Abstract
Regulatory science, a complex field which draws on science, law, and policy, is a growing discipline in medical‐related applications. Competencies help define both a discipline and the criteria to measure high‐quality learning experiences. This paper identifies competencies for regulatory science, how they were developed, and broader recommendations to enhance education and training in this burgeoning field, including a multifaceted training approach.
Keywords: regulatory science, translational science, training, competencies
Introduction
Regulatory science and translational science have shared goals to ensure that the significant investments and advances in basic science research are transformed into products that improve public health. The U.S. Food and Drug Administration (FDA) has defined regulatory science as “the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of FDA‐regulated products.” New tools and approaches to keep pace with or anticipate emerging technologies may reduce costs or increase the availability of safe and effective medical products. Because the goals of regulatory science align with those of translational science, the U.S. National Institutes of Health (NIH) and FDA have partnered on regulatory science initiatives, including a series of regulatory science research and education programs, and establishing the NIH‐FDA Joint Leadership Council to coordinate collaborative activities.1, 2, 3
The FDA has described the agency's vision and priority areas for regulatory science through its 2011 strategic plan.4 This effort was furthered by the awarding of four Centers of Excellence in Regulatory Science and Innovation (CERSIs) at academic institutions,5 which aim to promote regulatory science research, education, training, and professional development.
Regulatory science has received sustained attention from nongovernmental organizations as well. In 2011, the Institute of Medicine (IOM) hosted a workshop, Strengthening a Workforce for Innovative Regulatory Science in Therapeutics Development,6 to explore whether regulatory science represents a new discipline requiring unique educational needs and careers paths. The workshop included discussion about the need for “core competencies” as a means to define regulatory science as an academic discipline, although those specific competencies remained unidentified.
The focus of this paper is the subsequent development of competencies for regulatory science, initially by the Clinical and Translational Science Awards (CTSA) Regulatory Science Workgroup, and further refined through a 2014 workshop: Regulatory Science Core Competencies and Curricular Guidelines.
Development of Regulatory Science Competencies
Educational programs utilize competencies to define the knowledge, skills, and abilities that trainees should develop through a program. Competencies help shape curricula, aid in planning learning opportunities, and guide the application of metrics to assess the program and its components.7, 8 While the ongoing debate about the definition and scope of regulatory science along with its multidisciplinary nature make the development of competencies a challenge, it is a significant gap that must be addressed.
In 2013, the CTSA‐initiated Regulatory Science Workgroup began this process, utilizing the existing CTSA Competencies for Clinical and Translational Research as a framework.9, 10 With leadership from the University of Rochester, the Workgroup included representatives from NIH‐sponsored CTSA institutions, CERSI institutions, the FDA, and NIH. Through an iterative process, the Workgroup identified “Core Thematic Areas” (Figure 1) as a means to group related competencies. The Core Thematic Areas and associated competencies were shared with academic, industry, and government partners for additional refinement, and provided the basis for the 2014 workshop.
Figure 1.

Core Thematic Areas. These 11 areas will help shape the training experiences of biomedical researchers pursuing regulatory science. The core thematic areas are based on a subset of the FDA's regulatory science priority areas and define the areas where focused skills, knowledge and abilities can be addressed by specific competencies.
Workshop: Regulatory Science Core Competencies and Curricular Guidelines
An interdisciplinary group of experts convened in Washington, DC, on September 23, 2014, for a moderated discussion on the core competencies and curricular guidelines for regulatory science. The workshop included 36 leaders from academia (n = 14), government (n = 10), industry (n = 5), and related associations and foundations (n = 7). Represented disciplines included medicine, public health, pharmacy, pharmacology, bioengineering, clinical and translational research, regulatory science, and regulatory affairs.11, 12 The workshop focused on regulatory science as applied to medical products, including drugs, biologics, and devices. While the field of regulatory science is broad and encompasses areas such as food, cosmetics, veterinary products, and tobacco products, the focus of the working group was on biomedical products, and so these other areas were beyond the scope of discussion.
PhRMA Foundation hosted the workshop, building on its demonstrated interest in regulatory science education and training and its prior experience hosting a similar workshop for Comparative Effectiveness Research.13 The authors of this paper served on the workshop planning committee, developing the workshop concept and objectives. The workshop agenda included a panel discussion on workforce training needs among academia, industry, and government, as well as breakout group activities focused in these areas: (A) Teaching Methods and Case Studies, (B) Process Map for Curriculum Development, and (C) Career Development Pathways. In preparation for the workshop, a literature review was conducted and a landscape analysis was compiled, the latter comprising named “regulatory science” or related graduate education programs at US universities (Appendix 1).
Survey Methods and Results
Prior to the workshop, a survey was developed to query experts about the CTSA Regulatory Science Workgroup‐proposed Core Thematic Areas and related competencies. The survey asked respondents to apply a five‐point scale to rate their level of agreement with the inclusion of each competency as part of a required curriculum. Choosing “Disagree” or “Strongly Disagree” responses for any competency prompted the respondent to indicate whether the competency should be an elective component, rather than required. The overall response rate was 69% and survey data were collected and managed using the Research Electronic Data Capture tool.14
Survey respondents were from academia (55%), industry (21%), government (14%), nonprofit organizations (7%), and associations and foundations (3%). Respondents indicated engagement in regulatory science through participation in active or past research, education and training initiatives, or related mentorship. More than 70% of the respondents affirmed a need for regulatory science‐specific education and training.
The final list of 11 proposed Core Thematic Areas and the 68 related competencies are listed in Appendix 2 and were based on survey results and workshop discussion.
Panel Discussion: Training and Workforce Needs in Academia, Industry, and Government
As previously mentioned, a panel of experts from industry, government, and academia gathered during the workshop to discuss: “Clarifying Education and Workforce Training Needs in Regulatory Science.” Panelists universally agreed that experiential opportunities are a critical and central component of education and training in regulatory science. Chris Austin (National Center for Advancing Translational Science, NIH) and Michael Rosenblatt (Merck & Co., Inc.) echoed the need for hands‐on learning experiences and suggested that rotations in academia, industry, and at the FDA would be an ideal method of training to ensure a “regulatory science practitioner” fully understands the breadth of the discipline and the differences between it and regulatory affairs.
The FDA provides lengthy training curriculum for reviewers and other regulatory science staff. The typical reviewer training spans three years or more where formal courses are supplemented with guided experience, similar to an apprenticeship. This extensive training of new hires exacerbates a current and growing shortage of personnel, which significantly impacts the agency, as well as the sponsors who rely upon FDA regulatory decisions. Leslie Wheelock (FDA) suggested that regulatory science‐competent professionals who receive training prior to joining the FDA could help address the critical shortage in trained FDA personnel. Collaborative regulatory science programs linked to academic medical centers can help address these needs.15
UCSF and Stanford offer a master's degree program in bioengineering and a certificate program in regulatory affairs as a foundation for further regulatory science training.12 In describing these programs, Kathy Giacomini (UCSF) reinforced the need for the workshop, its discussion, and recommendations, as important resources for those developing new or transforming existing programs to include more robust regulatory science components.
While the panelists and other workshop participants agreed that experiential learning opportunities are critical, what is less certain (due to confidentiality issues) is the feasibility of a rotation‐based training program that includes time at the FDA. An in‐depth examination of the requirements and regulations around who may work for the agency was recommended to explore the practicality of such a program.
Key Takeaways and Recommendations
Application of the proposed regulatory science competencies
The workshop served as a means to achieve a level of agreement on the competencies, however it should not be taken as final. The nature of regulatory science and therefore regulatory science education will shift as science and scientific understanding continues to evolve. While the Core Thematic Areas do not cover all regulated products, the proposed competencies may serve as a basis for the development of competencies in other regulatory science areas. In addition, the level of training (i.e., Master of Science degree program versus certificate, etc.) will depend upon the milieu at each university.
Institutions should design and develop a program that meets the needs of its faculty and students, utilizing a process guided by these Core Thematic Areas and aligned with the institution's strategic mission and strengths. Based on workshop discussion and feedback from the survey, the following guidelines address practical issues that may arise as a regulatory science program is developed:
-
(1)
Adopt and adapt the competencies to align with institutional and program strengths. No one institution will cover the breadth of regulatory science concepts. However, several Core Thematic Areas are relevant across disciplines and should be included in every program, including regulatory science research questions, priorities and leadership, regulatory policies and process, research ethics, and communication (see Appendix 2).
-
(2)
Recruit faculty from diverse disciplines in pharmacology, pharmacy, bioinformatics, toxicology, law, and others. In addition to teaching and mentorship, faculty should be provided with opportunities to engage in regulatory science research and these efforts should be recognized in promotion and tenure decisions.
-
(3)
Apply the competencies in consideration of the global nature of regulatory science, including the roles of authorities such as FDA, international regulatory agencies, or global health agencies with regulatory authority. Recognize that the authority and requirements of any regulatory agency can be addressed in these competencies.
-
(4)
Design education and training programs with evaluation and assessment features to ensure the learning experiences effectively prepare trainees for regulatory science careers.
-
(5)
Include perspectives of all relevant stakeholders as education and training programs are developed. Relevant stakeholders include industry, regulatory agencies, clinicians, and patients.
The workshop discussion offered other ways to promote regulatory science research and education. The following themes and recommendations emerged from that discussion.
Experiential learning opportunities and team science
One of the key messages from the workshop participants was the need to provide regulatory science training with critical thinking skills necessary for team science settings, a shared priority adopted in translational science. Imparting knowledge in this way should rely on case study‐based learning strategies and experience‐based learning that can only occur outside of the classroom. Experiential learning opportunities should supplement didactic education methods, and these opportunities should be found among all key stakeholders, at all stages of the career lifecycle. The availability of internships or fellowships in academia, industry, and government are a critical component of a successful regulatory science program. Figure 2 depicts the multilateral training approach that was endorsed by workshop participants.
Figure 2.
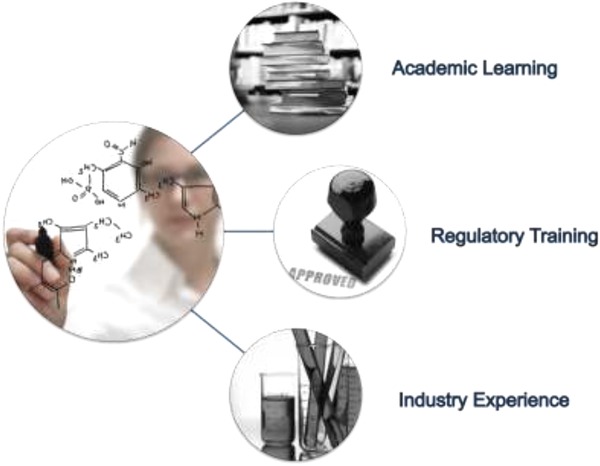
Multipart Training. These represent several components to the education and training lifecycle which would benefit the Regulatory Science Practitioner.
Consortium model
Several ideas were proposed and general agreement centered on a consortium‐based model leading to a comprehensive electronic collection of education and training resources. Since regulatory science is a multidisciplinary field, and relies on far‐ranging areas of expertise, a consortium model would allow for all regulatory science concepts to be addressed, without relying solely on any one institution. Creating alliances among potential competitors—whether in academia, industry, or government—would allow for wide distribution of curricula and opportunities as well as sharing of faculty to cover all areas of expertise. Furthermore, such a model would facilitate the portability of a curriculum. The CTSA Consortium was proposed as an academic home for regulatory science initiatives because of its established infrastructure that can readily support regulatory science education and training, although barriers within the academic centers would need to be addressed. Several nonprofit and nongovernmental organizations are also well suited to host this type of program, such as the Reagan‐Udall Foundation or PhRMA Foundation.
Funding for regulatory science research, education, and training
Another important topic of discussion focused on the practical means of financially developing and sustaining regulatory science education and training programs. Existing training grant mechanisms (e.g., TL1, KL2, R25) could be targeted to regulatory science to drive collaborative development of new regulatory science curriculum and training opportunities. NIH has taken this approach for data science training, and this could serve as a model for regulatory science as well. More broadly, the group agreed that all key stakeholders should address the question of funding, and who should sponsor and support regulatory science education and training, including through diverse business models. A formal economic analysis to measure and determine the value proposition of regulatory science education and training would demonstrate the value of a graduate degree or certificate in regulatory science to both the institution and to prospective students.
Communications
Related to the issues outlined above, workshop participants agreed that there is a missing link between the promise of regulatory science and what is understood about the discipline within the medical product development community and the public. Tied to the proposal for an economic analysis, a cohesive and deliberate communications strategy should promote the value proposition of regulatory science education and training.
Conclusion
The proposed regulatory science competencies can provide a useful guide to building or improving high‐quality education and training offerings for regulatory science. The Regulatory Science Workgroup is committed to continued engagement and implementation of the recommendations identified above. As discussed, several nonprofit organizations have aligned missions to promote regulatory science education and training and could serve as a critical mechanism to seed these efforts. Most importantly, the work achieved to date and described herein affirms the need for a coordinated and multiprong education and training initiative in regulatory science among all key stakeholders, including government, industry, academia, foundations, and other partners.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Appendix S1: Programs at a Glance: Regulatory Science Education Programs at US Academic Institutions. This summary of Regulatory Science programs was taken from coursework and curriculum details that are available online at institutions across the US. As new programs are developed, this landscape will be updated.
Appendix S2: Regulatory Science Competencies in 11 Core Thematic Areas. The 68 specific Regulatory Science Competencies are aligned with 11 broad Core Thematic Areas.
Acknowledgments
This work was supported by the PhRMA Foundation; the authors thank Eileen Cannon for her participation on the planning committee as well as her assistance with the workshop. The Regulatory Science workgroup was instrumental in the creation of these competencies and we would also like to thank the participants of the 2014 Regulatory Science Workshop. The authors thank Alicia Augustine, PhD, for assistance with the landscape research and survey design, Katie Libby for graphics assistance, as well as Robert Meyer, MD, and Carol Merchant, MD, MPH, for their review of the manuscript. Additional support came from the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health, and the Georgetown University Center of Excellence in Regulatory Science and Innovation award number U01 FD004319 from the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Food and Drug Administration.
References
- 1. Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. Jul 22 2010; 363(4): 301–304. [DOI] [PubMed] [Google Scholar]
- 2. NIH and FDA Announce Collaborative Initiative to Fast‐Track Innovations to the Public . 2010; February 24, 2010:http://www.nih.gov/news/health/feb2010/od‐24.htm. Accessed May 29, 2015.
- 3. The Common Fund Regulatory Science 2012; http://commonfund.nih.gov/regulatoryscience. Accessed May 29, 2015.
- 4. Advancing Regulatory Science at FDA: A Strategic Plan . 2011; http://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm267719.htm. Accessed May 29, 2015.
- 5. Regulatory Science Special Topics . 2011; http://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm301667.htm. Accessed May 29, 2015.
- 6. Strengthening a Workforce for Innovative Regulatory Science in Therapeutics Development . 2011; http://www.iom.edu/Reports/2011/Strengthening‐a‐Workforce‐for‐Innovative‐Regulatory‐Science‐in‐Therapeutics‐Development.aspx. Accessed May 29, 2015. [PubMed]
- 7. Diamond RM. Designing and Assessing Courses and Curricula: A Practical Guide. 3rd ed San Francisco: John Wiley & Sons, Inc; 2008. [Google Scholar]
- 8. Accreditation Council for Graduate Medical Education (ACGME) , Outcome Project. 2003; https://www.acgme.org. Accessed May 29, 2015.
- 9. CTSA Core Competencies . 2011; https://ctsacentral.org/consortium/best‐practices/335‐2/. Accessed May 29, 2015.
- 10. Meyers FJ, Begg MD, Fleming M, Merchant C. Strengthening the career development of clinical translational scientist trainees: a consensus statement of the Clinical Translational Science Award (CTSA) Research Education and Career Development Committees. Clin Transl Sci. Apr 2012; 5(2): 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Regulatory Science Workshop Agenda . 2014; http://www.phrmafoundation.org/regulatory‐science‐documents/. Accessed May 29, 2015.
- 12. Regulatory Science Workshop Participants . 2014; http://www.phrmafoundation.org/regulatory‐ science‐documents/. Accessed May 29, 2015.
- 13. Murray MD. Curricular considerations for pharmaceutical comparative effectiveness research. Pharmacoepidemiol Drug Saf. Aug 2011; 20(8): 797–804. [DOI] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Informat. Apr 2009; 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer RJ. The role of academic medical centers in advancing regulatory science. Clin Pharmacol Therapeut. May 2014; 95(5): 471–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Appendix S1: Programs at a Glance: Regulatory Science Education Programs at US Academic Institutions. This summary of Regulatory Science programs was taken from coursework and curriculum details that are available online at institutions across the US. As new programs are developed, this landscape will be updated.
Appendix S2: Regulatory Science Competencies in 11 Core Thematic Areas. The 68 specific Regulatory Science Competencies are aligned with 11 broad Core Thematic Areas.


