al, 2000), and a role for GluR6 in the facilitation of GABA release at inhibitory synapses on CA1 interneurons has also been proposed (Mulle et al, 2000; Cossart et al, 2001).
Here we have investigated the surface expression and fate of GluR6 in cultured hippocampal neurons. We show that GluR6-containing KARs are rapidly endocytosed and differentially sorted to either a recycling or degradative pathway depending on the endocytotic stimulus. Kainate activation causes a Ca2+- and PKA-independent but PKC-dependent internalization of KARs that are targeted to lysosomes for degradation. In contrast, NMDAR activation evokes a Ca2+-, PKA- and PKC-dependent endocytosis of KARs to early endosomes with subsequent reinsertion back into the plasma membrane. Our results demonstrate a previously unknown mechanism by which surface-expressed KARs can be regulated.
Results
Basal and activity-dependent GluR6 endocytosis
We first compared the time course of internalization of GluR6-containing KARs and GluR1-containing AMPARs. In these experiments, all surface proteins were biotinylated for 10 min at 4°C, washed extensively and returned to 37°C for various times to allow endocytosis. Biotinylated proteins remaining on the surface after incubation were debiotinylated by incubating the cells in glutathione (GSH) buffer at 4°C leaving only the endocytosed proteins biotinylated. The amount of biotinylated GluR6 was determined by binding cell lysate to streptavidin–agarose beads and Western blotting with anti-GluR6/7 antibody. Since GluR7 is present only in interneurons at relatively low levels in the hippocampus (Isaac et al, 2004) and our cultures comprise mainly of principal neurons, the anti-GluR6/7 antibody is an effective marker for GluR6 in our experiments. We quantified internalized (biotinylated) receptors under conditions of reduced (TTX, 2 μM), basal and activated (bicuculline, 10 μM) synaptic activity (Figure 1A and B). No endocytosis occurred in cells left at 4°C (data not shown). Under basal control conditions, GluR6-containing KARs were subject to time-dependent internalization that reached a plateau (17.3±4.9% at 15 min) that fitted a single exponential with a time constant (τ) of 2.9±0.9 min. Blocking synaptic activity with TTX markedly reduced the extent (11.4±1.2% after 30 min) and rate of endocytosis (τ=7.5±0.7 min). In contrast, increasing synaptic activity by blocking inhibitory GABAA receptors with bicuculline increased the amount (28.5±3.4% after 30 min) and rate of internalization (τ=2.5±0.7 min).
Figure 1.
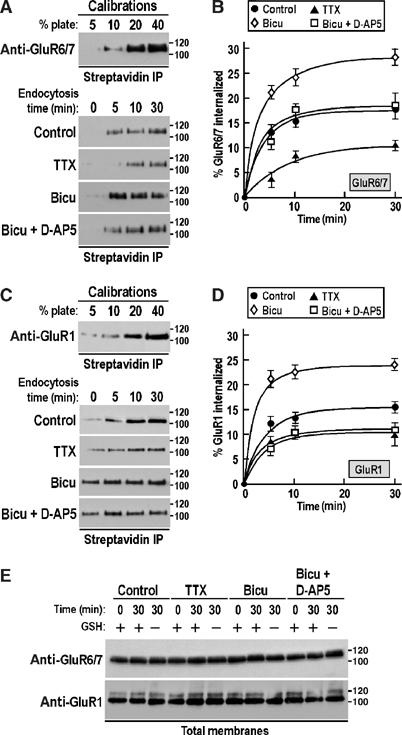
Basal and activity-dependent internalization of glutamate receptors in hippocampal neurons. (A, C) Upper panels: Calibration standards of total surface GluR6/7 (A) and GluR1 (C) corresponding to 5–40% of the cells used for experiment to determine the degree of endocytosis. Lower panels: Immunoblots of biotinylated GluR6/7 (A) and GluR1 (C) showing the time course of basal endocytosis and its modulation by enhancing or decreasing synaptic activity. The Na+ channel blocker TTX (2 μM) reduces synaptic activity, whereas the GABAA receptor antagonist bicuculline (10 μM) increases basal activity. The NMDA receptor antagonist D-AP5 (50 μM) was also tested in combination with bicuculline to determine the effect of NMDA receptors on kainate and AMPA receptor endocytosis. Molecular weights in kDa are shown. The blots are representative of at least three separate experiments. (B, D) Time course of GluR6/7 (B) and GluR1 (D) internalization in hippocampal neurons quantified by surface biotinylation assays from (A) and (C), respectively. Each time point represents mean±s.e.m. of at least three independent experiments. (E) Immunoblots of total proteins from control-, TTX-, bicuculline- and bicuculline+D-AP5-treated hippocampal neurons showing that the drugs have no effect on overall GluR1 and GluR6/7 protein expression. The blots are representative of three separate experiments.
The endocytosis rates for GluR1 (Figure 1C and D) were also rapid under all of the conditions investigated with values consistent with those obtained in a previous report of GluR1 internalization in cortical cultured neurons (Ehlers, 2000). As for GluR6, bicuculline resulted in the highest levels of internalization for AMPARs, but a striking difference between GluR6 and GluR1 was the observation that bicuculline plus D-AP5 caused AMPAR endocytosis to drop to levels similar to TTX, whereas for KARs the percentage of internalization was identical to control levels but significantly higher than in TTX (Figure 1A and B). There was no change in the total amount (surface plus intracellular) of either protein under any of the conditions (Figure 1E). These results suggest that NMDAR activation accounts for nearly all GluR1 internalization, but for GluR6 there is an NMDAR-independent component that is presumably due to direct activation of KARs by glutamate.
Recycling of endocytosed GluR6 back into the plasma membrane
We next measured the proportion of total GluR6 present at the plasma membrane (Figure 2A). Under all the conditions tested, the steady-state levels of surface-expressed GluR6 remained unchanged at ∼27% for GluR6. Since this value is lower than those reported for AMPARs (Ehlers, 2000), we also examined the cell surface expression of GluR1 (Figure 2B). The overall GluR1 surface expression was not changed by modulators of synaptic activity with ∼53% of GluR1 expressed at the plasma membrane in all conditions examined (Figure 2B). These results suggest that the changes in GluR6 and GluR1 endocytosis were counterbalanced by changes in exocytosis.
Figure 2.
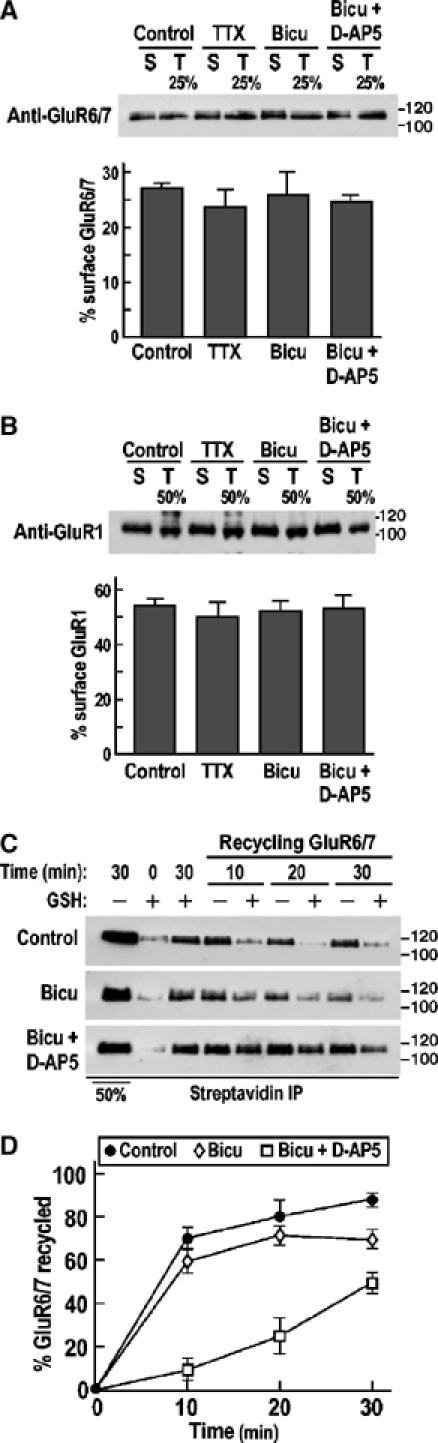
Basal and activity-dependent recycling of KARs in hippocampal neurons. (A, B) Immunoblots showing the effects of TTX, bicuculline and bicuculline+D-AP5 on GluR6/7 (A) and GluR1 (B) surface expression (see Materials and methods for details). Neurons were pretreated with the drugs indicated for 1 h in neurobasal medium at 37°C and then the biotinylated receptors (surface, S) were precipitated using streptavidin beads immediately after the biotinylation step. The surface expressions of GluR6/7 and GluR1 were compared to 25 or 50% plate fractions (T) for kainate and AMPA receptor, respectively. Molecular weights in kDa are shown. Histograms show mean±s.e.m. (n=3). (C) Modulation of GluR6/7 recycling by synaptic activity. Two rounds of debiotinylation were used to assess the recycling of internalized receptors to the cell surface (see Materials and methods for details). (D) Time course of GluR6/7 recycling in hippocampal neurons quantified by recycling biotinylation assays from (C). Each time point represents mean±s.e.m. of at least three independent experiments.
To determine if GluR6 internalized in response to changes in synaptic activity is reinserted back into the plasma membrane or whether new GluR6 is recruited, we investigated receptor recycling using biotinylation assays (Figure 2C and D). Neurons in which surface biotinylated GluR6 had been removed by incubating the cells in GSH buffer at 4°C were returned to 37°C for various times to allow reinsertion of biotinylated GluR6 back into the plasma membrane. Cells were then subjected to a second GSH treatment at 4°C to debiotinylate recycled GluR6 and compared to controls that did not undergo this second debiotinylation step. The difference in GluR6 retained by streptavidin precipitation between cells given the second GSH wash and those washed in control buffer indicates the amount of recycled GluR6.
Under basal conditions in the absence of TTX, there was a rapid and extensive recycling of GluR6 with 87±2% of the constitutively internalized receptor reinserted into the plasma membrane in 30 min (τ=4.6±1.2 min). Unexpectedly, the rate and extent of reinsertion were slightly but significantly (P<0.01) less in the presence of bicuculline with 68.8±2.8% of GluR6 recycled back into the plasma membrane (τ=5.6±0.8 min). When neurons were incubated with bicuculline plus the NMDAR antagonist D-AP5, a dramatically reduced GluR6 reinsertion was observed with only 52.4±4.4% of the receptors recycled after 30 min, suggesting a role of NMDAR in this process (Figure 2D). Thus, under basal synaptic activity, and especially under conditions where NMDARs are blocked, a proportion of the internalized receptors exit the recycling pathway. Therefore, given that ∼87% of internalized GluR6 are reinserted, the remaining receptors must be ‘new' to the plasma membrane. These data indicate that synaptic NMDAR activation is important for GluR6 surface expression as well as internalization and that processes exist to balance the total amount of surface-expressed receptors both in terms of endocytosis versus exocytosis and recycling versus recruitment of ‘new' GluR6.
Kainate-induced internalization of GluR6
The fact that the NMDAR antagonist D-AP5 did not reduce levels of GluR6 internalization to the same levels as TTX (Figure 1) indicates that NMDAR activation does not account for all of the synaptically evoked endocytosis. To analyse the effects of direct kainate- and NMDAR-evoked endocytosis, we bath applied drugs to the cultures. In these experiments, all surface-expressed receptors were modulated. Kainate-evoked internalization of GluR6 in hippocampal neurons was visualized by antibody feeding experiments where surface-expressed KARs were labelled with an N-terminal directed anti-GluR6 antibody (Fleck et al, 2003). After washing off excess antibody, the cells were incubated for 30 min at 37°C and then partially fixed. Antibodies remaining on the surface were labelled with a Cy3 (red) secondary antibody. Following further fixation and permeabilization with Triton X-100, the internalized anti-GluR6 antibodies were labelled with Cy2 (green) secondary antibody. Surface GluR6 showed a punctate distribution on the soma and processes of cultured hippocampal neurons (18–21 DIV; Figure 3). Consistent with the biotinylation data, in the presence of TTX little internalization was observed under nonstimulated conditions (Figure 3A). There was, however, an ∼6-fold increase in internalized receptor, seen as diffuse intracellular staining, following a 30 min application of kainate (10 μM) with loss of surface GluR6 immunofluorescence (Figure 3E; note that receptors exocytosed during the 30 min incubation are not detected with this technique). The kainate-evoked internalization was blocked in the presence of high concentrations (20 μM) of the AMPAR/KAR antagonist NBQX (Figure 3G), but was unaffected by low NBQX (2 μM, which blocks only AMPARs; Figure 3H) or the NMDAR antagonist D-AP5 (Figure 3F). Quantitative data are shown in Figure 3I, and Figure 3J–L shows the distribution of surface-associated GluR6 superimposed on a transmission image of a neuron.
Figure 3.
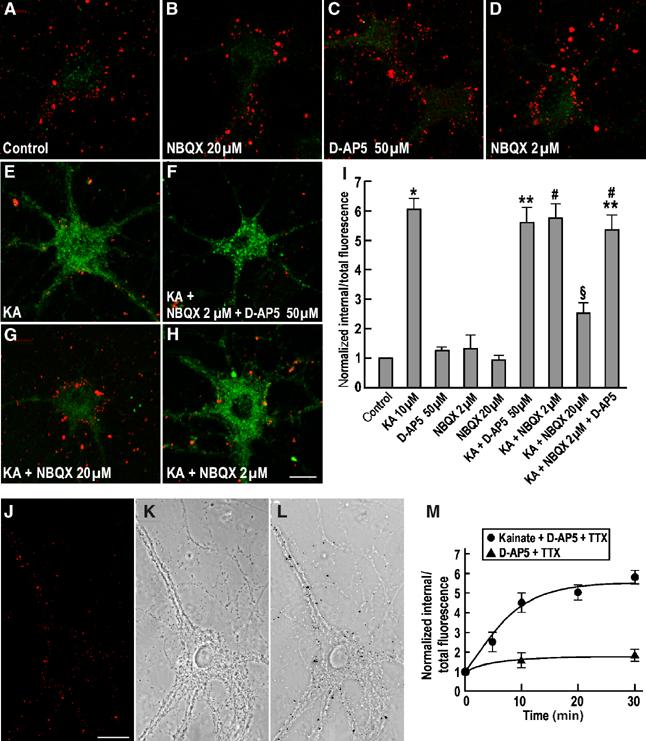
Pharmacological modulation of KAR endocytosis in cultured hippocampal neurons. Effect of drugs either alone or in combination with kainate (10 μM) on the endocytosis of GluR6 assessed by immunofluorescence assays (see Materials and methods). Internalized receptors after 30 min at 37°C are shown in green and the remaining prelabelled surface receptors are in red. (A) Constitutive GluR6 internalization at 30 min in TTX-treated conditions (control). (B) NBQX (20 μM), an AMPA and KAR competitive antagonist. (C) D-AP5 (50 μM), an NMDA receptor antagonist. (D) NBQX (2 μM), AMPAR-selective antagonist at this concentration. (E) Internalization of GluR6 induced by kainate (10 μM) at 30 min. (F) Kainate (10 μM)+NBQX (2 μM)+D-AP5 (50 μM). (G) Kainate (10 μM)+NBQX (20 μM). (H) Kainate (10 μM)+NBQX (2 μM). (I) Quantification of GluR6 endocytosis, measured as a ratio between internalized (green) and total labelled GluR6 (red and green), normalized to the 30 min control. The data are from at least three separate experiments and show mean±s.e.m. *P<0.001 compared with control; **P<0.001 compared with control, P<0.001 compared with D-AP5; ♯P<0.001 compared with control, P<0.001 compared with NBQX (2 μM). §P<0.001 compared with control, P<0.001 compared with kainate, P<0.001 compared with NBQX (20 μM), P<0.001 compared with NBQX (2 μM). (J–L) Surface GluR6 immunostaining as above showing the punctate distribution of GluR6 along an hippocampal neuron (J, in red) and the respective transmission image (K) showing that the labelling is associated with the membrane of the cell body and the dendritic arborization. (M) Time course of constitutive and kainate-induced GluR6 internalization in TTX- and D-AP5 treated hippocampal neuronal cultures measured by quantitative immunofluorescence assay. The data are the mean±s.e.m of three separate experiments. Scale bar, 20 μm.
We determined the time course of GluR6-containing KAR endocytosis by quantitative immunofluorescence assays in TTX- and D-AP5-treated neurons under basal or stimulated conditions (Figure 3M). In the presence of kainate (10 μM) plus D-AP5 (50 μM), GluR6 endocytosis reached a plateau by 15–20 min and the rate of internalization was rapid (τ=6.1±0.5 min). Under basal conditions, GluR6 endocytosis occurred to a much lesser extent reaching a plateau after 10 min. Kainate-evoked GluR6 endocytosis did not occur at 4°C and was blocked by expressing the dominant-negative (dynamin-K44E) form of dynamin (data not shown). These data suggest that in the absence of action potentials and NMDAR activation GluR6 is stable at the cell surface over 30 min and that direct stimulation of KARs evokes a rapid and substantial internalization.
Immunocytochemistry provides valuable information about localization, but can be difficult to quantify accurately. We therefore performed corresponding GluR6 internalization assays using surface biotinylation (Figure 4A and B; Supplementary Figure 1). We found that under kainate stimulation a plateau (29.3±0.9%) was reached by 10 min with a rate (τ=2.0±0.5 min) that was slightly more rapid than the time constant obtained using immunofluorescence assays (τ=6.1±0.5 min). In the absence of agonist and the presence of TTX and D-AP5, there was a modest endocytosis with ∼20% of surface receptors endocytosed after 30 min. These results using the more sensitive biotinylation assay indicate that there is a small component of spontaneous GluR6 internalization.
Figure 4.
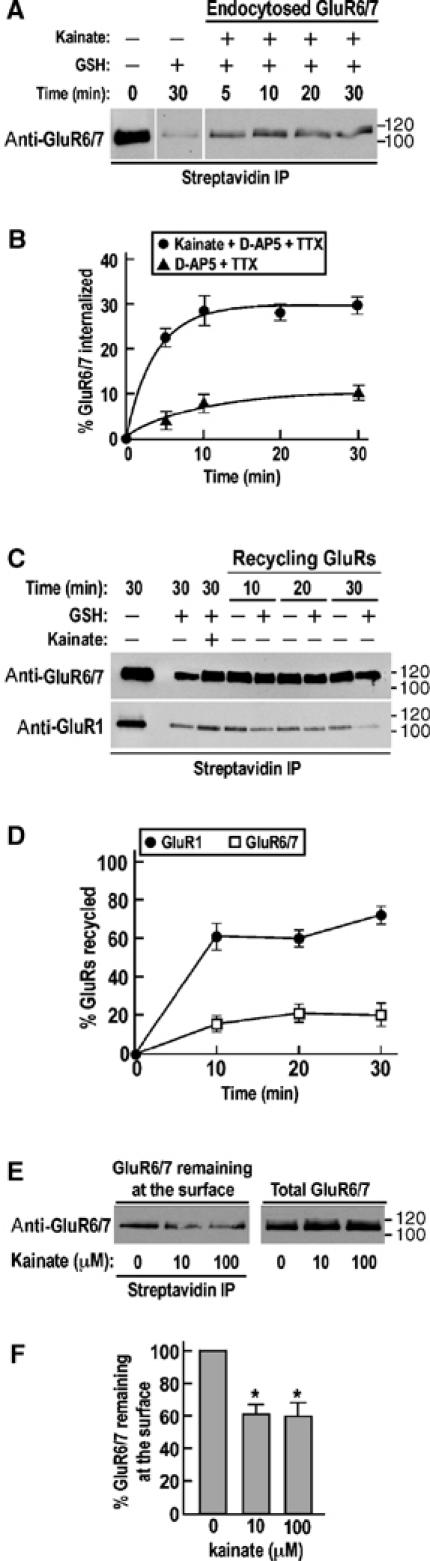
Recycling of AMPA and KARs after kainate-induced internalization in cultured hippocampal neurons. (A) Representative immunoblot of the time course of GluR6/7 endocytosis upon kainate stimulation in TTX- and D-AP5-treated hippocampal neurons using biotinylation assay (see Materials and methods for details). (B) Time course of kainate-induced GluR6/7 internalization measured by biotinylation assay from (A). Each time point represents the mean±s.e.m. of three different experiments. (C) Representative immunoblots of GluR6/7 (upper blot) and GluR1 (lower blot) recycling after kainate-induced internalization in neuronal culture pretreated with TTX and D-AP5. (D) Densitometric analysis of GluR1 and GluR6/7 recycling after kainate-induced internalization in TTX- and D-APV-treated hippocampal neurons measured by biotinylation assay from (C). Values represent the mean±s.e.m. of three separate experiments. (E) Representative Western blot showing the remaining surface-associated GluR6/7 after kainate stimulation. TTX- and D-AP5-pretreated neurons were incubated with either 10 or 100 μM kainate for 30 min and then surface biotinylated on ice. After streptavidin precipitation, samples were separated by SDS–PAGE and immunoblotted with GluR6/7 antibody and compared to the total amount of KARs. (F) Quantification of the remaining surface-associated GluR6/7 after a 30 min stimulation with different concentrations of kainate from (E). Histograms show mean±s.e.m. of three independent experiments. *P<0.001 compared to control in the absence of kainate.
Kainate is an agonist at both AMPARs and KARs (Lerma et al, 2001), and 10 μM kainate in the presence of D-AP5 (50 μM) for 30 min also evoked endocytosis of GluR1-containing AMPARs. GluR1 displays high levels of membrane recycling with 73.0±2.1% of receptors reinserted at the cell surface after 30 min. In contrast, only 20.7±6.4% of internalized GluR6 was recycled back into the plasma membrane following kainate-evoked internalization (Figure 4C and D). The finding that kainate stimulation caused a much higher degree of recycling of AMPARs compared to KARs was unexpected since it has been reported previously that only ∼50% of AMPARs return to the surface after 75 min following AMPA application (Ehlers, 2000). It should be noted, however, that in that study AMPA evoked 80–90% AMPAR internalization, whereas here kainate caused internalization of only about 20% of surface GluR1. Differences in the cultures, that is, hippocampal versus cortical, and/or experimental protocols may account for this difference, but another possibility is that there may be differences in agonist-induced trafficking such that kainate and AMPA stimulation of AMPARs leads to their entry into different sorting pathways.
In the 30 min time frame of these experiments, 10 and 100 μM kainate application reduced the amount of GluR6 at the cell surface to 61.5±7.5 and 60.7±8.4% of control (P<0.001), but did not change the total amount of GluR6 present in the neurons (Figure 4E and F). These results show that exposure to kainate effectively downregulates surface GluR6 and thus indicate that, unlike modulation of synaptic activity, the internalization is not compensated for by increased exocytosis.
NMDAR activation leads to GluR6 endocytosis
No role for NMDARs in KAR trafficking has been reported previously. We therefore further investigated the effect of NMDA (30 μM) in TTX-treated culture on the internalization–recycling of GluR6 using biotinylation assays (Figure 5). NMDA evoked an even greater endocytosis of GluR6 (43.7±1.7%; Figure 5A and C) than kainate (29.3±0.9%; Figure 4) with a time constant of 4.5±1.0 min. Importantly, almost no internalization of NR1 subunits was detected under these conditions (∼4%; Figure 5B and C). Most interestingly, however, and consistent with the modulation of synaptic activity data, a large percentage (78.2±4.3%) of the GluR6 endocytosed via the NMDAR-activated pathway was rapidly recycled back into the plasma membrane (τ=4.0±1.6 min; Figure 5A and D), whereas GluR6 internalized in response to kainate was not reinserted at the plasma membrane. These results demonstrate that internalized GluR6 can enter different recycling or retention pathways depending on the mode of stimulation.
Figure 5.
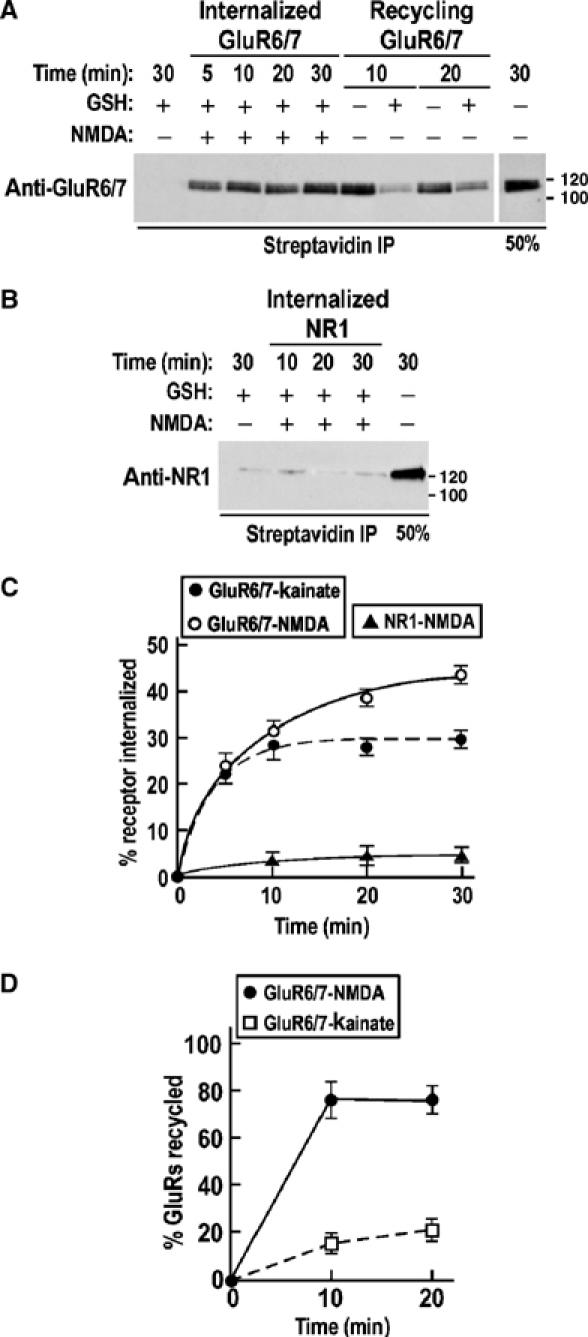
NMDAR-evoked internalization and recycling of GluR6/7. (A) Representative immunoblot of the time course of 30 μM NMDA-induced endocytosis and recycling of surface biotinylated GluR6/7. (B) Representative immunoblot of surface biotinylated NR1 internalization in response to NMDA (30 μM). (C) Time course of NMDA-induced GluR6/7 and NR1 internalization (from A and B) and compared to kainate-induced GluR6/7 endocytosis (dotted line; data replotted from Figure 4B) as in Figure 4. Each time point represents the mean±s.e.m. of three different experiments. (D) Time course of GluR6/7 recycling after NMDA-induced KAR internalization from (A). Data show mean±s.e.m. of three independent experiments. The dotted line represents the time course of GluR6/7 recycling after kainate-induced endocytosis as in Figure 4.
Differential sorting of endocytosed KARs
Similar to previous reports for AMPARs (Kameyama et al, 1998; Lee et al, 1998; 2002; 2004; Beattie et al, 2000; Ehlers, 2000; Colledge et al, 2003), NMDAR-mediated internalization and recycling of KARs indicates different sorting pathways for endocytosed receptors. We performed antibody-feeding experiments to identify the endocytic compartmentalization of GluR6 in response to NMDA or kainate (Figure 6A–E). Surface GluR6 was prelabelled with an N-terminal antibody and the neurons were exposed to NMDA (30 μM) or kainate (10 μM) for 3 min. Antibody remaining at the surface was removed by acid wash prior to fixation, permeabilization and probing with fluorophore-conjugated secondary antibody. This acid treatment effectively removed all surface labelling since no signal was observed in nonpermeabilized cells (Figure 6E). At 3 min after kainate- or NMDA-evoked internalization, GluR6-containing KARs initially redistributed to vesicular structures in dendrites that showed extensive colocalization with the early endosome marker EEA1 (Figure 6A and B; degree of colocalization=65.1±4.0 and 69.2±3.9% respectively). At 30 min, however, there was far less colocalization with EEA1 (19.2±8.3 and 27.3±3.1%, respectively). These data show that, irrespective of the stimulus, internalized receptors initially traffic to early endosomes that act as a transit point to downstream processing pathways.
Figure 6.
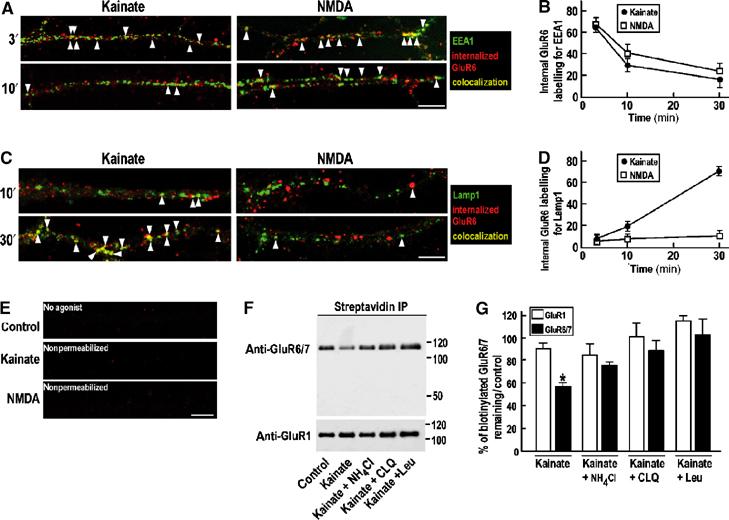
Differential sorting of internalized KARs following kainate or NMDA stimulation. (A, C) Confocal images showing colocalization (yellow) of kainate- and NMDA-induced internalized GluR6 (red) and the early endosomal marker EEA1 (A) or the lysosomal marker Lamp1 (C) (green) at different time points in TTX-treated hippocampal neurons. Scale bar, 10 μm. (B, D) Quantification of the percentage of internalized GluR6 that colocalizes with EEA1 (B) and with Lamp1 (D) after 3, 10 and 30 min of stimulation with kainate (10 μM kainate plus 50 μM D-AP5) or NMDA (30 μM). Data show mean±s.e.m. of three independent experiments. (E) Control experiments showing that almost no KARs are internalized after 30 min in the absence of agonist (upper panel) and that acid washing is effective in removing surface antibody labelling (centre and lower panels). (F) Representative immunoblots of kainate-induced GluR6/7 degradation in hippocampal neurons. TTX-pretreated cells were biotinylated on ice as described in Materials and methods and then incubated for 6 h with control (TTX, 2 μM; D-AP5, 50 μM) or kainate-containing media (TTX, 2 μM; D-AP5, 50 μM; kainate, 10 μM) plus or minus the lysosomal degradation inhibitor leupeptin (100 μM) or the compartment acidification blockers NH4Cl (50 mM) or chloroquine (200 μM). Note that almost no GluR1 is degraded after 6 h incubation following kainate stimulation (lower panel). (G) Quantification of KAR GluR6/7 degradation in response to kainate. The remaining biotinylated receptors are detected after streptavidin precipitation and immunoblotting using anti-GluR6/7 or anti-GluR1 antibodies. Histogram shows mean±s.e.m. (n=3). *P<0.01 compared to control.
Since the fate of GluR6-containing receptors internalized in response to KAR or NMDAR activation differs, they are likely to segregate to different compartments (e.g. lysosomes) downstream of early endosomes. We therefore determined the extent of colocalization of internalized GluR6 with the late endosome/lysosome marker Lamp1 (Figure 6C and D). At 3 min after agonist application, essentially no GluR6 localized to Lamp1-positive structures (not shown). After 30 min however, GluR6 internalized in response to kainate displayed extensive colocalization with Lamp1 (colocalization=70.7±3.0%). In marked contrast, GluR6 internalized by activation of NMDARs showed very little colocalization with Lamp1 (colocalization=12.1±0.9%). These results indicate that GluR6 endocytosed in response to agonist application exits from early endosomes to late endosomes/lysosomes and that this degradative pathway is comparatively slow. GluR6 internalized in response to NMDAR activation exits the early endosomes and rapidly recycles to the plasma membrane.
We next tested if long-term exposure of the cultures to kainate would lead to a reduction in the amount of GluR6 by driving internalized receptors into the degradative pathway (Figure 6F and G). To do this, TTX-treated neurons were biotinylated on ice and then stimulated for 6 h with 10 μM kainate (plus 50 μM D-AP5 to block NMDARs) plus or minus lysosomal degradation inhibitors. Long-term kainate application decreased (58.7±2.6%, P<0.01) biotinylated GluR6 immunoreactivity. GluR6 degradation was blocked by the lysosomal protease inhibitor leupeptin (102.4±16.7%), chloroquine (87.5±15.4%) and partially by ammonium chloride (74.6±2.9%), drugs that prevent lysosomal acidification (Figure 6F and G). In the same cultures, levels of the AMPAR subunit GluR1 were relatively unchanged (90±4%). This indicates that the effects on surface GluR6 were not due to cell death and reflect the fact that GluR1 internalized in response to kainate recycles back into the plasma membrane (Figure 4). These results confirm that kainate stimulation causes degradation of internalized GluR6 by trafficking to lysosomes.
Roles of Ca2+, PKC and PKA on kainate- and NMDA-induced KARs endocytosis
Calcium mobilization and protein phosphorylation are important mediators of AMPAR trafficking (Barry and Ziff, 2002), so we hypothesized that they may regulate surface expression of KARs. We therefore tested the effects of Ca2+ and selective activators and inhibitors of PKA and PKC on GluR6 endocytosis (Figure 7).
Figure 7.
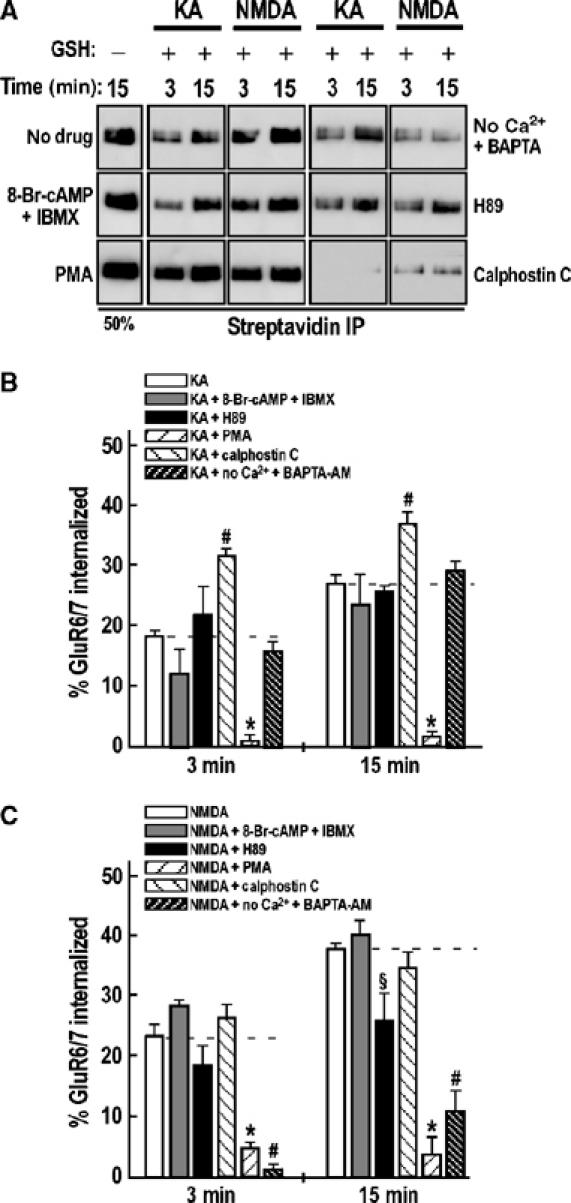
Effect of Ca2+, PKA and PKC on kainate- and NMDA-induced GluR6/7 endocytosis. (A) Representative immunoblots of kainate- and NMDA-induced GluR6/7 internalization in the presence or absence of PKA activators 8-Br-cAMP (100 μM) plus IBMX (100 μM), PKA inhibitor H89 (10 μM), PKC activator PMA (1 μM), PKC inhibitor calphostin C (1 μM) or the cell-permeable Ca2+ chelator BAPTA-AM (50 μM) in the absence of extracellular calcium. (B, C) Quantification of the effects of Ca2+ ions and PKA and PKC activators and inhibitors on GluR6/7 endocytosis following kainate (B) or NMDA (C) stimulation. The data show mean±s.e.m. of at least three independent experiments. *P<0.01 compared with kainate stimulation at 3 min; P<0.001 compared with kainate stimulation at 15 min; P<0.001 compared with NMDA treatment at 3 and 15 min. ♯P<0.05 compared with kainate stimulation at 3 and 15 min; P<0.01 compared with NMDA stimulation at 3 min; P<0.001 compared with NMDA stimulation at 15 min. §P<0.05 compared with NMDA stimulation at 15 min.
The Ca2+ chelator BAPTA-AM had no significant effect on kainate-evoked GluR6 endocytosis at 15 min (Figure 7A and B; 29.2±1.4% compared to 27.7±1.2% for kainate in the presence of Ca2+) showing that kainate-induced internalization is calcium independent. However, BAPTA-AM did significantly reduce NMDAR-evoked GluR6 internalization at both 3 min (3.3±1.9 versus 22.6±3.1% for NMDA treatment, P<0.01 compared to NMDA at 3 min) and 15 min (6.2±3.4 versus 37.8±1.5% for NMDA treatment, P<0.001 compared to NMDA at 15 min). The fact that some internalization still occurred in the absence of Ca2+ suggests that NMDAR-dependent GluR6 internalization also has a small Ca2+-independent component.
Recombinant GluR6 subunits are directly phosphorylated by PKA at serine 684 and homomeric GluR6 currents are potentiated by intracellular perfusion of PKA (Raymond et al, 1993; Wang et al, 1993). Neither the PKA activators 8-Br-cAMP plus IBMX nor the inhibitor H89 affected kainate-evoked GluR6 endocytosis in cultured hippocampal neurons (Figure 7A and B). For NMDAR-activated GluR6 internalization, there was a significant inhibition (P<0.05) by H89 at 15 min (26.2±6.9% compared to 37.8±1.5% for NMDA treatment) but not at 3 min. Surprisingly, activation of PKA did not increase NMDAR-evoked GluR6 internalization. These results suggest that PKA phosphorylation of GluR6 does not play a role in kainate-evoked internalization but that it may be permissive for NMDAR-induced endocytosis.
The PKC activator PMA and the inhibitor calphostin C had significant effects on kainate-evoked GluR6 endocytosis (Figure 7A and B). PKC activation significantly increased kainate-induced internalization at both 3 min (31.5±1.1 versus 17.9±1.1% for kainate, P<0.05 compared to kainate) and 15 min (36.9±3.1 versus 27.7±1.2% for kainate, P<0.05 compared to kainate), whereas inhibition of PKC using calphostin C abolished nearly all endocytosis (P<0.001 for both time points compared to kainate). Similarly, NMDAR-evoked GluR6 internalization was significantly inhibited by calphostin C at both 3 min (P<0.001 compared to NMDA at 3 min) and 15 min (11.1±3.0 versus 37.8±1.5% for NMDA treatment, P<0.001 compared to NMDA at 15 min). Increasing PKC activity using PMA, however, did not have significant effects on NMDA-induced GluR6 endocytosis (Figure 7A and C). These data suggest that PKC phosphorylation of GluR6 (or of receptor-associated protein) is required for agonist-evoked internalization. Under basal conditions, phosphorylation is incomplete since the PKC activator PMA caused increased internalization in response to kainate.
Discussion
The regulation of KAR responses has been shown at thalamocortical and mossy fibre/CA3 synapses (Kidd and Isaac, 1999; Hirbec et al, 2003). Despite progress identifying interacting proteins (Garcia et al, 1998; Hirbec et al, 2003) and elucidating some mechanisms of forward trafficking (Jaskolski et al, 2004; Yan et al, 2004) and ER retention/retrieval of these receptors (Gallyas et al, 2003; Hayes et al, 2003; Ren et al, 2003a; 2003b), understanding of the trafficking and targeting of KARs lags far behind that for AMPAR and NMDARs. Here we show that the expression of GluR6-containing KARs at the plasma membrane is dynamically regulated. In our hippocampal culture system, there is a relatively high level of spontaneous network activity (Noel et al, 1999) and the Na+ channel blocker TTX significantly reduced the rate and extent of GluR6 internalization. In contrast, increasing network activity with the GABAA receptor antagonist bicuculline significantly increased GluR6 endocytosis.
There were differences between NMDAR-evoked AMPAR (GluR1) and KAR (GluR6) internalization. D-AP5 treatment in the presence of bicuculline reduced GluR1 endocytosis to levels comparable to those in TTX, but only reduced GluR6 internalization to control levels. In the presence of both D-AP5 and TTX, some surface GluR6 (∼10%) was still constitutively internalized. Since the overall proportion of surface-expressed GluR6 remained unchanged, there must be increased levels of membrane insertion of GluR6 to compensate for increased internalization. Therefore, neurons possess mechanisms to balance receptor internalization with surface expression, processes that require extensive signalling.
AMPA application causes a slow and incomplete return of internalized AMPARs to the surface (Ehlers, 2000), but we found that kainate stimulation caused a high degree of recycling of AMPARs compared to KARs. However, kainate evoked only ∼20% internalization of surface GluR1, whereas AMPA evoked 80–90% internalization (Ehlers, 2000). This suggests that, if kainate is directly activating AMPARs, there is a difference in the degree of internalization (interestingly, AMPAR responses rapidly desensitize to kainate; Patneau et al, 1993) and that endocytosed AMPARs enter into different sorting pathways. Bath application of kainate did not markedly alter GluR1 surface expression, but effectively downregulated the total levels of surface GluR6 by ∼40% indicating that, in these circumstances, the GluR6 internalization is not compensated for by increased exocytosis.
Internalized GluR6-containing receptors initially traffic to early endosomes and subsequently enter recycling or degradation pathways. NMDAR activation leads to a high proportion (∼80%) of endocytosed GluR6 rapidly recycling back into the plasma membrane. GluR6 internalized in response to kainate, on the other hand, traffics to lysosomal compartments. This sorting of internalized KARs reveals an important aspect of KAR trafficking and provides a mechanism for the rapid and longer term activity-dependent regulation of KARs in neurons. Specifically, reinsertion of KARs allows rapid recovery, whereas degradation of internalized receptors would be expected to more chronically decrease the KAR-mediated responsiveness of the cell.
Ca2+, PKA and PKC have been shown to be important for AMPAR trafficking (Malinow and Malenka, 2002; Esteban et al, 2003; Terashima et al, 2004). Furthermore, all known KAR subunits have consensus phosphorylation sites for PKA and PKC. Previous studies on the recombinant GluR6 expressed in heterologous cells have shown that GluR6 can be phosphorylated by PKA on serine 684 resulting in an increase in the amplitude of glutamate response (Raymond et al, 1993; Wang et al, 1993) due to an increased channel open probability (Traynelis and Wahl, 1997). We show that neither activation nor inhibition of PKA affected kainate-evoked GluR6 endocytosis, suggesting that changes in channel properties are not associated with changes in the surface residence time of the receptor. Inhibition of PKA, however, significantly reduced the amount of NMDA-induced GluR6 internalization at 15 min, consistent with this kinase playing a permissive role in GluR6 internalization evoked by NMDAR-gated Ca2+ cascades.
We recently reported that the PKC-binding PDZ protein PICK1 interacts with GluR52b, GluR52c and GluR6, that PKC can directly phosphorylate the C-termini of these subunits and that PICK1 interactions maintain KAR-mediated synaptic function at mossy fibre–CA3 synapses (Hirbec et al, 2003). Furthermore, GluR5-containing KAR-mediated actions can be modulated by group 1 metabotropic glutamate receptors in a PKC-dependent manner (Cho et al, 2003). Here we show that PKC inhibition with calphostin C abolishes nearly all kainate- and NMDA-evoked GluR6 endocytosis, suggesting that PKC regulates GluR6 internalization. Increasing PKC activity with PMA, however, had differential effects. PMA increased kainate-induced GluR6 internalization, but had no effects on NMDA-induced GluR6 endocytosis. This could indicate a subpopulation of surface GluR6 that are amenable to kainate- (but not NMDA-) evoked internalization are prevented from undergoing endocytosis until they are phosphorylated by PKC. In this model, PKC phosphorylation would allow but not initiate GluR6 internalization. Thus, the roles of PKC in the surface expression of GluR52b- and GluR6-containing KARs and/or of GluR6 at different plasma membrane locations may be different, suggesting that PKC phosphorylation could act as a mechanism for regulating the subunit composition of surface-expressed KARs. It should be noted that a possible complication is that phorbol esters may activate other target proteins (Brose and Rosenmund, 2002). Nonetheless, a functional role for PKC in GluR6 metabotropic signalling has been reported. Activation of GluR6-containing KARs causes long-lasting inhibition of a postspike potassium current-mediated slow afterhyperpolarization (sAHP) in CA1 pyramidal cells via a pertussis toxin-sensitive and PKC-dependent mechanism (Melyan et al, 2002). These GluR6-containing KARs are activated by synaptically released glutamate, and this activation is increased in the presence of glutamate uptake inhibitors and blocked by the PKC inhibitor calphostin C (Melyan et al, 2004).
Removal of calcium had no effect on kainate-evoked GluR6 internalization (Figure 7). Consistent with analogous studies on AMPARs (Beattie et al, 2000; Ehlers, 2000; Lee et al, 2004), however, calcium influx through NMDARs was required for NMDAR-evoked GluR6 endocytosis. Our results showing that GluR6 internalization was almost completely suppressed in the absence of extracellular calcium and in the presence of BAPTA-AM are consistent with an electrophysiological study showing that calcium influx following activation of NMDA receptors caused depression of kainate currents (Ghetti and Heinemann, 2000).
Here, we report the differential endocytic sorting of endogenous GluR6-containing KARs that advances understanding of the cellular mechanisms that control surface expression of these receptors (Figure 8). The balance between receptor recycling and degradation is most likely a key determinant of the level of plasma membrane KARs. AMPARs and KARs have distinct physiological roles, and our results showing that GluR6-containing KARs differ markedly from AMPARs in their membrane stability and internalization properties, although there are similar pathways and processes involved, provide a mechanistic explanation for the independent regulation of these receptors in cultured neurons.
Figure 8.
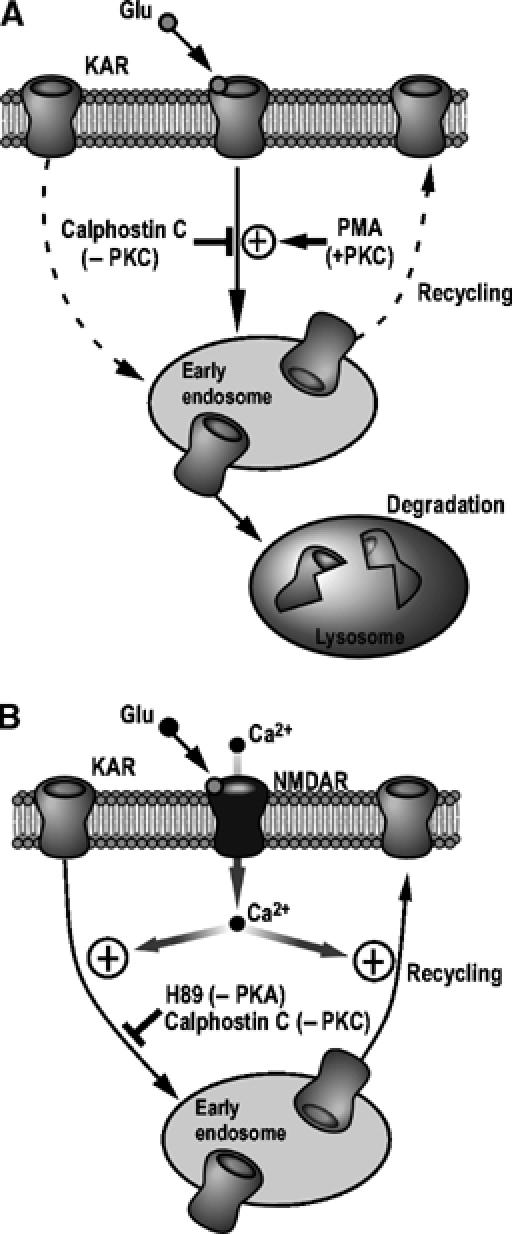
Proposed model for KAR trafficking in hippocampal neurons. GluR6-containing KARs are internalized in response to kainate or NMDA stimulation and rapidly targeted to the early endosomal compartment. They are then differentially sorted to either a recycling (in A, under basal conditions (dashed lines) and in B, following NMDARs activation) or a degradative pathway (in A, following direct KARs activation). The NMDAR-dependent GluR6 endocytosis requires Ca2+ (B), whereas direct activation of GluR6-containing KARs occurs in the absence of extracellular Ca2+. PKC plays a key role in the KAR endocytosis since its inhibition by calphostin C almost completely abolished both NMDA- and kainate-induced GluR6 endocytosis whereas its activation using PMA leads to an increase of GluR6 internalization only when neurons are stimulated with kainate (A). The inhibition of PKA using the specific inhibitor H89 also reduces the amount of GluR6 internalized in response to NMDAR activation (B).
Materials and methods
Dissociated hippocampal cultures
Hippocampal cultures were prepared using a modified published protocol (Noel et al, 1999), and further details are given in Supplementary Materials and methods.
Biotinylation experiments
Live hippocampal neurons (18–21 DIV) were preincubated for 1 h at 37°C. The cells were then biotinylated using the membrane-impermeant sulpho-NHS-SS-biotin for 10 min on ice, washed three times and subsequently incubated in the absence or in the presence of drugs for times indicated in Results. The cells were then washed again, remaining surface biotin removed with GSH and then lysed and incubated with streptavidin beads to bind biotinylated proteins. After washing, proteins were eluted from the streptavidin beads and used for Western blots. For recycling assays, surface-expressed proteins were then debiotinylated and the internalized receptor fraction was ‘chased' with a second incubation at 37°C. Surface-expressed proteins were debiotinylated again to remove the label from proteins recycled to the surface (see Supplementary Materials and methods).
Fluorescence endocytosis and localization experiments
Experiments were performed using a previously described protocol (Lin et al, 2000). Internalization was assessed using an extracellular epitope-directed anti-GluR6 antibody and appropriate Cy3-conjugated secondary and, where appropriate, Cy2-conjugated secondary antibodies to label anti-cell compartment marker antibodies. A Zeiss LSM 510 confocal microscope was used for imaging (see Supplementary Materials and methods).
Receptor degradation experiments
TTX-treated hippocampal neurons were preincubated for 1 h at 37°C in control or lysosome inhibitor-containing medium. After surface biotinylation, the cells were incubated in the absence or presence of lysosomal inhibitors and the amount of biotinylated GluR6 remaining was determined (see Supplementary Materials and methods).
Modulation of endocytosis by Ca2+, PKA and PKC
The roles of PKA and PKC on kainate- or NMDA- induced endocytosis were assessed by preincubation with selective activators and inhibitors. The cell-permeable Ca2+ chelator BAPTA-AM was used to determine the effects of Ca2+ on endocytosis (see Supplementary Materials and methods).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Mark W Fleck for the generous gift of anti-GluR6 antibody and Mike Ashby, Tristan Bouschet, Jon Hanley, Jack Mellor and Claire Palmer for helpful comments. We gratefully acknowledge the Wellcome Trust, the MRC and the EU (KAR-TRAP) for financial support. SM was supported by a Wellcome Trust Fellowship.
References
- Barry MF, Ziff EB (2002) Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12: 279–286 [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda A, von Zastrow M, Malenka RC (2000) Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3: 1291–1300 [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C (2002) Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci 115: 4399–4411 [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA (1997) Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388: 182–188 [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VRJ, Henley JM (1999) Kainate receptors: subunits, synaptic localisation and function. Trends Pharmacol Sci 20: 544–553 [DOI] [PubMed] [Google Scholar]
- Cho K, Francis JC, Hirbec H, Dev K, Brown MW, Henley JM, Bashir ZI (2003) Regulation of kainate receptors by protein kinase C and metabotropic glutamate receptors. J Physiol 548: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD (2003) Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40: 595–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C (2001) Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29: 497–508 [DOI] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF (2003) Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci 23: 8013–8019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28: 511–525 [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143 [DOI] [PubMed] [Google Scholar]
- Fleck MW, Cornell E, Mah SJ (2003) Amino-acid residues involved in glutamate receptor 6 kainate receptor gating and desensitization. J Neurosci 23: 1219–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallyas F Jr, Ball SM, Molnar E (2003) Assembly and cell surface expression of KA-2 subunit-containing kainate receptors. J Neurochem 86: 1414–1427 [DOI] [PubMed] [Google Scholar]
- Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J (1998) SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron 21: 727–739 [DOI] [PubMed] [Google Scholar]
- Ghetti A, Heinemann SF (2000) NMDA-dependent modulation of hippocampal kainate receptors by calcineurin and Ca(2+)/calmodulin-dependent protein kinase. J Neurosci 20: 2766–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DM, Braud S, Hurtado DE, McCallum J, Standley S, Isaac JT, Roche KW (2003) Trafficking and surface expression of the glutamate receptor subunit, KA2. Biochem Biophys Res Commun 310: 8–13 [DOI] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, Collingridge GL, Henley JM (2003) Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37: 625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JTR, Mellor J, Hurtado D, Roche K (2004) Kainate receptor trafficking: physiological roles and molecular mechanisms. Pharmacol Therapeutics (in press) [DOI] [PubMed] [Google Scholar]
- Jaskolski F, Coussen F, Nagarajan N, Normand E, Rosenmund C, Mulle C (2004) Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J Neurosci 24: 2506–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL (1998) Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron 21: 1163–1175 [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT (1999) Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400: 569–573 [DOI] [PubMed] [Google Scholar]
- Lee HK, Kameyama K, Huganir RL, Bear MF (1998) NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21: 1151–1162 [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M (2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36: 661–674 [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M (2004) Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron 43: 221–236 [DOI] [PubMed] [Google Scholar]
- Lerma J (2003) Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4: 481–495 [DOI] [PubMed] [Google Scholar]
- Lerma J, Morales M, Vicente MA, Herreras O (1997) Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci 20: 9–12 [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC (2001) Molecular physiology of kainate receptors. Physiol Rev 81: 971–998 [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M (2000) Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci 3: 1282–1290 [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126 [DOI] [PubMed] [Google Scholar]
- Melyan Z, Lancaster B, Wheal HV (2004) Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci 24: 4530–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B (2002) Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34: 107–114 [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF (1998) Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392: 601–605 [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF (2000) Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28: 475–484 [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM (1999) Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 23: 365–376 [DOI] [PubMed] [Google Scholar]
- Patneau DK, Vyklicky L, Mayer ML (1993) Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci 13: 3496–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond LA, Blackstone CD, Huganir RL (1993) Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature 361: 637–641 [DOI] [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Garcia EP, Sanders JM, Swanson GT, Marshall J (2003a) Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J Neurosci 23: 6608–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Needleman LA, Sanders JM, Swanson GT, Marshall J (2003b) Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J Biol Chem 278: 52700–52709 [DOI] [PubMed] [Google Scholar]
- Ruano D, Lambolez B, Rossier J, Paternain AV, Lerma J (1995) Kainate receptor subunits expressed in single cultured hippocampal neurones: molecular and functional variants by RNA editing. Neuron 14: 1009–1017 [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA (2001) Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA 98: 11003–11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT (2004) Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci 24: 5381–5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wahl P (1997) Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol 503 (Part 3): 513–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL (1997) The synaptic activation of kainate receptors. Nature 388: 179–182 [DOI] [PubMed] [Google Scholar]
- Wang LY, Taverna FA, Huang XP, Macdonald JF, Hampson DR (1993) Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science 259: 1173–1175 [DOI] [PubMed] [Google Scholar]
- Yan S, Sanders JM, Xu J, Zhu Y, Contractor A, Swanson GT (2004) A C-terminal determinant of GluR6 kainate receptor trafficking. J Neurosci 24: 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


