Abstract
This double‐blind crossover clinical trial randomized 12 adult males to receive 200 mg of caffeine from a green coffee extract, a guayusa leaf extract, and a synthetic control to compare their safety, absorption, and effect on neurotransmitters. The results showed no statistically significant changes in blood pressure or heart rate from baseline to 120 min postdose of each natural source compared with changes from baseline in the control (0.094 < = P < = 0.910). The ratios of Cmax, AUC0‐4, and AUC0‐∞ of each natural source to the control were bioequivalent by US Food and Drug Administration standards (90% CI within 80–125%). The guayusa leaf extract stimulated a significantly lower increase in epinephrine compared with the control (+0.5 vs. +2.78 μg/gCr, P = 0.04), while the green coffee extract provoked an increase in epinephrine similar to the control (+3.21 vs. +2.78 μg/gCr, P = 0.569). Implications for future clinical research are discussed.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ The current research on this topic shows that natural sources of caffeine may have influences on the body similar to those of synthetic caffeine when absorbed in comparable doses.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ This study addressed the question of whether two natural sources of caffeine, green coffee extract and guayusa leaf extract, are safe to consume and what impact their consumption has on the body's neurotransmitter system and vital signs compared with synthetic caffeine.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
✓ This study has several implications for future clinical research on the safety and neurological impact of natural caffeine sources. The findings suggest that the natural caffeine extracts behave like synthetic caffeine with respect to their effect on the cardiovascular system and their pharmacokinetic absorption profiles. However, the guayusa leaf extract may be less stimulating to the release of epinephrine compared with the green coffee extract and synthetic caffeine, which has implications for its therapeutic applications and safety.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ This study generates a hypothesis for the future study of clinical applications for natural caffeine sources—that tea‐leaf sourced caffeine may have differential impacts on excitatory neurotransmitters, particularly the adrenaline response, from the previously studied effects of synthetic caffeine. In addition, this study adds to the FDA's investigation of caffeine metabolism in conjunction with food substances, since it shows that natural sources of caffeine plus carbohydrates are absorbed similarly to synthetic caffeine.
Caffeine is one of the most common stimulants consumed worldwide and can originate from both plant‐based and synthetic sources.1, 2 The average daily caffeine intake in the United States ranges between 210 and 238 mg per day, which can be consumed through a variety of dietary supplements, beverages, and food products.3 Although caffeine has historically not been regulated as a drug by government agencies, its diverse interactions with neurotransmitters in the brain have been shown to have both therapeutic and harmful effects. From a therapeutic perspective, caffeine's role as an adenosine A2A antagonist has been linked to a lower risk of Parkinson's disease and other neurodegenerative disorders such as Alzheimer's disease.3, 4, 5 At the same time, caffeine's stimulation of the body's adrenaline response has been associated with acute increases in blood pressure and heart rate, although caffeine consumers seem to develop tolerance to these effects after prolonged regular consumption.6, 7
As caffeine continues to be added to a growing number of food products, it has become increasingly important for regulatory bodies to understand the clinical implications of caffeine's effect on the brain and the body. In recognition of this, the US Food and Drug Administration (FDA) announced in May 2013 that it would begin a coordinated effort to investigate the safety of caffeine.8 This includes an investigation into plant‐based caffeine sources, which have identical chemical structures to synthetic caffeine, but also contain other ingredients that may differentiate some effects of plant‐based caffeine from those of synthetic caffeine.9 Substances other than caffeine in natural sources such as coffee and green tea have been proposed as a mechanism linking caffeine consumption to a variety of health outcomes, from reduced rate of metabolic syndrome through lowered serum triglyceride levels, to increased performance on memory and attention tasks.10, 11
In response to the need for more research on the safety and neurological mechanisms of natural caffeine sources, the current study investigated the safety, pharmacokinetics, and nervous system effects of two natural sources of caffeine compared with a synthetic control: a green coffee extract (JAVA.g) that was derived from 100% green C. arabica and/or C. robusta coffee, and a guayusa (Illex guayusa) tea‐leaf extract (AMATEA). These extracts are intended as replacements for synthetic caffeine in general circulation. Consumption of each extract in quantities comparable to synthetic caffeine has been generally recognized as safe (GRAS) by a panel of independent experts based on data available and the intended use of the product,9, 12 but there is no human clinical evidence to date examining the safety, pharmacokinetics, and nervous system effects of either caffeine source. This randomized, double‐blind, crossover clinical trial provides human clinical evidence of the safety, pharmacokinetics, and nervous system effects of a green coffee extract and a guayusa leaf extract in healthy adult males.
METHODS
Subjects
Subjects consisted of 12 nonsmoking male volunteers aged 21 to 34 years old (mean 24.33 years old). Their body weight ranged from 60.10 to 96.00 kg (mean 78.91 kg). Subjects were in good health as determined by a physical examination, medical history, and ECG, and at the time of the study had been engaged in aerobic exercise for at least 150 min per week for the prior 6 months. Subjects who regularly consumed more than 500 mg of caffeine per day were excluded from the study. Subjects agreed not to use any new vitamins and/or minerals until after the study's completion, and to stop taking any other dietary and/or herbal supplements 7 days prior to the first visit and through the end of the study. They were also instructed to abstain from caffeine consumption 24 h prior to each study visit. All subjects provided informed consent before beginning the study. This study was approved by the Aspire IRB (Santee, CA) on 28 April 2015.
Protocol
The study followed a randomized, double‐blind, three‐period crossover design to compare the pharmacokinetics, neurological effects, and safety of the green coffee extract (GCE) and guayusa leaf extract (GLE) to synthetic caffeine. All eligible subjects were randomized in a block‐6 design to receive one of the three caffeine sources at each of three scheduled pharmacokinetic visits separated by at least 48 h. Subjects were required to abstain from caffeine and caffeine‐containing products, as well as any marketed “energy” beverages and alcohol, for 24 h prior to each visit. At each visit, subjects received one of the three caffeine sources as per the randomization schedule. The study was double‐blind, so neither the subjects nor the staff knew the order in which the three caffeine sources were provided. The treatments were administered in bottled liquid form with their unaltered odor and taste, and the subjects were required to drink the study product in 5 min or less. Each caffeine source contained 200 mg of caffeine per 4 fluid ounces, which was chosen to approximate the average daily caffeine intake in the United States.3 The GCE contained 30% caffeine and 40% polyphenols by weight and the GLE contained 20% caffeine and 30% polyphenols by weight. Both supplements also contained a balance of plant‐based compounds that include carbohydrates, protein, fiber, and trace minerals.
Baseline measurements of serum caffeine levels (processed by LabCorp), the urinary neurotransmitters serotonin, GABA, dopamine, epinephrine, norepinephrine, and glutamate (processed by Labrix Laboratory), blood pressure, and heart rate were obtained. Postdose measurements were taken as follows: for serum caffeine levels, measurements were done 30, 60, 120, 180, and 240 min postdose; for all neurotransmitters, measurements were done 60 min postdose; for blood pressure and heart rate, measurements were done at 60 and 120 min postdose; for adverse events and subjective comments, incidences over 240 min (the duration of the visit) were noted. All subjects completed the study per protocol, with the exception of one subject during his GCE visit who had nonzero levels of caffeine at baseline (serum caffeine concentration of 2.80 at baseline). This subject was included in the per‐protocol population since the statistical analysis accounted for differences in baseline levels.
Statistical analysis
The safety of the caffeine sources was assessed by measuring the mean change from baseline in blood pressure and heart rate over the duration of the visit. Adverse events were listed, MedDRA‐encoded, and rated for severity and relationship with the study product by the principal investigator. Subjective remarks were categorized to the extent possible and assessed qualitatively for evidence of safety concerns. The pharmacokinetics of the caffeine sources were assessed using the standard noncompartmental pharmacokinetic end points: the maximum concentration observed during the postdose samples (Cmax), the time at which the maximum concentration was observed (tmax), and the area under the concentration‐vs.‐time curve above the baseline (time 0) concentration, integrated from time 0 to 4 h postdose by trapezoidal‐rule integration (AUC0–4h). The half‐life (t1/2) and AUC0–∞ were estimated using the following formulas for total exposure (13):
| (1) |
| (2) |
where ke represents the elimination rate constant calculated by a first‐order exponential equation Ct = Cmax*e −ke*t on the descending part of the concentration‐vs.‐time curve, and Ct is the last measurable concentration. ke could not be calculated for one subject postsynthetic caffeine dose because the subject reached the maximum concentration at the last measurable timepoint, so there was not enough data to observe the descending part of the concentration‐vs.‐time curve. Therefore, n = 11 for the synthetic caffeine measurements of t1/2 and AUC0–∞ and comparisons between the natural sources and the synthetic control on these measurements. The mean change from baseline in serum caffeine levels was also assessed up to 240 min postdose for each source. The effect of the two caffeine sources on nervous system function was assessed by measuring the mean change from baseline in each of the neurotransmitters (serotonin, GABA, dopamine, epinephrine, norepinephrine, and glutamate) to 60 min postdose.
All statistical analysis was completed using R v. 3.1.2.14 Mean changes from baseline and the mean difference in changes from baseline between the natural sources and control were tested for statistical significance by the paired Student's t‐test, unless significant nonnormality was detected in the distribution by the Shapiro–Wilk normality test, in which case the Wilcoxon signed rank test was used instead (noted in the tables). To assess the bioequivalence of the natural caffeine sources to synthetic caffeine, 90% confidence intervals for the ratios of the log‐transformed Cmax, AUC0–4h, and AUC0–∞ to the control were compared with the FDA's 80–125% range for bioequivalence of log‐transformed data, and the median difference in tmax and t1/2 from the control was tested for significance from zero using the Wilcoxon signed rank test according to the FDA guidelines for discrete time interval data.15, 16
Statistical power and Type I error rates
No formal power analysis was conducted. The sample size of 12 participants was based on prior published and unpublished studies conducted on the pharmacokinetics of caffeine.17, 18, 19, 20 When multiple comparisons were made for each area of investigation, the sequentially rejective Bonferroni–Holm test was applied to ensure that the Type I error resulting from multiple tests never exceeded the preset level of statistical significance at α = 0.05.21 Interpretations of the individual unadjusted P‐values are made according to this paradigm.
RESULTS
Safety
As shown in Table 1, there were no statistically significant changes in blood pressure or heart rate from baseline levels at 60 and 120 min post‐GCE dose (0.074 < = P < = 0.515). These changes were also statistically no different from the changes in blood pressure or heart rate postsynthetic control dose (0.237 < = P < = 0.910). At 60 min post‐GLE dose, there was a decrease in heart rate that remained statistically significant among all the multiple safety comparisons (–5.83 beats per min, P = 0.006), but it was not statistically different from the change from baseline in heart rate 60 min postsynthetic control dose (P = 0.547). No changes from baseline in blood pressure were statistically significant at 60 and 120 min post‐GLE dose (0.450 < = P < = 0.938), and none of these changes was statistically different from the changes in blood pressure postsynthetic control dose (0.094 < = P < = 0.845).
Table 1.
Safety of vital signs (n = 12)
| Baseline | 60 min postdose | 120 min postdose | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Safety measure | Guayusa leaf | Green coffee | Synthetic | Guayusa leaf | Green coffee | Synthetic | Guayusa leaf | Green coffee | Synthetic |
| Systolic blood pressure (mmHg) | 115.17 ± 6.12 | 113.42 ± 8.85 | 109.50 ± 9.20 |
|
|
|
|
|
|
| Diastolic blood pressure (mmHg) | 72.25 ± 5.50 | 70.92 ± 4.64 | 68.75 ± 5.33 |
|
|
|
|
|
|
| Heart rate (beats per min) | 62.33 ± 8.29 | 58.83 ± 10.12 | 59.58 ± 7.25 |
|
|
|
|
|
|
Results are presented as mean ± standard deviation.
pb tests for a significant change from baseline levels within product. pc tests for a significant difference in the change from baseline between product and synthetic control.
+ Where noted, P–values are from a Wilcoxon paired test because of nonnormality. Otherwise, P–values are from a paired Student's t–test.
Four adverse events were reported across the three study visits: a fractured clavicle and right toe abrasion at the GCE visit, an upper respiratory tract infection at the GLE visit, and right ankle pain at the synthetic control visit. All four events were determined to be definitely unrelated to the caffeine sources by the principal investigator. No subjects made subjective comments regarding adverse effects related to the products.
Pharmacokinetics
Table 2 shows that, from baseline to 4 h postdose, levels of serum caffeine significantly different from baseline were detected in the subjects after consuming each caffeine source (P < 0.005 for all timepoints). As shown in Figure 1, at the end of the 4‐h period, nonzero levels of serum caffeine were still present in the body at an average of 2.54 μg/mL for GCE, 2.50 μg/mL for GLE, and 2.36 μg/mL for the synthetic control above baseline levels (P < 0.001). No changes from baseline serum caffeine levels were significantly different from the synthetic control for either product (0.095 < = P < = 0.892).
Table 2.
Serum caffeine concentration above baseline (μg/mL) over 240 min postdose (n = 12)
| Time Point | Guayusa leaf | Green coffee | Synthetic |
|---|---|---|---|
| Baseline | 0.00 ± 0.00 | 0.23 ± 0.81 | 0.00 ± 0.00 |
| 30 min |
|
|
|
| 60 min |
|
|
|
| 120 min |
|
|
|
| 180 min |
|
|
|
| 240 min |
|
|
|
Results are presented as mean ± standard deviation.
pb tests for a significant change from baseline levels within product. pc tests for a significant difference in the change from baseline between product and synthetic control.
+ Where noted, P–values are from a Wilcoxon paired test because of nonnormality. Otherwise, P–values are from a paired Student's t–test.
Figure 1.
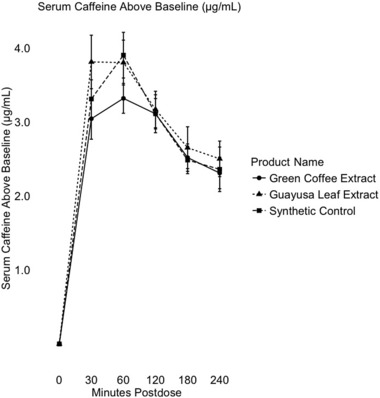
Mean serum caffeine concentration above baseline (μg/mL) from 0 to 240 min postdose of the green coffee extract, guayusa extract, and synthetic control (n = 12). Error bars represent one standard error from the mean.
As shown in Table 3, the average maximum concentration (Cmax) observed after baseline was 3.95 μg/mL for GCE, 4.13 μg/mL for GLE, and 4.12 μg/mL for the synthetic control. The average timepoint at which the maximum concentration was observed (tmax) occurred at 60 min for GCE, 47.50 min for GLE, and 72.50 min for synthetic caffeine. AUC0‐4h was calculated at an average of 647.25 μg/mL*min for GCE, 709.25 μg/mL*min for GLE, and 681 μg/mL*min for the synthetic control. The estimated half‐life (t1/2) of the caffeine sources was 341.19 min for GCE, 284.20 min for GLE, and 281.60 min for the synthetic control. AUC0–∞ was estimated at an average of 1,916.04 μg/mL*min for GCE, 1,789.55 μg/mL*min for GLE, and 1,785.43 μg/mL*min for the synthetic control.
Table 3.
Pharmacokinetic parameters over 240 min postdose (n = 12)
| Source | Cmax (μg/mL) | tmax min | AUC0–4h (μg * min/mL) | t1/2 min | AUC0−∞ (μg * min/mL) |
|---|---|---|---|---|---|
| Guayusa leaf |
|
|
|
|
|
| Green coffee |
|
|
|
|
|
| Synthetic |
|
|
|
|
|
Ratios are presented as mean ± standard deviation with 90% confidence intervals.
* n = 11.
Table 4 shows that the bioequivalence ratios of the log‐transformed Cmax, AUC0‐4h, and AUC0–∞ values relative to the control were within the FDA's standard equivalence range of 80–125% for log‐transformed data. Table 4 also shows that the difference in the untransformed tmax and t1/2 values between each caffeine source and the synthetic control were statistically not different from zero when analyzed by the nonparametric Wilcoxon signed rank test (0.178 < = P < = 0.966).
Table 4.
Pharmacokinetic bioequivalence tests compared with synthetic control over 240 min postdose (n = 12)
| Source | Cmax ratio | tmax difference | t1/2 difference | AUC0–4h ratio | AUC0–∞ ratio |
|---|---|---|---|---|---|
| Guayusa leaf |
|
|
|
|
|
| Green coffee |
|
|
|
|
Ratios are presented as mean ± standard deviation with 90% confidence intervals. Cmax and AUCs were log transformed before taking the ratios.
+ P–values are from a Wilcoxon paired test compared with synthetic control.
* n = 11.
Nervous system function: Serotonin, GABA, dopamine, epinephrine, norepinephrine, and glutamate
As shown in Table 5, no statistically significant changes from baseline in any of the urinary neurotransmitters were observed post‐GLE dose (0.233 < = P < = 0.913). The change from baseline in epinephrine post‐GLE dose was statistically significantly smaller than the change from baseline in epinephrine postsynthetic control dose in a single test comparison (+0.5 vs. +2.78, P = 0.04), but was not at the level of statistical significance needed to reject the null hypothesis given the set of comparisons. Post‐GCE dose, levels of epinephrine increased on average by 96% from baseline to 60 min postdose (+3.21 μg/gCr, P = 0.034), which was not at the level of statistical significance needed to reject the null hypothesis given the set of comparisons, and levels of glutamate increased post‐GCE dose by 35% on average from baseline to 60 min (+20.70 μMol/gCr, P = 0.005), which remained statistically significant among the multiple comparisons. Neither increase was statistically different from the corresponding increases in those neurotransmitters postcontrol dose (0.098 < = P < = 0.569). Postsynthetic control dose, an 8.6% decrease in dopamine was observed that was individually statistically significant (−11.94 μg/gCr, P = 0.035), but not at the level needed to reject the null hypothesis given the set of comparisons, and levels of epinephrine increased by 64% from baseline (+2.78 μg/gCr, P = 0.01), which remained marginally statistically significant among the set of comparisons. No other statistically significant changes from baseline were observed in any of the other neurotransmitters.
Table 5.
Neurotransmitters from baseline to 60 min postdose dose (n = 12)
| Baseline | 60 min postdose | |||||
|---|---|---|---|---|---|---|
| Neurotransmitter | Guayusa leaf | Green coffee | Synthetic | Guayusa leaf | Green coffee | Synthetic |
| Serotonin (μg/gCr) | 75.01 ± 19.41 | 72.19 ± 19.50 | 73.76 ± 24.02 |
|
|
|
| Dopamine (μg/gCr) | 145.59 ± 42.55 | 137.20 ± 42.76 | 138.97 ± 45.23 |
|
||
| Norepinephrine (μg/gCr) | 58.85 ± 37.89 | 59.55 ± 41.51 | 51.62 ± 26.24 |
|
|
|
| GABA (μMol/gCr) | 2.86 ± 0.97 | 2.65 ± 0.94 | 3.00 ± 1.38 |
|
|
|
| Glutamate (μMol/gCr) | 58.71 ± 35.39 | 59.25 ± 34.11 | 59.43 ± 34.14 |
|
|
|
| Epinephrine (μg/gCr) | 4.99 ± 3.24 | 4.86 ± 2.45 | 4.35 ± 3.55 |
|
|
|
Results are presented as mean ± standard deviation.
pb tests for a significant change from baseline levels within product. pc tests for a significant difference in the change from baseline between product and synthetic control.
+ Where noted, P–values are from a Wilcoxon paired test because of non–normality. Otherwise, P–values are from a paired Student's t–test.
DISCUSSION
This prospective randomized safety clinical trial yielded preliminary evidence of no safety concerns for GCE and GLE at the doses consumed during the study (4 fluid ounces containing 200 mg of caffeine, or 2.5 mg/kg on average). The statistically significant decrease in heart rate post‐GLE dose was determined by the principal investigator to be of no clinical significance, as the absolute values of the 60‐min postdose heart rates for GLE and control were within 1 bpm of each other and the decrease was due to elevated baseline levels for GLE rather than depressed postdose levels compared with the control. The fact that there were no clinically significant acute changes in blood pressure or heart rate postdose for any of the three caffeine sources suggests that the subjects may have developed tolerance to caffeine's cardiovascular effects due to their history of prior caffeine consumption outside the study.6, 7
The pharmacokinetic analysis showed significant absorption of caffeine in the body over 4 h for both natural caffeine sources, with maximum levels of serum caffeine reaching about the reference maximum from previous pharmacokinetic studies of caffeine and bioequivalence to the maximum concentration observed postsynthetic control dose.22 Caffeine concentration reached its peak for both sources between 45 and 60 min on average, which is consistent with other studies of comparable oral doses of caffeine and was not statistically different from the peak concentration time postsynthetic control dose.17 The AUC0‐4h and estimated AUC0–∞ for both natural caffeine sources met the FDA standards for bioequivalence to the synthetic control, suggesting no statistical difference in absorption between the natural and synthetic caffeine sources. This may have important implications for the FDA's investigation into caffeine as an additive to food products, since ingesting the combination of carbohydrates, protein, fiber, and trace minerals present in the natural sources did not seem to impact the body's absorption and elimination of caffeine. However, because the duration of the study visits did not extend beyond the estimated average half‐life of the caffeine sources, longer pharmacokinetic time windows are suggested for future research on the absorption profiles.
The neurotransmitter analysis showed no statistically significant postdose changes from baseline levels in serotonin, dopamine, norepinephrine, or GABA for either natural caffeine source. A marginally significant increase in epinephrine (+3.21 μg/gCr, P = 0.034) and a statistically significant increase in glutamate (+20.70 μMol/gCr, P = 0.005) post‐GCE dose are consistent with evidence that certain doses of caffeine can augment the release of excitatory neurotransmitters.23 A similar increase in epinephrine was observed postcontrol dose but not post‐GLE dose. This provides preliminary evidence that the GLE caffeine source differs from the synthetic and GCE caffeine sources with respect to epinephrine, which may imply a reduced chance of adrenaline‐related side effects. Since there is evidence that the effect of caffeine on excitatory neurotransmitters is dose‐dependent, more investigation is needed to clarify whether the observed differences are present with varying doses of each caffeine source.24
Acknowledgments
The authors thank the following people who were also involved in the research that led to this article: Isabel Pino, Athena Vanzant, Lara Paraskos, Adriana Acosta, Jorge Caso, Martha Hernandez, Robert Salzman, and John C. Pezzullo. The research and writing for this study was funded by Applied Food Sciences Inc, the manufacturer of the JAVA.g and AMATEA extracts.
Author Contributions
S.N. wrote the manuscript; D.Kr., D.Ka., and S.F. designed the research; L.A., D.G., and O.G. performed the research; S. N., D.Kr., and S.F. analyzed the data.
Conflict of Interest/Disclosure
This research was sponsored by Applied Food Sciences Inc., the manufacturer of the JAVA.g and AMATEA extracts. Applied Food Sciences Inc. was involved in the research design, protocol formation, and review of the final manuscript, but QPS MRA (Miami Research Associates) was exclusively responsible for the execution of the clinical trial, analysis of the data and the interpretation of the results.
References
- 1. Frary, C.D. , Johnson, R.K. & Wang, M.Q. Food sources and intakes of caffeine in the diets of persons in the United States. J. Am. Diet. Assoc. 105, 110–113 (2005). [DOI] [PubMed] [Google Scholar]
- 2. Alonso‐Salces, R.M. , Serra, F. , Reniero, F. & Heberger, K. Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): chemometric evaluation of phenolic and methylxanthine contents. J. Agric. Food Chem. 57, 4224–4235 (2009). [DOI] [PubMed] [Google Scholar]
- 3. Fredholm, B. , Battig, K. , Holmen, J. , Nehlig, A. & Zvartau, E. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev. 51, 83–133 (1999). [PubMed] [Google Scholar]
- 4. Chen, J.F. et al Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson's disease. J. Neurosci. 21:RC143, 1–6 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eskelinen, M.H. & Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer's disease. J. Alzheimers Dis. 20, S167–S174 (2010). [DOI] [PubMed] [Google Scholar]
- 6. Robertson, D. , Wade, D. , Worman, R. & Woosley, R.L. Tolerance to the humoral and hemodynamic effects of caffeine in man. J. Clin. Invest. 67, 1111–1117 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nawrot, P. , Jordan, S. , Eastwood, J. , Rotstein, J. , Hugenholtz, A. & Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 20, 1–30 (2003). [DOI] [PubMed] [Google Scholar]
- 8. U.S. Food and Drug Administration . FDA to investigate added caffeine. [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; 2013 May [cited 2016 Apr 29]. Available from: http://www.fda.gov/forconsumers/consumerupdates/ucm350570.htm.
- 9. AIBMR Life Sciences, Inc. The Generally Recognized as Safe (GRAS) Status of Java.g. (2015, February).
- 10. Takami, H. et al Inverse Correlation Between Coffee Consumption and Prevalence of Metabolic Syndrome: Baseline Survey of the Japan Multi‐Institutional Collaborative Cohort (J‐MICC) Study in Tokushima, Japan. J. Epidemiol. 23, 12–20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camfield, D.A. , Silber, B.Y. , Scholey, A.B. , Nolidin, K. , Goh, A. & Stough, C. A randomized placebo‐controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS ONE 8, e82897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration . How U.S. FDA's GRAS Notification Program Works. [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; 2005 Dec [cited 2016 Apr 29]. Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ucm083022.htm.
- 13. U.S. Food and Drug Administration . Bioavailability and bioequivalence studies for orally administered drug products–general considerations. [Internet]. Rockville, MD: U.S. Food and Drug Administration; 2002 July [cited 2016 Apr 29]. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM154838.pdf.
- 14. Duncan Murdoch . R‐3.1.2 for Windows (32/64 bit). [Internet]. 2014 October [cited 2016 Feb 10]. Available from: https://cran.r‐project.org/bin/windows/base/old/3.1.2/
- 15. Rani, S. & Pargal, A. Bioequivalence: An overview of statistical concepts. Indian J. Pharmacol. 36, 209–216 (2004). [Google Scholar]
- 16. U.S. Food and Drug Administration . Statistical approaches to establishing bioequivalence. [Internet]. Rockville, MD: U.S. Food and Drug Administration; 2001 Jan [cited 2016 Apr 29]. Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070244.pdf.
- 17. Teekachunhatean, S. , Tosri, N. , Rojanasthien, N. , Srichairatanakool, S. & Sangdee, C. Pharmacokinetics of Caffeine following a Single Administration of Coffee Enema versus Oral Coffee Consumption in Healthy Male Subjects. ISRN Pharmacol, Article ID 147238. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ivy, J.L. et al Improved Cycling Time‐Trial Performance After Ingestion of a Caffeine Energy Drink. Int. J. Sport Nutr. Exerc. Metab. 19, 61–78 (2009). [DOI] [PubMed] [Google Scholar]
- 19. Mohr, M. , Nielsen, J.J. & Bangsbo, J. Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J. Appl. Physiol. 111, 1372–1379 (1985). [DOI] [PubMed] [Google Scholar]
- 20. Kamimori, G.H. et al The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int. J. Pharm. 234, 159–167 (2002). [DOI] [PubMed] [Google Scholar]
- 21. Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 6, 65–70 (1979). [Google Scholar]
- 22. Nehlig, A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav. Rev. 23, 563–576 (1999). [DOI] [PubMed] [Google Scholar]
- 23. Ferre, S. Role of the Central Ascending Neurotransmitter Systems in the Psychostimulant Effects of Caffeine. J. Alzheimers Dis. 20, S35–S49 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solinas, M. , Ferre, S. , You, Z. , Karcz–Kubicha, M. , Popoli, P. & Goldberg, S. Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens. J. Neurosci. 22, 6321–6324 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]


