Abstract
RADA16‐I is a synthetic type I self‐assembling peptide nanofiber scaffold (SAPNS) which may serve as a novel biocompatible hemostatic agent. Its application in neurosurgical hemostasis, however, has not been explored. Although RADA16‐I is nontoxic and nonimmunogenic, its intrinsic acidity may potentially provoke inflammation in the surgically injured brain. We conducted an animal study to compare RADA16‐I with fibrin sealant, a commonly used agent, with the hypothesis that the former would be a comparable alternative. Using a standardized surgical brain injury model, 30 Sprague–Dawley rats were randomized into three treatment groups: RADA16‐I, fibrin sealant or gelatin sponge (control). Animals were sacrificed on day 3 and 42. Astrocytic and microglial infiltrations within the cerebral parenchyma adjacent to the operative site were significantly lower in the RADA16‐I and fibrin sealant groups than control. RADA16‐I did not cause more cellular inflammatory response despite its acidity when compared with fibrin sealant. Immunohistochemical studies showed infiltration by astrocytes and microglia into the fibrin sealant and RADA16‐I grafts, suggesting their potential uses as tissue scaffolds. RADA16‐I is a promising candidate for further translational and clinical studies that focus on its applications as a safe and effective hemostat, proregenerative nanofiber scaffold as well as drug and cell carrier.
Keywords: fibrin sealant, hemostasis, inflammation, nanomaterial, RADA16‐I, surgical brain injury, SAPNS
Introduction
Hemostasis is an important aspect of neurosurgery. An ideal hemostat should be able to stop bleeding effectively without causing additional trauma while also being conducive to healing and regeneration. In recent years, advances in nanotechnology have led to the development of novel biocompatible nanomaterials including hemostats.1 One candidate is RADA16‐I, a synthetic type I self‐assembling peptide nanofiber scaffold (SAPNS).2, 3 It consists of ionic self‐complementary oligopeptides that would undergo spontaneous assembly when exposed to physiologic conditions. The resultant hydrogel is highly porous and provides a three‐dimensional nanofiber scaffold that can potentially facilitate axonal regrowth and repopulation.4, 5, 6 Interestingly, RADA16‐I can also stop bleeding upon self‐assembly.7 Since it is nontoxic, nonimmunogenic and biodegradable, RADA16‐I is potentially fit for clincal use.8 Its application in neurosurgical hemotasis has not been explored and is the focus of this study.
RADA16‐I can be applied directly onto operative sites as an injectable liquid, akin to fibrin sealants that are already widely used in clinical practices. But RADA16‐I is comparatively more acidic (pH < 4) and there is a concern that this may provoke inflammation in neural tissue.9 We therefore conducted an animal study to compare RADA16‐I with a conventional fibrin sealant, with the hypothesis that RADA16‐I would not cause excessive cellular inflammatory responses. Our aim was to provide preclinical evidence to support and inform future translational studies on nanopeptide hemostats.
Materials and Methods
Animal model
We used a cortical resection model that has been previously described by our group.10 Adult Sprague–Dawley (SD) rats (220–250 g) were anesthetized with 80 mg/kg ketamine and xylazine (10 mg/kg). The animal head was fixed on a stereotactic frame and a right frontal craniotomy was made. After dura opening, a 1 mm × 2 mm × 2 mm block of cortical tissue, with its postereomedial corner being at 1 mm lateral and anterior to the bregma, was resected from the right frontal cortex. Hemostasis was achieved by applying (i) RADA16‐I (n = 10); (ii) fibrin sealant (n = 10); (iii) gelatin sponge (n = 10) into the cavity. (Figure 1 ) The latter served as the control. The dura was closed with 10–0 suture (EthilonTM Nylon Suture, Sommerville, NJ, USA) and reinforced with a dural substitute (DURAFOAMTM, Raynham, MA, USA), followed by skin closure with 4–0 suture (EthilonTM). The sample size was calculated to be n = 5 per group per time‐point, provided by a mean percentage of cellular infiltration of 10% in the control, an effect size of 20%, a power of 0.8, and a p value of 0.05.
Figure 1.
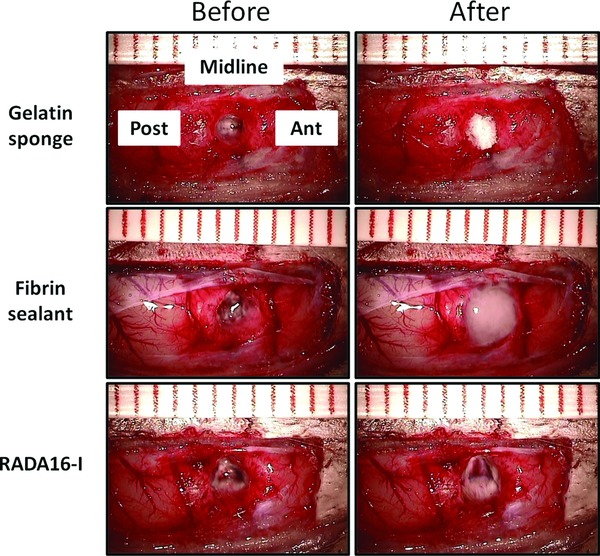
Intraoperative photographs showing the cortical resection cavities before (left column) and after (right column) the applications of gelatin sponge, fibrin sealant and RADA16‐I. The cavities in the left column were temporarily filled with normal saline for clearer illustration. Ant, anterior; Post, posterior.
Animals were allowed to survive until postoperative day 3 and day 42 (n = 5 per group per time‐point) when they were sacrificed with transcardial perfusion using 4% paraformaldehyde in 0.1 M PBS (pH 7.4) after an overdose of intraperitoneal pentobarbital. The animal brains were postfixed with 4% paraformaldehyde, cryoprotected in 30% sucrose overnight at 4°C, and embedded in optimum cutting temperature compound (OCT; Thermo Scientific, Waltham, MA, USA). Horizontal cryosections of 25 μm were mounted onto gelatin‐coated slides and stored at −20°C with cryoprotectant (30% Ethylene glycol, 30% sucrose in 0.04 M PB).
Surgical hemostats
RADA16‐I solution (BDTM PuraMatrixTM Peptide Hydrogel, BD Biosciences, MA, USA) was prepared by dissolving 10 mg of the powder in 1 mL of autoclaved Milli‐Q water (Millipore Corp., Billerica, MA, USA) in eppendorf tubes as previously described.11 The 1% wt/vol solution was sonicated for 5 minutes, filtered and stored until use. Each animal in the RADA16‐I group received 20 μL of it applied with a micropipette. The fibrin sealant (TISSEEL®, Baxter, Deerfield, IL, USA) was commercially available as prefilled double‐chamber syringes that contained human fibrinogen, thrombin, calcium chloride and synthetic antifibrinolytic. When injected together, they combined to achieve hemostasis. Gelatin sponge (Gelfoam®, Pfizer, Pharmacia & Upjohn Co., NY, USA) was cut into 1 mm × 2 mm × 2 mm pieces and inserted into the brain resection cavity to achieve hemostasis in the control group.
Immunohistochemistry
Frozen tissue sections were dried at room temperature for 30 minutes, washed, and rehydrated with 0.01 M PBS for 10 minutes, and examined with haematoxylin and eosin (H&E) staining. For immunohistochemical studies, sections were incubated with 1% hydrogen peroxide in 0.01 M PBS (pH 7.4) for 10 minutes at room temperature for endogenous peroxidase blockade, and then with 1% bovine serum albumin, 10% normal goat serum and 0.3% Triton X‐100 in PBS for 1 hour to block nonspecific antibody binding. Primary antibodies, including mouse anti‐GFAP (1:300; Cell Signaling Technology, Danvers, MA, USA) for astrocytes and rabbit anti‐IBA1 (1:500; Wako, Osaka, Japan) for microglia, were applied overnight at 4°C after washing with PBS for 5 minutes. The slides were washed and incubated with fluorescent Alexa Fluor® 555 goat antimouse (1:400; Abcam, Cambridge, England) or Alexa Fluor® 488 goat antirabbit secondary antibodies (1:500; Invitrogen, Grand Island, NY, USA) for 2 hours at room temperature. Cell nuclei were counterstained with 4′,6‐diamidino‐2‐phenylin‐dole (DAPI; Sigma, Sigma‐Aldrich, St. Louis, MO, USA). Images were captured with a Carl Zeiss Axioport microscope equipped with SPOT Advanced Imaging software. Positive reactivities were quantified on four consecutive sections per brain. Six images (200 μm × 200 μm) were randomly selected and the percentage of positive reactivities was calculated using the following formula: A p/A f × 100, where A p is the total area of positive reactivities, and A f is area of the field.
Statistical analysis
GraphPad Prism (version 5.0, GraphPad) software was used for statistical analysis. All results were presented by means ± standard deviation (SD). The difference between groups will be analyzed by two‐tailed, one‐way analysis of variance (ANOVA) with post hoc test. The alpha level was set at 0.05 for statistical significance.
Results
All three treatments could stop bleeding upon the first application. (Figure 1 , right column) Figure 2 A shows the macroscopic appearance of harvested brains. Residual hematomas were observed on postoperative day 3. These resolved by day 42, when the brain resection cavities became clearly identifiable in all groups. Residual hemostatic agents were identified microscopically within the cavities. (Figure 2 B) Fibrin sealant and RADA16‐I, but not gelatin sponge, were heavily infiltrated by astrocytes and microglia. (Figure 2 C)
Figure 2.
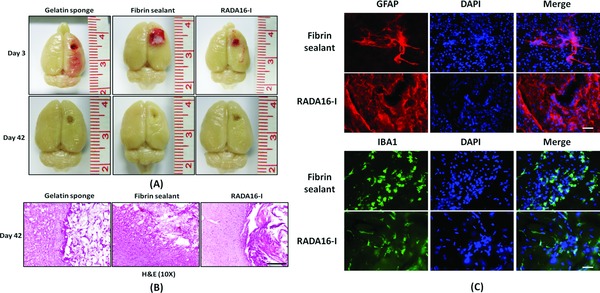
(A) Macroscopic appearances of representative brain specimens on day 3 and 42; (B) H&E examination of the boundary zone between the brain and the lesion cavities (*) on day 42; (C) Immunohistochemical studies of the fibrin sealant and RADA16‐I in situ, showing infiltration by microglia and astrocytes on day 42 (40×). Scale bar: B = 50 mm, C = 20 μm.
The cerebral parenchyma surrounding the resection cavity was studied for cellular inflammatory response. Overall, astrocytic infiltration was observed on day 3, and became more pronounced on day 42. When compared with control, the degree of astrocytosis was significantly less after treatments with fibrin sealant and RADA16‐I at both time‐points (both p < 0.001). No significant difference was found between the last two treatments on day 3 (p = 0.089) or day 42 (p = 0.715). (Figure 3 )
Figure 3.
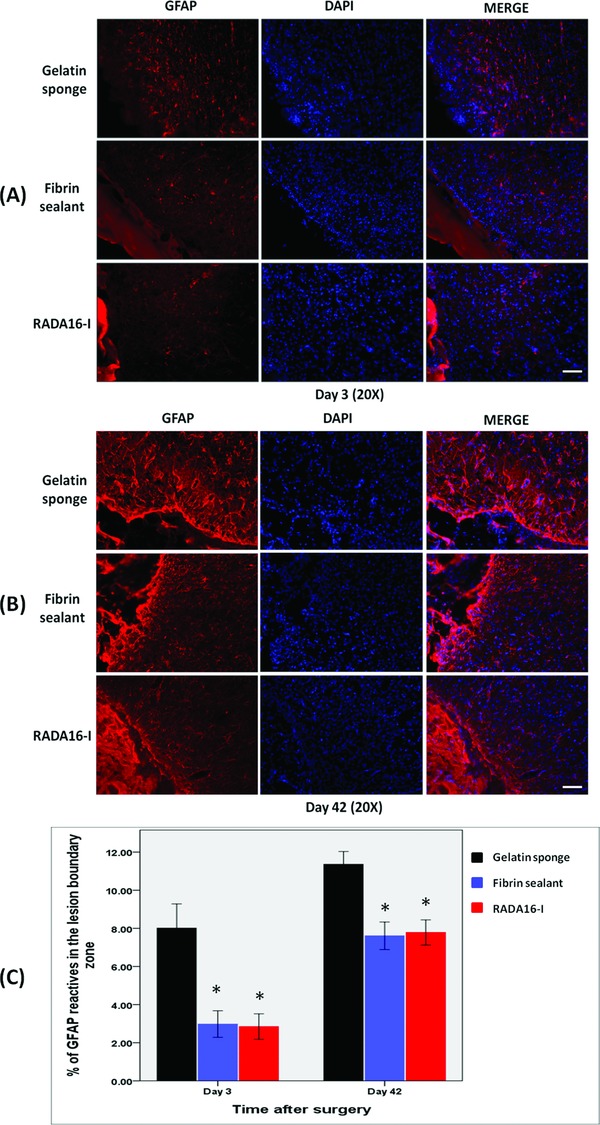
Immunohistochemical studies showing astrocyte infiltration in the cerebral parenchyma adjacent to the lesion cavity on (A) day 3 and (B) day 42. (C) The degree of infiltration was more extensive after treatment with gelatin sponge than with fibrin sealant or RADA16‐I. *p < 0.001. Scale bar: A–B = 100 μm.
In terms of microglia infiltration, fibrin sealant caused significantly less response than control and RADA16‐I on day 3 (p = 0.008). There was no difference between the last two groups (p = 0.474). By day 42, both fibrin sealant and RADA16‐I caused significantly less reaction than control (both p < 0.001). There was no difference between the first two groups (p = 0.461). (Figure 4 )
Figure 4.
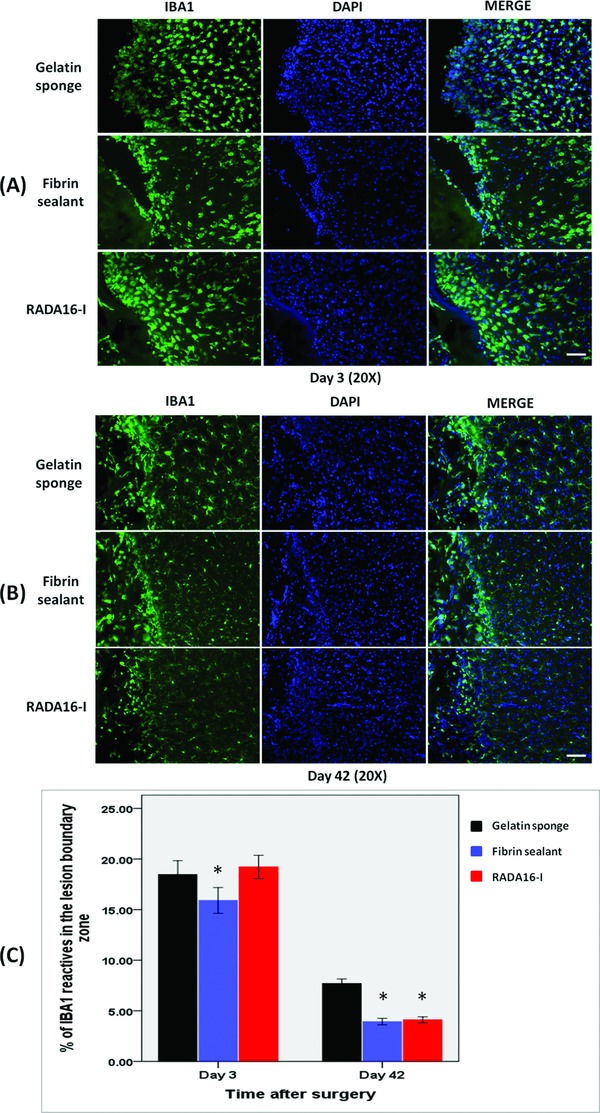
Immunohistochemical studies showing microglial infiltration in the cerebral parenchyma adjacent to the lesion cavity on (A) day 3 and (B) day 42. (C) The degree of infiltration was more extensive after treatment with gelatin sponge than with fibrin sealant or RADA16‐I. *p < 0.001. Scale bar: A–B = 100 μm.
Discussion
The name RADA16‐I refers to its constituent oligopeptides, each made up of 16 alternating hydrophobic and hydrophilic amino acids in the repeated sequence of Arg–Ala–Asp–Ala (RADA).8 When exposed to physiologic media, the electrostatic repulsion between the oligopeptides is neutralized, resulting in the formation of complementary ionic bonds. This leads to a polymerization‐like process that forms a hydrogel with nanofibers of 50–200 nm in diameters. By serendipity, this gelation process was also found to achieve hemostasis. Subsequent works showed that RADA16‐I could stop bleeding from the brain within 8.4 seconds, compared with 227.0 seconds with saline‐treatment. The underlying mechanism was incompletely understood and was probably unrelated to thrombus formation or platelet aggregation.7, 12, 13
Fibrin sealants are widely used as topical hemostats. They cause little tissue reactions,14 and is safe on neural tissue.15 Modern commercial products have eliminated bovine‐derived components, but they still carry clotting factors obtained from human plasma pool, hence the potential risks of disease transmission. This contrasts with RADA16‐I, which is entirely synthetic and made up of naturally occurring and reuseable amino acids. No prion‐like substances in animals receiving RADA16‐I have been identified.11 Its low acidity, however, is a potential drawback. Guo et al. used RADA16‐I to treat experimental spinal cord and brain injuries, and reported pronounced inflammatory response in the former.9, 10 This study showed that RADA16‐I indeed did not cause excessive inflammation in the brain when compared with fibrin sealant, and may represent a novel class of surgical hemostats for further translational studies and possibly clinical use.
RADA16‐I has other advantages. In promoting healing and regeneration, it can carry drugs16 and transplantable cells.17 Preclinical studies have already demonstrated its efficacy in various neurologic conditions.9, 10, 11, 18 We found extensive glial cell infiltration within RADA16‐I for up to 6 weeks after surgery, lending further support to its role as a tissue scaffold in the treatment of surgical brain injury. The higher porosity offered by the nanostructure of SAPNS may also explain why microglial infiltration was more pronounced in SAPNS than in fibrin sealant on postoperative day 3. Like other SAPNSs, RADA16‐I is subject to tailored designs and modifications (e.g., peptide length and sequence), and future research effort will continue to provide new candidates with improved hemostatic and regenerative properties.19, 20
This pilot study has several limitations. First, we focused on cellular inflammatory response from two cell types only. Other indicators such as proinflammatory cytokine expression and brain edema were not addressed. Second, the experimental model produced cell death mainly by necrosis; apoptosis was minimal and was not formally assessed. Similarly, functional loss was also minimal. Thirdly, RADA16‐I at other concentrations, hence of different acidity and hemostatic properties, was not tested. This could have important impact on its clinical applications.
Conclusion
When applied as a topical hemostat to the surgically injured brain, RADA16‐I was associated with a similar degree of cellular inflammatory response as fibrin sealant despite the former's intrinsic acidity. Given its many superior properties as a synthetic nanomaterial, RADA16‐I deserves to be further investigated as an alternative to conventional neurosurgical hemostats. Future studies may investigate the effects of different concentrations of RADA16‐I, other parameters of surgical brain injury, axonal regrowth and functional recovery.
Conflict of Interest
None declared.
Acknowledgments
We thank Professor George Tsao, Ms Connie W.M. Wong, and Dr Wen Li of the Department of Anatomy, as well as Dr. C.F. Fung and Jensen Y.T. To of the Department of Surgery, The University of Hong Kong, for their support and guidance. We are also grateful for the support from the Health and Medical Research Fund (project no: 02132186), Food and Health Bureau, The Government of the HKSAR.
References
- 1. Webber MJ, Kessler JA, Stupp SI. Emerging peptide nanomedicine to regenerate tissues and organs. J Intern Med. 2010; 267: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cormier AR, Ruiz‐Orta C, Alamo RG, Paravastu AK. Solid state self‐assembly mechanism of RADA16‐I designer peptide. Biomacromolecules. 2012; 13: 1794–1804. [DOI] [PubMed] [Google Scholar]
- 3. Zhang S, Marini DM, Hwang W, Santoso S. Design of nanostructured biological materials through self‐assembly of peptides and proteins. Curr Opin Chem Biol. 2002; 6: 865–871. [DOI] [PubMed] [Google Scholar]
- 4. Cigognini D, Satta A, Colleoni B, Silva D, Donega M, Antonini S, Gelain F. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self‐assembling scaffold. PloS One. 2011; 6: e19782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cunha C, Panseri S, Villa O, Silva D, Gelain F. 3D culture of adult mouse neural stem cells within functionalized self‐assembling peptide scaffolds. Int J Nanomed. 2011; 6: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellis‐Behnke RG, Liang YX, Guo J, Tay DK, Schneider GE, Teather LA, Wu W, So KF. Forever young: how to control the elongation, differentiation, and proliferation of cells using nanotechnology. Cell Transpl. 2009; 18: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 7. Ellis‐Behnke RG, Liang YX, Tay DK, Kau PW, Schneider GE, Zhang S, Wu W, So KF. Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine. 2006; 2: 207–215. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self‐complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA. 1993; 90: 3334–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo J, Su H, Zeng Y, Liang YX, Wong WM, Ellis‐Behnke RG, So KF, Wu W. Reknitting the injured spinal cord by self‐assembling peptide nanofiber scaffold. Nanomedicine. 2007; 3: 311–321. [DOI] [PubMed] [Google Scholar]
- 10. Guo J, Leung KK, Su H, Yuan Q, Wang L, Chu TH, Zhang W, Pu JK, Ng GK, Wong WM, et al. Self‐assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine. 2009; 5: 345–351. [DOI] [PubMed] [Google Scholar]
- 11. Ellis‐Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci USA. 2006; 103: 5054–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song H, Zhang L, Zhao X. Hemostatic efficacy of biological self‐assembling peptide nanofibers in a rat kidney model. Macromol Biosci. 2010; 10: 33–39. [DOI] [PubMed] [Google Scholar]
- 13. Wang T, Zhong X, Wang S, Lv F, Zhao X. Molecular mechanisms of RADA16–1 peptide on fast stop bleeding in rat models. Int J Molecul Sci. 2012; 13: 15279–15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng H, Almstrom S, Olson L. Fibrin glue used as an adhesive agent in CNS tissues. J Neural Transpl Plasti. 1995; 5: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kassam A, Nemoto E, Balzer J, Rao G, Welch WC, Kuwabara H, Boada F, Horowitz M. Effects of Tisseel fibrin glue on the central nervous system of nonhuman primates. Ear, Nose, Throat J. 2004; 83: 246–248, 250, 252 passim. [PubMed] [Google Scholar]
- 16. Nishimura A, Hayakawa T, Yamamoto Y, Hamori M, Tabata K, Seto K, Shiata N. Controlled release of insulin from self‐assembling nanofiber hydrogel, PuraMatrix: application for the subcutaneous injection in rats. Eur J Pharmaceut Sci. 2012; 45: 1–7. [DOI] [PubMed] [Google Scholar]
- 17. McGrath AM, Novikova LN, Novikov LN, Wiberg M. BD PuraMatrix peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain Res Bull. 2010; 83: 207–213. [DOI] [PubMed] [Google Scholar]
- 18. Ellis‐Behnke RG, Teather LA, Schneider GE, So KF. Using nanotechnology to design potential therapies for CNS regeneration. Curr Pharmaceut Design. 2007; 13: 2519–2528. [DOI] [PubMed] [Google Scholar]
- 19. Hartgerink JD, Beniash E, Stupp SI. Peptide‐amphiphile nanofibers: a versatile scaffold for the preparation of self‐assembling materials. Proc Natl Acad Sci USA. 2002; 99: 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leung GK, Wang YC, Wu W. Peptide nanofiber scaffold for brain tissue reconstruction. Methods Enzymol. 2012; 508: 177–190. [DOI] [PubMed] [Google Scholar]


