Abstract
As the number of clinical trials conducted in China increases, understanding Chinese attitudes toward clinical research is critical for designing effective and ethical studies. Two survey studies were conducted in 2012 and 2013 to compare patient attitudes toward clinical research and factors affecting research participation in the United States and urban and rural China. We surveyed 525 patients in 2012 (186 US, 186 urban, 153 rural China) and 690 patients in 2013 (412 US, 206 urban, 72 rural China). US patients were more likely to have no concerns regarding research participation than Chinese patients. Most common concerns of US patients were safety, privacy and confidentiality, and time required. Safety was a top concern for many Chinese. Chinese patients, particularly rural Chinese, were more concerned about the likelihood of self‐benefit, and receiving free medical care and financial incentive had greater influence on their participation. Being informed of the freedom to choose whether to participate or to leave a study was less important to Chinese patients. Our study provides important insights into Chinese patients' attitudes toward clinical research and the need to educate them about their rights. These findings help in designing cross‐cultural clinical studies that maximize enrollment while upholding Western ethical standards.
Keywords: enrollment, informed consent, safety, privacy, financial incentives, cultural differences
Introduction
Clinical research is undergoing a major shift. No longer are clinical trials primarily conducted in developed countries. In the past 10 years, the number of clinical research sites outside the United States has more than doubled.1 China, with its large population and increasing importance as a key player in global health, has become an attractive country for conducting clinical trials.2 Among countries active in research, China boasts the highest average relative annual growth rate for research.3 As clinical trials are increasingly expanded to developing nations, researchers must ensure cultural relevance of subject recruitment and the consent process and preservation of Western ethical standards of patient autonomy and disclosure of relevant information. Understanding patients' attitudes toward participation in clinical research in different countries is critical to the effective and ethical design of cross‐cultural collaborative studies.4
The majority of studies on patient attitudes toward clinical research have been conducted in Western countries. Surveys of US and European patients show generally favorable attitudes toward clinical trials with patients citing altruism and personal benefit as primary reasons for participation.5, 6, 7, 8, 9, 10, 11, 12 However, studies show that when approached in both hypothetical and real scenarios less than half of eligible patients chose to participate; unease with randomization, desire for another treatment, concerns about treatment risks, lack of awareness about clinical research, and physician‐related factors have been identified as reasons for patients refusing participation.7, 8, 10, 12, 13, 14, 15 Importantly, attitudes of patients in different countries may vary due to differences in health literacy, cultural values, trust in physicians and the healthcare system, and access to care.
The limited data available about Chinese patients' attitudes toward clinical research suggest possible mistrust of clinical trials, differing motivations for participation, and misgivings regarding the consent process.16, 17, 18 However, little can be concluded from such a small number of studies. In 2012 and 2013, we surveyed patients in urban and rural China and the United States to compare their attitudes toward clinical research and factors that motivate or deter their participation.
Methods
Study design and site selection
Two comparative survey studies of patients in the United States and China were conducted between April and November of 2012 and June and July of 2013. The 2012 study surveyed patients with hepatitis C being followed in hepatology clinics at the University of Michigan Health System (UMHS) in Ann Arbor, Michigan, United States; People's Hospital, Peking University Health Science Center (PUHSC) in Beijing, China; and Guan County Center for Disease Control and Prevention in Hebei, China. The investigators at UMHS and PUHSC have been collaborating on a study of hepatitis C disease progression in the United States and China. The original intention was to conduct this survey at the two hepatology clinics run by the investigators. The protocol was later revised to include a third site in rural China to determine whether responses obtained from patients in Beijing are representative of patients in China. Hebei was chosen because the PUHSC investigators had been following a cohort of patients who acquired hepatitis C virus infection through contaminated plasma. Hebei is a rural area 35 miles from Beijing and most residents there are farmers with limited education.
The 2013 study surveyed patients not under the care of the investigators to eliminate biases due to patients' relations with the investigators and to elicit responses of patients that might have less prior exposure to research. Three sites were selected: a general medicine clinic at an UMHS off‐site multidisciplinary clinic 5 miles from the main hospital in Ann Arbor, Michigan, United States and two multidisciplinary clinics in Beijing, China: one at Zhan Lan Lu Hospital in Beijing city and another one at Sha He Hospital in Chang Ping, a rural town 27 miles from the Beijing metropolis. Both study sites in Beijing are affiliated with PUHSC.
Adults were randomly recruited in‐person at the time of their clinic visits after informed consent was obtained. In the United States, the English survey was self‐administered by patients. At all sites in China, a researcher administered the survey to patients in Chinese due to concerns of low literacy. Data were entered into a secure online database accessible by the research team in both countries. The research protocols were reviewed and approved by the Institutional Review Boards of both the University of Michigan and Peking University.
Survey design
Both surveys were initially prepared in English by UMHS investigators, reviewed and approved by the PUHSC investigators, and then translated into Mandarin Chinese by professional translators at PUHSC. Accuracy of the translation was verified by two native Chinese speaking investigators at UMHS and minor revisions were made. Both surveys were pilot tested in patients in the United States and patients in Beijing and revised based on feedback from these patients. The 2013 survey included most of the questions in the 2012 survey with minor changes based on feedback from the 2012 participants.
In both surveys, patients were asked about prior participation in research and their greatest concern about participating in research. Five‐point Likert scales were used for responses to statements about the likelihood of participating in clinical research based on incentives/disincentives and importance of various factors when asked to join a research study. Two sections on perceived benefits of research participation and privacy concerns were added to the 2013 survey. Both 2012 and 2013 surveys contained a section on demographics (see supplementary material for a copy of the surveys).
Data analyses
Data from all three sites in both surveys were downloaded from the electronic database and analysis was performed using Statistical Package for the Social Science (SPSS version 20; IBM, Armonk, NY, USA). During implementation of the 2013 survey at Chinese sites, it was discovered that the revised translation of questions regarding the influence of incentives/disincentives on decision to participate in a research study based on feedback from 2012 participants resulted in ambiguity and responses to those questions in the 2013 study were not included in this analysis.
Data were presented as median and range or number and percent. Categorical variables were compared using chi‐squared tests and continuous variables using t‐tests. Responses from each of the three sites in the 2012 survey and in the 2013 survey were compared. Univariate and multivariate logistic regression were conducted to explore the effects of study site, patient gender, age, education, and prior participation in research on concerns over personal safety and benefit and importance of being informed of risks of participating in research. Note that p values <0.05 were considered statistically significant.
To further explore factors associated with responses, patient responses to the 2012 survey at all three sites and to the 2013 survey in the United States between patients with and without prior research participation, and patient responses to the 2012 survey in the United States between white and African Americans were compared. The impact of prior research participation on responses to the 2013 survey in China was not examined because >90% of patients had no prior research participation. The impact of race on responses to the 2013 survey in the United States was also not examined because information on race was not collected.
Results
The 2012 study surveyed 525 patients (186 US, 186 urban China, 153 rural China). The 2013 study surveyed a total of 769 patients (437 US, 240 urban China, 92 rural China), but 5.7% of US patients, 14.2% of urban China, and 21.7% of rural China surveys were discarded because responses to questions in the demographics section were incomplete. Therefore, only 690 patients from the 2013 study (412 US, 206 urban China, 72 rural China) were included in the analyses.
Characteristics of patients analyzed
Characteristics of the patients analyzed are shown in Table 1 . Roughly half of the patients surveyed in 2012 were men with a significantly higher percent of men at the US site (62.9% US vs. 52.2% urban China and 41.2% rural China). By contrast, more than 60% of the patients surveyed in 2013 were women with a similar gender distribution across the three sites. The median ages of the six cohorts of patients were 51–56 years (range 19–86). In both surveys, US patients were significantly more likely to have attended at least some college than those in urban China, and urban Chinese patients were significantly more likely to have attended at least some college compared to those in rural China. At all three sites, patients in the 2013 survey were significantly more likely to have attended at least some college than those in the 2012 survey (p < 0.001). In the 2012 survey, 77.3% of US patients were white and 16.8% were African American; >90% of Chinese patients were Han Chinese. Race was not captured in the 2013 survey.
Table 1.
Characteristics of patients analyzed.
| 2012 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|
| United States | Urban China | Rural China | p value | United States | Urban China | Rural China | p value | |
| Total | 186 | 186 | 153 | 412 | 206 | 72 | ||
| Gender, n (%) | ||||||||
| Male | 117 (62.9) | 97 (52.2) | 63 (41.2) | <0.001 | 148 (35.9) | 86 (41.7) | 30 (41.7) | 0.306 |
| Female | 69 (37.1) | 89 (47.8) | 90 (58.8) | 264 (64.1) | 120 (58.3) | 42 (58.3) | ||
| Age, years, median, range | 56, 20–84 | 53, 19–85 | 56, 36–77 | 0.002 | 53, 19–86 | 55, 21–84 | 51, 19–76 | 0.054 |
| Race/nationality, n (%) | ||||||||
| White, 143 (77.3) | Han Chinese, 171 (91.9) | Han Chinese, 150 (98.0) | NA* | NA* | NA* | |||
| African American, 31 (16.8) | ||||||||
| Other, 11 (5.9) | Other, 15 (8.1) | Other, 3 (2.0) | ||||||
| Education, n (%) | ||||||||
| High school or less | 69 (37.1) | 113 (60.8) | 150 (99.3) | <0.001 | 64 (15.5) | 86 (41.8) | 52 (72.2) | <0.001 |
| College | 85 (45.7) | 68 (36.5) | 1 (0.7) | 166 (40.3) | 107 (51.9) | 17 (23.6) | ||
| Graduate or professional school | 32 (17.2) | 5 (2.7) | 0 (0) | 182 (44.2) | 13 (6.3) | 3 (4.2) | ||
| Prior participation in research | ||||||||
| No | 90 (48.4) | 119 (64.0) | 30 (19.6) | <0.001 | 209 (51.1) | 189 (91.7) | 70 (97.2) | <0.001 |
| Yes | 96 (51.6) | 67 (36.0) | 123 (80.4) | 200 (48.9) | 17 (8.3) | 2 (2.8) | ||
NA = not available.
*Information was not collected in 2013 survey.
Previous participation in research
A significantly higher percent of patients in the 2012 survey had previously participated in research studies than those in the 2013 survey (54.5% vs. 31.9%, p < 0.001; Table 1 ). In the 2012 survey, rural Chinese patients were most likely to have previously participated in clinical research (80.4% vs. 51.6% US [51.7% of white and 48.4% of African American] and 36% urban Chinese). In the 2013 survey, the proportion of US patients who had previously participated in clinical research was similar to that in the 2012 survey (48.9% vs. 51.6%) while the proportion of Chinese patients who had previously participated in clinical research was markedly lower (8.3% vs. 36% urban and 2.8% vs. 80.4% rural Chinese) than those in the 2012 survey.
Most important concern about participation in research
When asked the biggest concern about participating in a research study, both surveys showed that US patients were far more likely to have no concerns (21.6–32.4%) compared to Chinese patients (1.6–2.9% urban and 1.4–10.7% rural Chinese, p < 0.001 and p < 0.001, respectively for 2012 and 2013; Figure 1 ). The most common concerns of US patients in both surveys were safety, privacy and confidentiality, and time required. Personal safety was a top concern for many urban and rural Chinese patients, particularly those in the 2013 survey (62.1% urban and 64.8% rural Chinese). Chinese patients, particularly rural Chinese in the 2012 survey (63.3%) were more concerned about the likelihood of self‐benefit from research participation while few US patients (8.1%) were concerned about self‐benefit. Few patients at any of the three sites in either survey had major concerns about the possibility of receiving placebo treatment, trust in the study and the research team, or cost to them or their health insurance. In the United States, patients with prior research participation had more concerns about safety (22.9% prior participation vs. 10.1% no participation, p = 0.02) in the 2012 survey but not in the 2013 survey (17.8% prior participation vs. 24.1% no participation). Responses to this question were similar between Chinese patients with and without previous exposure to research.
Figure 1.
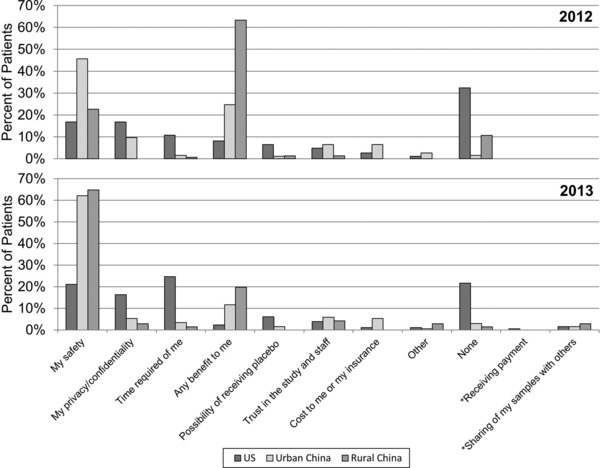
Greatest concern about research participation. Patients in 2012 and 2013 were asked to select one response only to the question “What is your biggest concern about participating in a research study?”. *These survey options were added in 2013.
Importance of information disclosed about research
Patients from the United States ranked the importance of being informed on all aspects of a research study higher than patients in China in both 2012 and 2013 surveys, and responses of urban and rural Chinese patients were similar in both surveys (Figure 2 ). The differences between US and Chinese patients' responses were most pronounced regarding being informed of their freedom to choose whether to participate in a study and to leave a study at any time with Chinese patients ranking the importance of those options lower than US patients (25.4–52.9% Chinese patients vs. 80.7–83.3% US patients considered them important or very important). The vast majority of the patients at all three sites in both surveys considered it important or very important to be informed of the side effects and risks of participating in a research study with patients from rural China in the 2012 study ranking this lower than the other five cohorts. In the 2012 survey, Chinese patients without prior research participation felt it was more important to be informed that they could speak with others about the study (61.5–73.3% no participation vs. 46.3–52.1% prior participation). Other responses in patients with or without prior research participation were similar at all three sites in the 2012 survey and in the United States in the 2013 survey. The 2013 survey included an additional question regarding the importance of having a committee review the research study. Over 95% of the US patients compared to 77.1% of urban and 78.9% of rural Chinese patients ranked this as important or very important.
Figure 2.
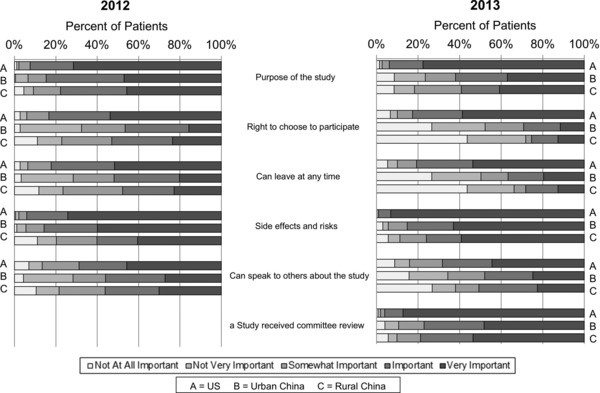
Perceptions about informed consent process. Patients in 2012 and 2013 were asked to respond using Likert scale to questions on “How important to you is it that you are informed of…when asked to join a research study?” Patients selected one response per question. aThis survey option was added in 2013.
Factors influencing likelihood of participation in research
The 2012 survey showed that roughly 70% of Chinese and US patients indicated that their doctor mentioning or recommending a research study was likely to influence their participation in a research study (Figure 3 ). Receipt of free medical care and financial incentive had greater influence on rural Chinese patients' participation in a research study (>80%) compared to 56.8–79.9% in urban Chinese and 54.1–67.8% in US patients. Access to better care had a stronger positive influence while need for collection of blood samples and genetic information had a stronger negative influence on urban and rural Chinese patients' participation in a research study than US patients. Their doctor mentioning or recommending the study had greater influence on the decision to participate in a research study among urban Chinese patients and US patients with prior research participation but not among rural Chinese patients (data not shown).
Figure 3.
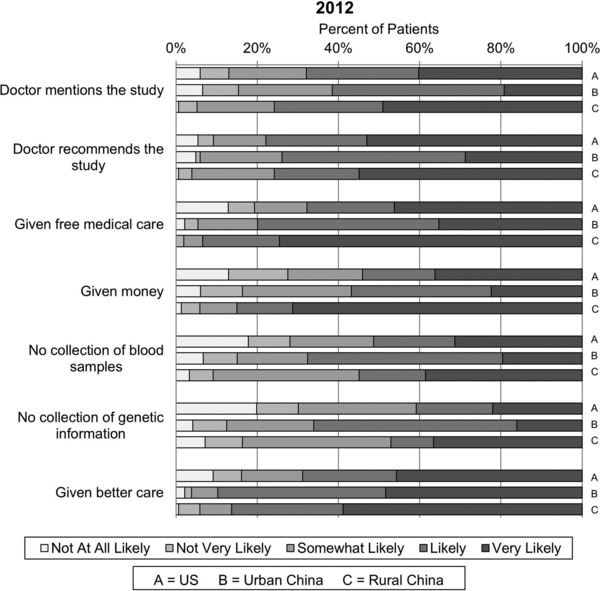
Perceptions about research participation. Patients in 2012 were asked to respond using a Likert scale to the question “How likely are you to participate in a research study if…?”
Benefit of participation in research
When asked about the greatest benefit of participating in a research study, roughly 25% of patients from all three sites in the 2013 survey chose helping others with a similar condition (Figure 4 ). More than half of the US patients while only 29.3% of urban and 19.4% of rural Chinese patients chose contributing to advances in medicine as the most important benefit of participating in a research study. Approximately one‐third of Chinese patients (26.8% urban and 40.3% rural) reported that improving one's own health was the most important benefit in contrast to 4.1% of US patients. Neither access to treatments not widely available, receipt of free medical care or financial incentive, improved access to specialist care, nor decreased wait time in clinics were considered to be important benefits of participating in a research study for either US or Chinese patients.
Figure 4.
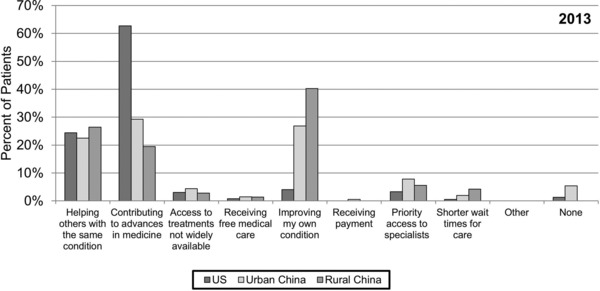
Greatest benefit of research participation. Patients in 2013 were asked to select one response only to the question “What do you think is the greatest benefit of participating in a research study?”
Concern about release of information
US patients were generally more concerned about confidentiality (Figure 5 ). In the 2013 survey, only 13.1% of US patients had no concerns about privacy compared to 19.9% of urban Chinese and 26.8% of rural Chinese. Most patients at all three sites (79.8% US, 74.6% urban and 64.8% rural Chinese) had concerns about release of their personal information. A higher percent of US patients was concerned about release of their medical records or financial status compared to Chinese patients.
Figure 5.
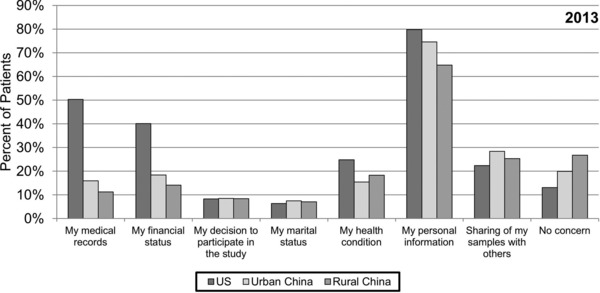
Concerns about release of information. Patients in 2013 were asked to select all responses that apply to the question “Which of the following information would you be worried about being released?”
Factors associated with responses to the survey
Multivariate analyses showed that study site was a significant predictor of patients' concerns over personal safety and self‐benefit on research participation (p < 0.001; Table 2 ). Study site (and education in 2012 survey only) was also a significant predictor of patients' assessment of the importance of being informed of side effects and risks of the research (p < 0.001). However, while Chinese patients had greater concerns about their personal safety, they ranked the importance of being informed of risks of research participation lower than US patients.
Table 2.
2012 and 2013 surveys: factors associated with concerns regarding self‐benefit, safety, and risks.
| Greatest concern | Consent process | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any benefit to me | My safety | Risks | ||||||||||
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |||||||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Site | ||||||||||||
| Urban China | 3.57 (1.87, 6.86) | <0.001 | 7.26 (2.78, 18.95) | <0.001 | 4.11 (2.46, 6.89) | <0.001 | 5.82 (3.78, 8.94) | <0.001 | 0.48 (0.23, 1.04) | 0.06 | 0.09 (0.03, 0.28) | <0.001 |
| Rural China | 15.30 (7.38, 31.75) | <0.001 | 14.32 (4.72, 43.41) | <0.001 | 1.27 (0.67, 2.41) | 0.46 | 6.09 (3.30, 11.24) | <0.001 | 0.20 (0.09, 0.43) | <0.001 | 0.06 (0.02, 0.21) | <0.001 |
| United States | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Age, years | ||||||||||||
| ≥55 | NA† | NA† | 0.75 (0.50, 1.12) | 0.15 | 1.16 (0.82, 1.64)* | 0.41 | NA† | NA† | ||||
| <55 | Ref | Ref | ||||||||||
| Education | ||||||||||||
| High school or less | 1.50 (0.41, 5.48) | 0.54 | 1.44 (0.45, 4.59) | 0.54 | 0.91 (0.37, 2.21)* | 0.83 | 1.23 (0.72, 2.09) | 0.45 | 0.25 (0.11, 0.57) | 0.001 | 0.41 (0.11, 1.54) | 0.19 |
| College | 1.26 (0.34, 4.65) | 0.73 | 1.55 (0.52, 4.65) | 0.43 | 0.71 (0.29, 1.73)* | 0.45 | 1.12 (0.70, 1.78) | 0.65 | Ref‡ | 0.74 (0.20, 2.83) | 0.66 | |
| Graduate/professional school | Ref | Ref | Ref | Ref | Ref | |||||||
| Prior participation in research | ||||||||||||
| No | 0.88 (0.54, 1.39) | 0.55 | 0.47 (0.19, 1.15)* | 0.10 | 0.92 (0.60, 1.41)* | 0.70 | 1.14 (0.73, 1.76) | 0.58 | 1.13 (0.66, 1.95) | 0.66 | 0.55 (0.15, 2.00) | 0.36 |
| Yes | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Gender | ||||||||||||
| Female | NA† | NA† | NA† | NA† | 1.02 (0.63, 1.66)* | 0.94 | 2.06 (1.11, 3.84) | 0.02 | ||||
| Male | Ref | Ref | ||||||||||
Results were similar when all variables were inserted into the multivariate model.
CI = confidence interval; NA = not applicable.
*Although variable was not significant in univariate analysis, variable was included in multivariate analysis for equal comparison.
†Variable not included in multivariate analysis as the univariate analysis was not significant.
‡Only two groups, high school or less compared to any college, included in analysis. Unable to run analysis with three groups because too few patients in graduate/professional school group.
Discussion
In this survey study comparing patient attitudes toward clinical research in the United States, urban China, and rural China, responses of patients at all three sites to many items were comparable but there were also some striking differences. Overall, the responses of urban and rural Chinese patients were more similar to each other than to responses of US patients. Responses of patients at each site were similar in the 2012 and 2013 surveys although the participants were enrolled from the investigators' clinics in 2012 and from clinics with no affiliation to the investigators in 2013.
Several factors impacted willingness to participate in research studies although 20–30% of US patients and 1–10% of Chinese patients had no concerns regarding research participation. The higher percent of US patients with no concerns may be related to a higher frequency of prior participation in research and greater familiarity and trust in research and regulatory oversight.
Safety was the biggest concern of Chinese patients more so in the 2013 survey than the 2012 survey (>60% 2013 vs. 23–46% 2012). This is possibly because fewer Chinese patients in the 2013 survey had prior exposure to clinical research (3–8% 2013 vs. 36–80% 2012). Among Chinese patients in the 2012 survey, rural Chinese had less concerns about safety than urban Chinese possibly because most had been followed by the investigators for many years and 80% had previously participated in research. Lack of prior participation in research was, however, not an independent predictor of safety being ranked as the top concern in research participation in both 2012 and 2013 surveys. Overall, patients without prior participation in research had similar responses to those who had previously participated in research. Of note, a higher percent of US patients in the 2012 survey with prior research experience had major concerns about safety although these findings were not replicated in the 2013 survey.
In our study, Chinese patients were less likely than US patients to consider review and approval of research studies by ethics committees as important and ranked the importance of disclosure of risks lower than US patients. These differences may be related to Chinese patients' unfamiliarity with the role of ethics committee oversight, mistrust of the review process, or their assumption that physicians would not present risky studies to their patients. It is also possible that Chinese patients may be influenced by reports of corruption scandals involving physicians and pharmaceutical companies in China although we are not sure if patients particularly those in rural China were aware of these reports.16, 19, 20, 21 Financial disclosures of relationships between the pharmaceutical industry and physicians have come under increasing scrutiny in Western countries but these policies are more lax in other countries including China. Nevertheless, few (<10%) cited trust in the study or the research team as their biggest concern about research participation. More efforts are needed to elucidate which aspects of research pose a safety concern to patients.
Not surprisingly, US patients had greater concerns about their privacy than Chinese patients. When this question was probed more deeply in the 2013 survey, most (>60%) patients from all three sites worried about release of their personal information but Chinese patients worried less about release of their medical records or financial status. The latter may be related to differences in clinical practice with Chinese patients hand‐carrying their own medical records which do not contain any financial information to clinic visits while US patients' personal, medical, and financial information are stored in electronic records that are more easily accessed by others. Furthermore, Chinese patient visits are often conducted in open clinic rooms whereas US clinic rooms are kept private for individual consultations, thus leading to differing expectations of privacy and confidentiality.
Some studies have shown that the possibility of receiving placebo or being randomized to a less desirable treatment can be a barrier to research participation.15, 22 We did not find this to be the case in any of the six cohorts of patients studied possibly because we were asking a hypothetical question and the responses might have been different if the patients were invited to participate in a randomized controlled trial. Traditionally, Chinese patients have a greater aversion to blood draw,23 but we did not observe a difference between Chinese and US patients when asked whether collection of blood samples or genetic testing would impact their likelihood of research participation. It is, however, possible that Chinese patients particularly those in rural China do not fully understand the implications of genetic testing.
Whether research participation would lead to self‐benefit was a bigger concern and a stronger motivating factor among Chinese than US patients. The importance of self‐benefit was particularly pronounced among rural Chinese in the 2012 survey (>60%), possibly because of their lower socioeconomic status and limited access to medical care. This is supported by a higher percent of this cohort indicating that free medical care or payment would increase their likelihood of research participation. We did not specify the amount of financial remuneration in our study nor collect information on receipt of payment from those who had previously participated in research. A survey of Chinese patients from predominantly low socioeconomic backgrounds in Shandong province similarly revealed that financial incentives were the main reason for clinical research participation.18 These patients may be less aware of the risks of clinical research or more inclined to make trade‐offs for a potential gain and are therefore more vulnerable to coercion. In our study, significantly fewer rural Chinese patients felt it was important for researchers to inform them of all the possible side effects or risks of participating in a research study. Both urban and rural Chinese patients would also be more willing to participate in research to obtain better care from their physicians compared to US patients. In China, patient encounters with physicians in clinics are usually limited to a few minutes. Participation in clinical research often provides greater access to physicians.
A greater emphasis on self‐benefit was also observed among Chinese patients in the 2013 survey with 27–40% of Chinese patients but only 4% of US patients ranking improvement of their own health condition as the greatest benefit of participating in a research study. A similar proportion of US and Chinese patients considered helping others with the same condition as the greatest benefit while a higher proportion of US patients cited contributions to scientific advances in medicine as the greatest benefit of participating in research. Our findings are similar to other studies showing both altruism and personal benefit as reasons for research participation.5, 6, 7, 8, 9, 10, 11, 12
Conducting research abroad often leads to the question whether consent is voluntary particularly in a culture that places less value on individual autonomy.24 While all six cohorts of our patients considered it important to be informed of the purpose and risks of research studies, Chinese patients were less interested in being informed of their freedom to choose whether to participate in a study or to leave a study at any time. Our findings may be related to Chinese culture being more paternalistic with less emphasis on individual autonomy; however, China is rapidly evolving. Contrary to common belief, we found that physicians mentioning or recommending a study did not have a greater influence on research participation for Chinese patients than US patients. In fact, the 2012 survey found that the influence of physicians in Beijing was less than that in Ann Arbor indicating a change in physician–patient relation. We did note that among those who had previous research participation, their doctors mentioning or recommending the study had a greater influence on patient participation for both US and urban Chinese patients. Informed consent is still a new concept in China even among physicians.25 Our results suggest that more efforts are needed to educate Chinese patients of their rights and researchers must not use cultural differences to justify conducting research in developing countries without adhering to ethical standards upheld in Western countries.26
To determine factors associated with responses to the survey, we focused on three items deemed central to patient participation in clinical research: concerns regarding self‐benefit and safety when considering participation in a research study and importance of being informed of the risks of a research study. Univariate analysis showed that study site, patient education, and prior participation in clinical research were associated with responses to the items selected, while study site was the most important predictor of response on multivariate analysis. Our findings indicate that while education and prior exposure to research are important, factors associated with the patients' place of residence, such as the community they identify with and access to resources (including medical care and information and knowledge through media, friends, and family) have greater influence on patients' perceptions about clinical research. These findings provide insight regarding measures to educate the public about risks and benefits of clinical research and strategies to improve recruitment of patients from diverse socioeconomic backgrounds.
Studies have shown that racial minorities are underrepresented in clinical research compared to white patients; however, they are just as willing as whites to participate.27, 28 In our 2012 survey, a similar proportion (roughly half) of African Americans from the United States had previously participated in research as whites. More African Americans had concerns about participation in research, particularly with safety and trust in the study and research staff. Interpretation of these data is limited by the small number of African Americans in the study, but these findings merit further investigations which may shed light on how to improve enrollment of African Americans and other minorities into future research studies.
Several limitations of this study must be considered. The US surveys were conducted in Ann Arbor, Michigan, a university town with a higher‐than‐average education level and the Chinese surveys were conducted in Beijing and communities close to Beijing. The rural Chinese sites in the two studies were also different. Chang Ping, the 2013 site, is a small town closer to Beijing and patients were better educated compared to Hebei, the 2012 site. The study cohorts may not be representative of US or Chinese patients, although we found a striking similarity between the results of the 2012 and 2013 surveys, which enrolled patients from different clinics with and without relations to the investigators. Administration of the survey also differed between US and Chinese sites. Trained researchers administered the survey to patients at the four Chinese sites due to concerns of low literacy while patients completed the survey independently at the two US sites. While the original intention was to replicate the 2012 survey with two additional sections in the 2013 survey, ambiguity due to rewording one section of the Chinese version of the 2013 survey forced us to abandon analysis of that section; thus, responses to some items were not available for comparison.
Conclusions
In this study involving more than 1,200 patients in the United States and China, we found that Chinese patients had greater concerns about safety while US patients had more concerns about privacy regarding participation in clinical research. Self‐benefit was a more important motivator for participation among Chinese patients while altruism and advancing science were more important motivators among US patients. While Chinese patients had greater concerns about safety, they ranked the importance of being informed of risks, freedom to leave the study, and ethics committee review and approval of research study lower. These findings highlight the need to educate Chinese patients about their rights. Financial incentive was a greater motivator for participation in clinical research among patients from lower socioeconomic areas. This is likely true not only in China but also in the United States and should serve as a reminder that these patients are vulnerable and may become targets of coercion. In summary, our study provided important insights into attitudes of Chinese patients toward clinical research that would help in the design of cross‐cultural clinical research studies to maximize enrollment while upholding Western ethical standards.
Sources of Funding
Support for this study was provided by the University of Michigan Health System—Peking University Health Science Center Joint Institute for Clinical and Translational Research (LW and ASL) and the Tuktawa Foundation (ASL). The University of Michigan Medical School (XC, ZG, and CC) and Global REACH Program (TW and TL) provided travel funds for this M1 medical student summer project.
Supporting information
Additional supporting information may be found in the online version of this paper.
2012 Survey
2013 Survey
References
- 1. Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, Schulman KA. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009; 360(8): 816–823. [DOI] [PubMed] [Google Scholar]
- 2. Harmer A, Xiao Y, Missoni E, Tediosi F. ‘BRICS without straw’? A systematic literature review of newly emerging economies’ influence in global health. Global Health. 2013; 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chakma J, Sun GH, Steinberg JD, Sammut SM, Jagsi R. Asia's ascent—global trends in biomedical R&D expenditures. N Engl J Med. 2014; 370(1): 3–6. [DOI] [PubMed] [Google Scholar]
- 4. Dawson L, Kass NE. Views of US researchers about informed consent in international collaborative research. Soc Sci Med. 2005; 61(6): 1211–1222. [DOI] [PubMed] [Google Scholar]
- 5. Cassileth BR, Lusk EJ, Miller DS, Hurwitz S. Attitudes toward clinical trials among patients and the public. JAMA. 1982; 248(8): 968–970. [PubMed] [Google Scholar]
- 6. Ellis PM. Attitudes towards and participation in randomised clinical trials in oncology: a review of the literature. Ann Oncol. 2000; 11(8): 939–945. [DOI] [PubMed] [Google Scholar]
- 7. Halpern SD, Karlawish JH, Casarett D, Berlin JA, Townsend RR, Asch DA. Hypertensive patients’ willingness to participate in placebo‐controlled trials: implications for recruitment efficiency. Am Heart J. 2003; 146(6): 985–992. [DOI] [PubMed] [Google Scholar]
- 8. Jones JM, Nyhof‐Young J, Moric J, Friedman A, Wells W, Catton P. Identifying motivations and barriers to patient participation in clinical trials. J Cancer Educ. 2006; 21(4): 237–242. [DOI] [PubMed] [Google Scholar]
- 9. Madsen S, Holm S, Riis P. Ethical aspects of clinical trials: the attitudes of the public and out‐patients. J Intern Med. 1999; 245(6): 571–579. [DOI] [PubMed] [Google Scholar]
- 10. Madsen SM, Mirza MR, Holm S, Hilsted KL, Kampmann K, Riis P. Attitudes towards clinical research amongst participants and nonparticipants. J Intern Med. 2002; 251(2): 156–168. [DOI] [PubMed] [Google Scholar]
- 11. Rojavin MA, Downs P, Shetzline MA, Chilingerian R, Cohard‐Radice M. Factors motivating dyspepsia patients to enter clinical research. Contemp Clin Trials. 2006; 27(2): 103–111. [DOI] [PubMed] [Google Scholar]
- 12. Slevin M, Mossman J, Bowling A, Leonard R, Steward W, Harper P, McIllmurray M, Thatcher N. Volunteers or victims: patients’ views of randomised cancer clinical trials. Br J Cancer. 1995; 71(6): 1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lara PN Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, Wun T, Welborn J, Meyers FJ, Christensen S, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001; 19(6): 1728–1733. [DOI] [PubMed] [Google Scholar]
- 14. Llewellyn‐Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients’ willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Soc Sci Med. 1991; 32(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 15. Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, Ellis P, Wright JR. Barriers to participation in clinical trials of cancer: a meta‐analysis and systematic review of patient‐reported factors. Lancet Oncol. 2006; 7(2): 141–148. [DOI] [PubMed] [Google Scholar]
- 16. Su L, Huang J, Mellor D, Yang W, McCabe M, Shen Y, Li H, Wang W, Xu Y. The rights of psychiatric patients in China: a survey of medical staff and consumers’ attitudes toward patient participation in clinical trials. Soc Sci Med. 2012; 75(5): 823–827. [DOI] [PubMed] [Google Scholar]
- 17. Tu WJ, Li Y, Shi XD. The views of Chinese parents on research participation and informed consent. J Paediatr Child Health. 2012; 48(2): 186–187. [DOI] [PubMed] [Google Scholar]
- 18. Wang B, Cao Y, Li C, Wei S, Chen X. Analysis of the status of subject recruitment in clinical trials in Shandong, China. J Empir Res Hum Res Ethics. 2012; 7(1): 9–16. [DOI] [PubMed] [Google Scholar]
- 19. Hennig W. Bioethics in China: although national guidelines are in place, their implementation remains difficult. EMBO Rep. 2006; 7(9): 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Z, Fan D. How to solve the crisis behind Bribegate for Chinese doctors. Lancet. 2012; 379(9812): e13–e15. [DOI] [PubMed] [Google Scholar]
- 21. Henning PJ. Lessons from the Glaxo case in China. The New York Times. July 29, 2013. [Google Scholar]
- 22. Meropol NJ, Buzaglo JS, Millard J, Damjanov N, Miller SM, Ridgway C, Ross EA, Sprandio JD, Watts P. Barriers to clinical trial participation as perceived by oncologists and patients. J Natl Compreh Cancer Network. 2007; 5(8): 655–664. [DOI] [PubMed] [Google Scholar]
- 23. Wong ML, Chia KS, Yam WM, Teodoro GR, Lau KW. Willingness to donate blood samples for genetic research: a survey from a community in Singapore. Clin Genet. 2004; 65(1): 45–51. [DOI] [PubMed] [Google Scholar]
- 24. Angell M. Ethical imperialism? Ethics in international collaborative clinical research. N Engl J Med. 1988; 319(16): 1081–1083. [DOI] [PubMed] [Google Scholar]
- 25. Cong Y. Doctor‐family‐patient relationship: the Chinese paradigm of informed consent. J Med Philos. 2004; 29(2): 149–178. [DOI] [PubMed] [Google Scholar]
- 26. Doring O. China's struggle for practical regulations in medical ethics. Nat Rev Genet. 2003; 4(3): 233–239. [DOI] [PubMed] [Google Scholar]
- 27. Lang R, Kelkar VA, Byrd JR, Edwards CL, Pericak‐Vance M, Byrd GS. African American participation in health‐related research studies: indicators for effective recruitment. J Public Health Manage Pract. 2013; 19(2): 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wendler D, Kington R, Madans J, Van Wye G, Christ‐Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006; 3(2): e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this paper.
2012 Survey
2013 Survey


