Abstract
The protein kinase C (PKC) signaling system plays a role in mood disorders and PKC inhibitors such as endoxifen may be an innovative medicine for bipolar disorder (BP) patients. In this study we show for the first time the antimanic properties of endoxifen in patients with bipolar I disorder (BPD I) with current manic or mixed episode. In a double‐blind, active‐controlled study, 84 subjects with BPD I were randomly assigned to receive endoxifen (4 mg/day or 8 mg/day) or divalproex in a 2:1 ratio. Patients orally administered 4 mg/day or 8 mg/day endoxifen showed significant improvement in mania assessed by the Young Mania Rating Scale as early as 4 days. The effect remained significant throughout the 21‐day period. At study end point, response rates were 44.44% and 64.29% at 4 mg/day and 8 mg/day of endoxifen treatment, respectively. Thus, endoxifen has been shown as a promising novel antimanic or mood stabilizing agent.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ PKC inhibitors are new compounds for the treatment of bipolar I disorder and mood‐stabilizing agents.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ The study addressed the efficacy and safety of endoxifen at two doses in the treatment of patients with bipolar disorder I.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
✓ This prospective clinical trial demonstrated that endoxifen, a protein kinase C inhibitor, acts rapidly and demonstrated for the first time an antimanic activity in patients with bipolar disorder I. Endoxifen was well tolerated by patients. Furthermore, the endoxifen amount required for the antimanic activity is 125–250‐fold less than divalproex (active‐control), a commonly used drug for the treatment of this disease.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ This is the first clinical study to elucidate the safety and antimanic effects of endoxifen in patients with bipolar I disorder. These findings on endoxifen deserve to be studied in a phase III trial.
The protein kinase C (PKC) is a family of serine/threonine kinases, which are known to play a vital role in cell signaling pathways. It regulates multiple neuronal processes implicated in mood regulation.1, 2 In current clinical practice, antidepressants and mood stabilizers have been shown to modulate the PKC pathway. Disrupted PKC activity has been found both in postmortem brains and platelet from patients with mood disorders. Accumulating evidence suggests an imbalance of the PKC signaling system in mood disorders. Thus, PKC may be a novel molecular target for the development of innovative medicine for bipolar disorder (BP). This is a chronic, debilitating illness that affects 0.4% to 4% of the US population.3, 4 The causes of BP are still unknown and no agent has been specifically developed on the basis of an understanding of the pathophysiology of the illness or mechanism of action for effective treatments. However, several drugs have been approved such as lithium, valproate, carbamazepine, and atypical antipsychotics for the treatment of acute bipolar mania.5 While these drugs have provided relief for many individuals with BP, significant issues with tolerability and efficacy still remain. The clinicians, for example, may find themselves in situations in which better‐tolerated agents are less effective, and vice versa. Also, the adherence to the treatment is affected by adverse effects such as sedation and weight gain. Therefore, there is an urgent need to develop novel and more effective treatments for BP.
Two placebo‐controlled, randomized trials of a PKC inhibitor drug, tamoxifen, were carried out independently.6, 7 These studies indicated that tamoxifen has strong antimanic properties both in men and women. Tamoxifen is extensively metabolized predominantly by the cytochrome P450s (CYP450) system to several primary and secondary metabolites including active metabolite endoxifen.8 We reported earlier the endoxifen (Figure 1) synthesis and its superior inhibitory PKC activity compared with tamoxifen. Endoxifen showed fourfold higher potency in inhibiting the PKC activity compared with tamoxifen.9 Endoxifen, being the active metabolite of tamoxifen, is not dependent on drug‐metabolizing enzymes such as CYP450 and especially major polymorphic isozyme CYP2D6. In addition, the avoidance of CYP2D6‐mediated drug metabolism represents an early Go / No Go decision criteria in central nervous system (CNS) drug discovery efforts because of its potential for variable patient safety and drug efficacy arising from genetic polymorphisms and its involvement in the metabolism of many existing drugs.
Figure 1.
Chemical structure of endoxifen.
To the best of our knowledge, this is the first report that describes the findings of a randomized, double‐blind, active‐controlled clinical trial to evaluate efficacy and safety of endoxifen in BPD I patients with current manic or mixed episode.
METHODS
Conduct of the clinical study
Written informed consent was obtained from all patients before enrollment. The clinical study was initiated as per the protocol after approval from the Independent Ethics Committee or Institutional Review Board. In addition, the study was conducted as per the International Conference on Harmonization Good Clinical Practice based on the basic principles of Good Laboratory Practice, Indian Council of Medical Research Guidelines for Biomedical Research on human subjects, and the Declaration of Helsinki (Seoul, 2008) on the rights of research participants. Safety assessments were based on adverse event (AE) reporting, laboratory testing, daily physical examination, recording of vital signs, and electrocardiograms.
Patients
Inclusion criteria: Male and female patients, 18 to 65 (both inclusive) years of age willing to give written informed consent along with at least one first‐degree relative / legally acceptable representative (LAR), who were capable of understanding the purposes and risks of the trial and had given written informed consent, which included compliance with the study requirements and restrictions listed in the consent form, patients who were diagnosed of BPD I and having displayed an acute manic or mixed episode (with or without psychotic features) according to DSM‐IV‐TR as judged by the investigator. The patients were previously treated with at least one of the drugs, viz., lithium, valproate, carbamazepine, or an atypical (except for clozapine) or typical antipsychotic at some time during the course of their bipolar illness. Their last intake of the medication(s) for BPD I was within 2–7 days prior to randomization, depending on the individual drug's plasma half‐life. The male patients of child‐begetting potential and female patients of child‐bearing potential, who were practicing adequate contraception, were enrolled in the study. Female patients were not pregnant or lactating and had a negative serum pregnancy test at the time of screening and negative urine pregnancy test at the time of randomization. The patients had a Young Mania Rating Scale (YMRS) total score of ≥20 and a score of ≥4 on the Clinical Global Impressions – Severity of Illness (CGI‐S) Scale at the time of screening and at randomization (baseline).
Exclusion criteria: Newly diagnosed and not having any suitable treatment exposure in the past for their bipolar mood disorder; clinically significant suicidal or homicidal ideation; serious, unstable illnesses including hepatic, renal, gastroenterologic, respiratory, cardiovascular (including ischemic heart disease), endocrinologic, neurologic, immunologic, or hematologic disease as per history and medical examination.
Methodology
This was a randomized, double‐blind, double‐dummy, active‐controlled, two‐arm, 3‐week active treatment, two‐stage parallel assignment, inpatient study. The study was coordinated by a Clinical Research Organization. This multisite study compared fixed‐doses of endoxifen (4 mg or 8 mg) and extended release tablets of divalproex 1,000 mg for the treatment of BPD I. Before randomization to the 3‐week double‐blind treatment phase, the subjects underwent a screening and a washout period of 1 week after signing a written informed consent with at least one first‐degree relative / LAR staying with the patient. The schema of this trial is shown in Figure 2.
Figure 2.

Study design.
Patients entering the study were randomly assigned 2:1 (endoxifen:divalproex) in randomized and double‐blinded fashion for 21 days. Endoxifen was given orally as enteric coated tablets at two fixed doses (4 mg/day or 8 mg/day) to enhance the bioavailability.
Blinding
The actual treatment given to individual patients was determined by a randomization schedule prepared at Lambda Therapeutic Research, India. The randomization schedule was generated using SAS v. 9.3 (Cary, NC) by an unblinded biostatistician before commencement of the study.
The pharmacy custodian verified the randomization schedule for correctness. The randomization schedule was kept under controlled access, which was handled only by the pharmacy custodian or designate, until the blind was broken. A control copy of the randomization schedule was provided as per the access level ensuring the blind. Study medication for each individual patient was prepackaged and prenumbered and provided to each participating site according to the randomization schedule. A designated pharmacy employee dispensed the study medication serially at the site.
The blind was to be broken only if knowledge of the treatment regimen assisted medical management of the patient in an acute emergency. The date, time, and reason for breaking the code had to be documented in the source documents and on the Case Report Form, and a serious adverse event was to be reported if the event met the serious criteria. On breaking the treatment randomization code in case of an emergency, the patient was to be withdrawn from the study.
Concomitant medications
All psychotropic medications except benzodiazepines (lorazepam/diazepam only) were discontinued at least 2 days before randomization. Benzodiazepines (lorazepam/diazepam only) (up to 5 mg/day, preferably in divided doses) were allowed as adjunctive medication as needed at the discretion of the investigator from 2 days prior to randomization but not beyond the first 10 days of investigational medicinal product dosing. Benzodiazepines were avoided within 12 h of scheduled mania ratings. The usage of two benzodiazepines was permitted to reduce undue excitement by using these adjuvants in an appropriate manner while avoiding efficacy or safety overlap with the endoxifen or divalproex. Several reports have been published about evaluation of mood‐stabilizing drugs where concomitant intake of benzodiazepines was permitted during the study.10, 11, 12
Pharmacokinetic assessments
Predose PK samples were collected. The treatment was started by orally administering investigational medications from Day 0 onwards. Four mL blood for the Day 0 sample and 2 mL blood samples on Day 4, Day 7, Day 14, and Day 20 were collected from patients just before dosing the investigational medications at the clinic/hospital site. The last sample of 2 mL was collected on Day 49 when the patient visited the site for posttreatment safety follow‐up. A total of six samples were collected per patient for PK assessment.
The blood samples were processed to separate plasma and blood cells. The plasma samples were then frozen at –20°C until use. Plasma samples of patients were analyzed using a validated LC‐MS/MS method13, 14 for endoxifen at the bioanalytical facility of Lambda Therapeutic Research Ltd., Ahmedabad, India. The concentration data were tabulated using WinNonlin Professional Software v. 5.3 (Pharsight, Princeton, NJ).
The area under the curve (AUC) was estimated using Excel with the linear trapezoidal method. For a given time interval (t1 – t2), the AUC were calculated with the following equation:
Where C is the plasma concentration of endoxifen at a given time (t) of each patient. In this case, C1 = 0, t1 = 0. The dose–response relationship between mean YMRS score (change from baseline) and mean corresponding AUC at a certain time point was also analyzed using Excel and a linear regression that was illustrated in a semilogarithmic graph.
Efficacy and safety assessments
All psychiatrists participating in this double‐blind trial at different sites were well trained and had experience in using Diagnostic & Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV). All baseline scores were recorded by due administration of all defined scales. Efficacy and safety assessments were done based on evaluation parameters on Days 4, 7, 14, and 21 (end of treatment). Safety blood Clinical Global Impression of Improvement (CGI‐I) was collected on Day 21 / discharge day from hospital. The primary end point was defined as the proportion of responders in each arm on Day 21 based on change in the Young Mania Rating Scale (YMRS) total score (≥50% decrease from baseline). Secondary end points were mean change from baseline to the end of treatment in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score, Clinical Global Impressions–Severity of Illness Scale (CGI‐S) score, and Columbia–Suicide Severity Rating Scale (C‐SSRS) score.
All statistical and safety analysis was performed using SAS v. 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics and study design
A total of 84 patients who met the requirements for the study and provided informed written consent for participation were enrolled in this trial. The mean ± SD for age of these 84 patients was 36.8 ± 11.9 years. The study was conducted at various hospitals in India with a racial makeup of 100% Asian.
The study was conducted in two stages (Figure 2). A total of 42 patients were randomized in each stage of the study across two arms in a 2:1 ratio. In stage I, 27 (instead of 28) and 15 (instead of 14) patients were randomized to endoxifen 4 mg and divalproex 1,000 mg arms, respectively. This happened due to site‐specific randomization and the double‐blind, double‐dummy design of the study. In stage II, 28 patients were randomized to endoxifen 8 mg arm and 14 patients to divalproex 1,000 mg arm. A total of 84 patients were dosed in the trial (42 patients in each stage). Of these 84 patients, 6 patients were withdrawn/discontinued from the study. Two patients were withdrawn from the trial due to adverse event. Another two patients withdrew their consent from the trial and one patient lost to follow‐up.
Thus, 78 patients completed the clinical phase of the study at five sites. The plasma samples of all 84 patients were collected.
Efficacy
The mean change in the YMRS total score from baseline was used to evaluate the effectiveness of antimanic medications. Using the analysis of variance (ANOVA) model of SAS, the total YMRS score at baseline and end of trial assessment was found to be statistically significant (P < 0.0001) across the sites. The mean change in YMRS score from baseline was found to be –12.65, –16.21, and –16.38 in endoxifen 4 mg, 8 mg, and divalproex 1,000 mg arm, respectively (Figure 3 a). This percent change from baseline to each subsequent assessment in total YMRS score is shown in Figure 3 b. The mean change in the intent‐to‐treat (ITT) population was not statistically significant among different groups.
Figure 3.
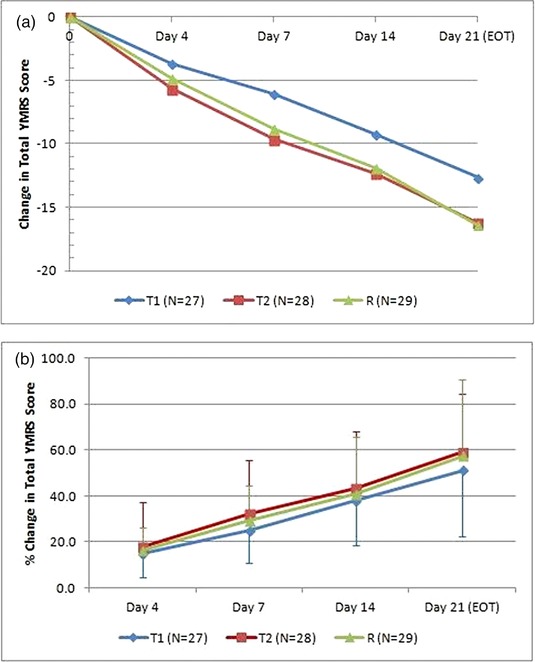
(a) Mean change in baseline from total YMRS score. (b) Percent change from the baseline to each subsequent assessment in the total YMRS score. T1 = endoxifen 4 mg/day; T2 = endoxifen 8 mg/day; R = divaloproex 1,000 mg/day.
The percent change in the YMRS total score from baseline to end of the treatment was 54.68% and 55.36% in the endoxifen 4 mg and divalproex arm, respectively, in the per protocol (PP) data analysis in stage I. In stage II, the percent change was 58.98% and 65.06% in the endoxifen 8 mg and divalproex arm, respectively. The proportion of responders based on YMRS total score for endoxifen 4 mg/day (stage I), endoxifen 8 mg/day (stage II), and divalproex 1,000 mg/day (stage I + stage II) data are presented in Table 1. Statistically, the differences among groups were not significant. The response rate with the use of lithium, valproate, and atypical antipsychotics such as olanzapine, risperidone, and paliperidone in White/Black/Asian was found to vary from 30–70%, which is comparable to this study.15, 16, 17, 18, 19
Table 1.
Proportion of responders based on YMRS Score (ITT set)
| Endoxifen | Endoxifen | Divalproex Rc | |
|---|---|---|---|
| 4 mg/day (N = 27) n (%) | 8 mg/day (N = 28) n (%) | 1000 mg/day (N = 29) n (%) | |
| Respondersa | 12 (44.44%) | 18 (64.29%) | 21 (72.41%) |
| Nonrespondersb | 15 (55.56%) | 10 (35.71%) | 8 (27.59%) |
Responders: ≥50% improvement for YMRS scores.
Nonresponders: ≤50% improvement for YMRS score.
R represents the combined data of stage I and stage II for divalproex used as reference.
Since a switch to depression remains a concern in all treatments of bipolar mania, depressive symptoms were assessed with the MADRS score throughout the trial. The result demonstrated the effectiveness of endoxifen 4 mg and 8 mg in alleviating depressive symptoms of mixed episode of BPD similar to that of divalproex.20, 21, 22
The CGI‐S score at baseline was comparable in two arms of stage I and II, reflecting that most patients had marked or moderate manic symptoms. At the end of the 3‐week study treatment, the CGI‐S scores of most patients in all arms showed reductions indicative of no illness to mild illness states. Similar results were also observed in the CGI‐I score in all arms. In the CGI‐I, the score of a majority of the patients shifted to “very much improved” and “much improved” categories in all arms. The CGI‐efficacy index is the scale that evaluates combined safety and efficacy of the medication. At the end of 3 weeks of treatment, this index was similar among the endoxifen 4 mg or 8 mg and divalproex arm. Similar results have been obtained with the use of other antimanic medications.6, 7, 23, 24, 25
It is concluded that endoxifen at 8 mg appears to be more efficacious than endoxifen 4 mg and as effective as divalproex sodium extended release 1,000 mg in the treatment of BPD I with concurrent manic or mixed episode.
Safety and tolerability
The safety analysis included all 84 patients who received at least one dose of the study medication. A total of 63 adverse events (AEs) were reported by 27 patients during the conduct of this study. Out of 63, 59 were mild, 2 were moderate, and 2 were severe in intensity. In all, 33 AEs were reported by 41.38% (n = 12) of 29 patients in the divalproex arm and 19 AEs were reported by 29.63% (n = 8) of 27 patients in the endoxifen 4 mg arm and 11 AEs were reported by 25.00% (n = 07) of 28 patients in the endoxifen 8 mg arm. There were two patients who left the study in the 4 mg/day group due to adverse effect, three patients withdrew consent, and one patient was lost to follow‐up. No patients left the study in the 8 mg/day endoxifen or in the divalproex arm. There were no deaths, other significant AEs, or serious AEs reported during the conduct of the study. Overall, endoxifen was well tolerated.
The most common AEs reported in the trial were psychiatric in nature. Insomnia and nausea were the most common AEs in the divalproex arm (Table 2). Headache was reported in two patients at endoxifen 4 mg, three patients at endoxifen 8 mg, and three patients in the divalproex 1,000 mg arm. Gastrointestinal disorders like dyspepsia, nausea, and vomiting were found in four, one, and seven patients in the endoxifen 4 mg, endoxifen 8 mg, and divalproex arm, respectively. Only two AEs were found to be severe in the 4 mg endoxifen group and one AE was moderate in the divaloproex group; all other AEs were mild in nature. The two severe AEs observed in the 4 mg endoxifen group were related to delusions in patients. Based on the above results, it is concluded that endoxifen 4 mg and 8 mg was well tolerated and safe as compared with divaloproex.
Table 2.
Incidence of treatment related adverse events in BPD I patients
| Endoxifen, 4 mg (N = 27) | Endoxifen, 8 mg (N = 28) | Divalproex, 1 gm (N = 28) | ||||
|---|---|---|---|---|---|---|
| Adverse event | N | % | N | % | N | % |
| Patients with at least one AE | 8 | 29.63 | 7 | 25 | 12 | 41.38 |
| Nausea | 1 | 3.7 | 1 | 3.57 | 5 | 17.24 |
| Headache | 2 | 7.4 | 3 | 10.71 | 3 | 10.34 |
| Insomnia | 1 | 3.7 | 2 | 7.14 | 5 | 17.24 |
Pharmacokinetics and pharmacokinetic‐pharmacodynamic (PK‐PD) relationship of endoxifen
The endoxifen plasma trough level concentration–time profile is shown in Figure 4 a for both the 4 mg and 8 mg doses. The mean trough concentration (Ctrough) of endoxifen (4 mg/day) for Day 14 and Day 20 were 28 ng/mL and 29 ng/mL, respectively. Similarly, the mean Ctrough of endoxifen (8 mg/day) for Day 14 and Day 20 were found to be 58 ng/mL and 59 ng/mL, respectively. This showed that there is no significant difference when average Ctrough concentrations of endoxifen were compared for Day 14 and Day 20, indicating that the steady‐state of endoxifen was achieved within 14 days of endoxifen administration. The mean exposure (AUC, day.ng/mL) values on Day 14 (AUC0–14 days) and Day 20 (AUC0–20 days) for endoxifen at the 4 mg/day dose were 203.01 and 282.82, respectively, whereas for endoxifen at 8 mg/day doses were 399.58 and 586.82, respectively. This observation demonstrates that the doubling of dose from 4 mg to 8 mg once daily results in ∼2‐fold increase in plasma AUC. We have shown in our earlier single‐dose study13 that AUC and maximum plasma concentration (Cmax) for endoxifen increased in proportion to the dose from 0.5–4 mg. The mean terminal elimination half‐life (t½) of endoxifen after a single dose of 4 mg was 52.05 h. Additionally, we also showed in a multiple‐dose study that at steady‐state rate endoxifen displays dose‐proportional PK with respect to Cmax and AUC to the dose ranging from 2–8 mg. The data obtained in the current study reflect a similar profile.
Figure 4.
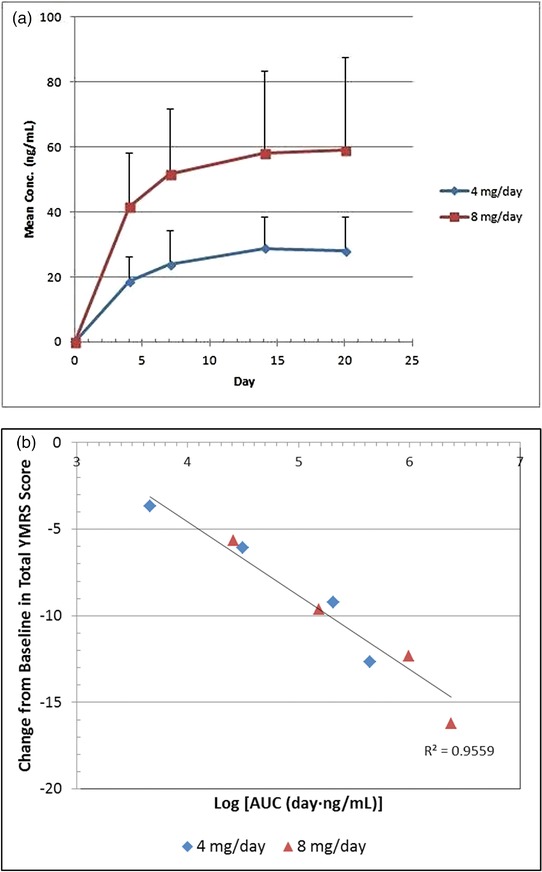
(a) Plasma concentration profile of endoxifen at two doses of 4 mg/day and 8 mg/day. (b) Pharmacokinetic–pharmacodynamic relationship. AUC values were calculated from plasma concentration–time curve of endoxifen. Each AUC value represents the average of those from 24 (4 mg/day group) or 28 (8 mg/day group) patients.
In order to investigate a potential relationship between exposure levels of endoxifen and its antimanic effect, an exploratory exposure–response analysis for endoxifen was performed using data from the exposure (AUC) and PD end point of antimanic activity (YMRS total score) data. Such exposure–response analysis is increasingly recognized as necessary to optimize the benefits of small drug molecules such as endoxifen for patients. As shown in Figure 4 b, the antimanic effect (response) of endoxifen monotherapy is manifested as the exposure is increased. Regression analysis of YMRS total score and AUC indicates that the relationship was linear, with R2 = 0.9559.
DISCUSSION
This is the first controlled clinical trial that demonstrates that endoxifen, when used alone, significantly (P < 0.0001) reduces the manic symptoms associated with BPD I. Endoxifen, at two fixed doses, produced significantly greater improvements on the Young Mania Rating Scale at almost every treatment evaluation. The evidence for efficacy and safety of endoxifen based on the controlled data observed in this trial provide an alternate treatment option to the physician and patients. The efficacy of endoxifen observed in this trial was not significantly different from divalproex, a “the first‐line” pharmacological treatment for patients with mania. The duration of this trial (21 days) may be insufficient to adequately make clinical decisions regarding the efficacy and safety of long‐term endoxifen treatment in patients with BPD I. Particularly, the effects of endoxifen on weight gain and metabolic parameters should be interpreted with caution.
As shown in Table 3, the magnitude of the endoxifen effect at 4 mg/day or 8 mg/day compares favorably with the magnitude of effect shown on the YMRS in recently conducted 3‐week monotherapy trials of BPD I with tamoxifen, aripiprazole, and olanzapine.6, 7, 22, 23, 24, 25 Similar to other BPD I studies, a 3‐week study duration was sufficient to assess endoxifen efficacy in the treatment of BPD I. Due to its PKC inhibitory as well as the antiestrogenic activity of endoxifen, the maximal utilization of this drug may be restricted. However, longer studies are needed to determine the efficacy and tolerability of endoxifen. In conclusion, orally administered endoxifen at 4 mg/day or 8 mg/day in this phase II clinical trial was well tolerated and showed significant improvement in manic or mixed episodes patients.
Table 3.
Monotherapy trials of drugs for the treatment of BP
| Drug and study | Trial duration (weeks) | Baseline Young Mania Rating Scale score | Change from baseline | Onset of action (day) |
|---|---|---|---|---|
| Tamoxifen | ||||
| Zarrate et al.6 | 3 | 30 | –18.3 | 4 |
| Yildiz et al.7 | 3 | 38 | –16.6 | 4 |
| Aripiprazole | ||||
| Sach et al.22 | 3 | 29 | –12.5 | 4 |
| Keck et al.23 | 3 | 28 | –8.2 | 4 |
| Olanzapine | ||||
| Tohen et al.24 | 3 | 28 | –10.3 | 7 |
| Tohen et al.25 | 4 | 29 | –14.8 | 7 |
| Endoxifen | ||||
| 4 mg/ day | 3 | 24 | –12.65 | 4 |
| 8 mg/day | 3 | 27 | –16.22 | 4 |
| Valproate | 4 | |||
| Divalproex (1000 mg/day) | 3 | 28 | –16.38 | 4 |
Author Contributions
A.A., S.S., and I.A. wrote the article; A.A., S.S., and I.A. designed the research; T.S., M.S.R., B.S.V.P., K.K.V., B.B.C., M.P., and P.K. performed the research; A.A., S.S., I.A., M.P., P.K., R.V.S., H.B., and R.P. analyzed the data.
Conflict of Interest/Disclosure
The authors declare no conflicts of interest.
References
- 1. Abrial, E. et. al Protein Kinase C Inhibition Rescues Manic‐Like Behaviors and Hippocampal Cell Proliferation Deficits in the Sleep Deprivation Model of Mania. Int. J. Neuropsychopharmacol. 18, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abrial, E. , Lucas, G. , Scama, H. , Haddjeri, N. , Lambas‐Senas, L. A role for the PKC signaling systems in pathophysiology and treatment of mood disorders: involvement of a functional imbalance? See comment in PubMed Commons below Mol. Neurobiol. 44, 407–419 (2011). [DOI] [PubMed] [Google Scholar]
- 3. Kessler, R.C. et al Prevalence, severity, and comorbidity of 12 month DSM‐IV disorders in the National Comorbidity Survey Replication [published correction appears in Arch. Gen. Psychiatry. 62, 709 (2005)]. Arch. Gen. Psychiatry 62, 617–627 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merikangas, K.R. et al Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch. Gen. Psychiatry 68, 241–251 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cousins, D.A. & Young, A.H. The armamentarium of treatments for bipolar disorder. A review of literature. Int. J. Neuropsychopharmacol. 10, 411–431 (2007). [DOI] [PubMed] [Google Scholar]
- 6. Zarate, C.A. Jr. et al Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 9, 561–570 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Yildiz, A. , Guleryuz, S. , Ankerst, D.P. , Ongür, D. & Renshaw, P.F. Protein kinase C inhibition in the treatment of mania: a double‐blind, placebo‐controlled trial of tamoxifen. Arch. Gen. Psychiatry 65, 255–263 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Mürdter, T.E. et al Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin. Pharmacol. Ther. 89, 708–717 (2011). [DOI] [PubMed] [Google Scholar]
- 9. Ali, S.M. , Ahmad, A. , Syed, S. , Ahmad, M.U. , Sheikh, S. & Ahmad Imran. Endoxifen is a new potent inhibitor of PKC: a potential therapeutic agent for bipolar disorder. Bioorgan. Med. Chem. Lett. 20, 2665–2667 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Khanna, S. et al Risperidone in the treatment of acute mania. Br. J. Psychiatry 187, 229–234 (2005) [DOI] [PubMed] [Google Scholar]
- 11. Gary S., Sachs et al Cariprazine in the treatment of acute mania in bipolar I disorder: A double‐blind, placebo‐controlled, phase III trial. J. Affect. Disord. 174, 296–302 (2015) [DOI] [PubMed] [Google Scholar]
- 12. Loebel, A. et al Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double‐blind, placebo‐controlled study. Am. J. Psychiatry 171, 160–168 (2014) [DOI] [PubMed] [Google Scholar]
- 13. Ahmad, A. et al Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin. Pharmacol. Ther. 88, 814–817 (2010). [DOI] [PubMed] [Google Scholar]
- 14. Ahmad, A. et al Endoxifen for breast cancer: Multiple‐dose, dose‐escalation study characterizing pharmacokinetics and safety in metastatic breast cancer patients. J. Clin. Oncol. 30, (suppl; abstr 3089) (2012). [Google Scholar]
- 15. Smulevich, A.B. , Khanna, S. , Eerdekens, M. , Karcher, K. , Kramer, M. , Grossman, F. Acute and continuation risperidone monotherapy in bipolar mania: a 3‐week placebo‐controlled trial followed by a 9‐week double‐blind trial of risperidone and haloperidol. Eur. Neuropsychopharmacol. 15, 75–84 (2005). [DOI] [PubMed] [Google Scholar]
- 16. Yatham, L.N. , Grossman, F. , Augustyns, I. , Vieta, E. , Ravindran, A. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double‐blind, randomized controlled trial. Br. J. Psychiatry J. Ment. Sci. 182, 141–147 (2003). [DOI] [PubMed] [Google Scholar]
- 17. Sachs, G. et al Quetiapine with lithium or divaloproex for the treatment of bipolar mania: a randomized, double‐blind, placebo‐controlled study. Bipolar Disord. 6, 213–223 (2004). [DOI] [PubMed] [Google Scholar]
- 18. Berwaerts, J. , Lane, R. , Nuamah, I.F. , Lim, P. , Remmerie, B. , Hough, D.W. Paliperidone extended‐release as adjunctive therapy to lithium or valproate in the treatment of acute mania: a randomized, placebo‐controlled study. J. Affect. Disord. 29, 252–260 (2011). [DOI] [PubMed] [Google Scholar]
- 19. Vieta, E. , Mullen, J. , Brecher, M. , Paulsson, B. , Jones, M. Quetiapine monotherapy for mania associated with bipolar disorder: combined analysis of two international double‐blind, randomized, placebo‐controlled study. Curr. Res. Med. Opin. 6, 923–934 (2005). [DOI] [PubMed] [Google Scholar]
- 20. Tohen, M. et al Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially non responsive to valproate or lithium monotherapy. Arch. Gen. Psychiatry 59, 62–69 (2002). [DOI] [PubMed] [Google Scholar]
- 21. Hirschfeld, R.M. et al Rapid antimanic effect of risperidone monotherapy: a 3‐week multicenter, double‐blind, placebo‐controlled trial. Am. J. Psychiatry 6, 1057–1065 (2004). [DOI] [PubMed] [Google Scholar]
- 22. Sachs, G. et al Aripiprazole vs placebo in acute mania: results from a second study, in New Research from the 55th Institute on Psychiatric Services, Oct 29‐Nov 2, 2003. Arlington, VA: APA (2003).
- 23. Keck, P.E Jr . et al A placebo‐controlled, double‐blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am. J. Psychiatry 160, 1651–1658 (2003). [DOI] [PubMed] [Google Scholar]
- 24. Tohen, M. et al Olanzapine versus placebo in the treatment of acute mania. Am. J. Psychiatry 156, 702–709 (1999). [DOI] [PubMed] [Google Scholar]
- 25. Tohen, M. et al Efficacy of olanzapine in acute bipolar mania: a double‐blind, placebo‐controlled study. Arch. Gen. Psychiatry 57, 841–849 (2000). [DOI] [PubMed] [Google Scholar]


