Abstract
Hibernating brown bears (Ursus arctos) and black bears (Ursus americanus) spend half of the year in a physically inactive state inside their winter dens without food intake and defecating and no or little urination. Under similar extreme conditions, humans would suffer from loss of lean body mass, heart failure, thrombosis, azotemia, osteoporosis, and more. However, bears exit the den in the spring strong without organ injuries. Translational animal models are used in human medicine but traditional experimental animals have several shortcomings; thus, we believe that it is time to systematically explore new models. In this review paper, we describe physiological adaptations of hibernating bears and how similar adaptations in humans could theoretically alleviate medical conditions. The bear has solved most of the health challenges faced by humans, including heart and kidney disease, atherosclerosis and thrombosis, and muscle wasting and osteoporosis. Understanding and applying this library of information could lead to a number of major discoveries that could have implications for the understanding and treatment of human disease.
Keywords: arteriosclerosis, heart rate, bone loss
Introduction
The use of experimental models in medical research typically adheres to the following chain of actions: (1) select a model organism, (2) induce disease in this model organism, (3) interpret the results, and (4) develop a treatment. The most frequently used model animals in research laboratories are rodents, especially mice and rats. However, these models have a number of shortcomings. Experimental mice and rats are inbred, hypertensive, glucose‐intolerant, prone to cancer and kidney failure, and on a trajectory to premature death.1 Most often these animals are kept at room temperature, which is well below their thermoneutral zone (30–32°C for mice) that in combination with light and absence of hiding space cause constant physiological stress.2 Focusing on a few animal species confines the providence to those particular organisms,3 which could partly explain the decline in medical research breakthroughs that lead to major disease elimination.4
In this paper, we use brown bears (Ursus arctos) and black bears (Ursus americanus) as experimental animal models as inspiration for understanding human medicine. The pursuit of learning from Nature's solution is termed biomimicry—a discipline successfully exploited within engineering, architecture, and medicine.
Brown and black bears remain physically inactive inside their winter dens for half a year without eating, defecating, and with no or intermittent urination. Under similar conditions, humans would develop cardiovascular disease, kidney failure, muscle loss (sarcopenia), osteoporosis, and other deleterious conditions; however, bears readily exit their dens when spring arrives and show no signs of organ damage. In this review, we systematically discuss a number of key areas in which bear physiology might lead us to discover new ways to understand and treat human disease.
Methods
We executed the literature searches using medical subjects headings terms in the PubMed database and free text searches in Google Scholar. To frame the search systematically, we identified Population, Intervention, Comparative intervention, and Outcomes (PICO) in the examined articles. These PICO components defined the inclusion for our bibliographic search strategy, identifying research articles of relevance. We included research papers involving markers for heart failure, atherosclerosis, kidney failure, sarcopenia, and osteoporosis, which also showed relevance to the effects of hibernation in bears with comparisons to active bears or other mammals (including humans). Table 1 summarizes our literature search results.
Table 1.
Hibernating bear physiology and human health challenges. The human diseases of chamber dilatation, atherosclerosis, thrombosis, azotemia, muscle atrophy, osteoporosis are all disabilities that hibernating bears manage to avoid. The right column summarizes hypotheses that explain these scenarios
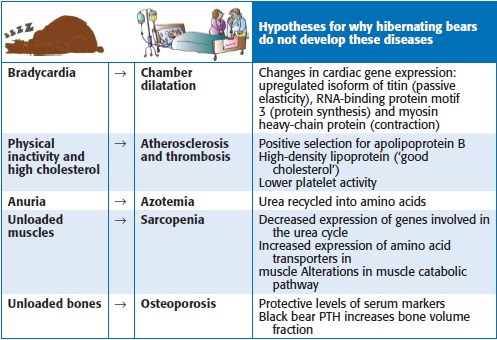
|
Results
Down‐regulated heart rate with respiratory sinus arrhythmia
Extreme heart rate reductions with profound respiratory sinus arrhythmia have been documented in hibernating bears (Figure 1).5, 6, 7, 8, 9 Respiratory sinus arrhythmia has dramatic manifestations, where the duration of the cardiac cycle rises up to three times longer than the cycle length in active state (cycle length variations of 20% would be normal in humans) and up to 13‐ to 14‐second episodes of asystole have been reported in studies of free‐ranging black bears (n = 37; n = 15).6, 10 These effects are accompanied by extremely low respiratory rates.6 The change in heart rhythm is probably primarily mediated by cardiac vagal tone. The biological function of respiratory sinus arrhythmia is to maintain interplay between cardiovascular and respiratory systems in order to meet metabolic demands over highly variable conditions.11
Figure 1.
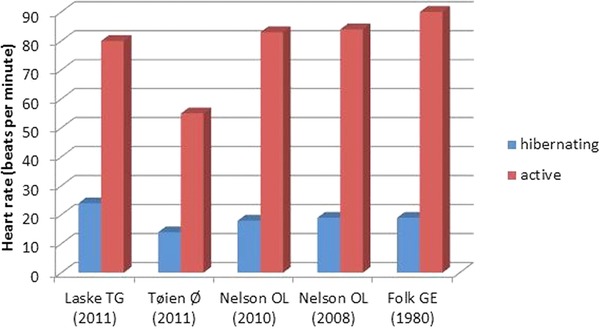
Heart rates in the hibernating and active states. Average heart rate values in brown and black bears during the hibernation and active states. The study of Laske TG included free‐ranging black bears (n = 8), and that of Tøien Ø examined captive black bears (n = 4). The papers of Nelson OL (n = 4, n = 8) and Folk GE (n = 2) are based on samples from captive brown bears. The heart rates reported from the group of Tøien Ø were unexpectedly low compared to those of other studies. This could be related to the captive environment combined with the small sample size, since heart rate is strongly affected by activity.
As a response to their low respiratory rate, bears have optimized oxygen transport during hibernation. Erythrocyte count and hemoglobin are increased during hibernation in captive brown bears (n = 7)12 and free‐ranging black bears (n = 48).13 We recently documented that bears have higher oxygen affinity when hibernating compared to active state. This increased affinity is associated with a significant decrease in red cell hemoglobin‐cofactor 2,3‐diphosphoglycerate, which binds to the low‐affinity conformation of mammalian hemoglobin and halves hemoglobin‐oxygen affinity compared to active state. These changes may be crucial for maintaining adequate tissue oxygen tension during hibernation.14
Protective cardiac gene expression
Humans suffering from bradycardia may develop cardiac chamber dilatation due to volume overload during the long diastolic pauses. Studies of captive grizzly bears (n = 4 [9]; n = 8 [8]) have revealed no significant reduction in ejection fraction during hibernation (although the left ventricular mass decreases), indicating sustained heart function.
One way to investigate how bear myocardium tolerates bradycardia is to study myocardial gene expression during hibernation compared to the active state. Such examinations have revealed significant alternations in the expressions of several heart‐related genes. Several groups have investigated the genes titin (involved in muscle passive elasticity) and RNA‐binding protein motif 3 (enhances protein synthesis at mildly hypothermic temperatures). Expression of the stiffer N2B isoform of titin is significantly elevated during hibernation, indicating increased ventricular passive rigidity,9 which could reduce the risk of chamber overload. This finding is supported by faster ventricular filling deceleration. A collaborative study has shown that phosphorylating titin in animal models with diastolic heart failure could restore diastolic function by reducing ventricular stiffness.15 RNA‐binding protein motif 3 is expressed at a sevenfold higher level during hibernation.16 Elevated levels of this gene were also seen in hibernating ground squirrels.17 It has been suggested that RNA‐binding protein motif 3 facilitates global protein synthesis under hypothermia by binding to 60S ribosomal subunits and reducing levels of microRNAs. Therefore, the protein biosynthesis of RNA‐binding protein motif 3 may prevent myocardial atrophy and atrophy of skeletal muscle over an extended period of bradycardia and immobility as during hibernation.18
In hibernating bears, atrial strain imaging have showed decreased atrial contractility and increased atrial stiffness. The reportedly increased alpha‐myosin heavy‐chain protein expression in the atria (n = 14) may influence cardiac contraction characteristics. Atrium‐specific expression of creatine kinase, consistent with lowered energy requirements, was reduced by 50% (n = 17), indicating depressed atrial emptying. These reported molecular changes of the atria would reduce ventricular overload and seem to be a protective response.19
Human heart failure is a clinical challenge that requires effective interventions. Therefore, the genes which seem to contribute to endured heart function in bears during hibernation could be suggested for therapeutic targets in experimental studies.
Resistance to atherosclerosis and thrombosis
Metabolism is mainly based on lipids from stored fat during hibernation leading to increases in plasma lipid levels. Most important, groups have noted increased plasma levels of low‐density lipoprotein (“bad cholesterol”) and triglycerides20, 21 in hibernating bears. In humans, long periods of bed rest and high plasma lipid concentrations are associated with atherosclerosis.22 However, no atherosclerotic histological changes—such as fatty streaks, cell infiltration, or inflammation—were detected in the coronary arteries and aortic arches of bears aged 1.5–12 years,21 thus suggesting atherosclerotic resistance (although atherosclerosis in bears of high age has been described in one report).23
Genome analyses of polar bears and brown bears have revealed positive selection for mutated apolipoprotein B (which encodes the primary lipoprotein component of low‐density lipoprotein). This may explain how polar bears can cope with enhanced cholesterol without a build‐up of arterial plaque, despite their hyper‐lipid diet.24 However, brown bears have higher cholesterol levels than polar bears,21 which challenge this statement.25 The absence of atherosclerosis could alternatively be explained by the high levels of high‐density lipoprotein (“good cholesterol”).21
Immobilization is also associated with thrombus formation, for which atrial chamber hypokinesia is an additional risk factor. Compared to human controls, hibernating bears exhibit lower platelet activity (less than half), using either adenosine diphosphate, arachidonic acid, or thrombin receptor‐activating peptide as agonists.26 This may explain how the bears endure hibernation without obvious thrombus building.
It is also possible that bears benefit from the immunosuppressive effect of reduced body temperature27 or the extreme consumptions of berries (Vaccinum), containing antioxidants such as vitamin C and polyphenols,28 which have shown favorable effects on high‐density lipoprotein, blood pressure, and platelet function. A berry‐rich diet may reduce cardiovascular risk factors for patients suffering from, for example, hyperlipidemia and hypertension.29
Suppressed glomerular filtration rate
The glomerular filtration rate (GFR; the flow rate of plasma filtered through the kidney) decreases by about 70% in hibernating bears, resulting in anuria with a very low urine production, which is reabsorbed across the urine bladder.30 Despite GFR suppression no clinical signs of azotemia are seen in hibernating bears. The serum urea levels remain low20 in free‐ranging bears (n = 71),31 which could be explained by urea being recycled back into amino acids. Please see recent review on kidney function in bears for a detailed overview.32
Limited muscle atrophy
Despite months of inactivity and starvation during hibernation, skeletal muscle mass33, 34 and strength35 are not reduced to the same extents as observed in humans during bed rest. Free‐ranging black bears (n = 7) and captive brown bears (n = 6) do not exhibit changes in muscle‐fiber number and cross‐sectional area between seasons.33, 34 Studies of muscle biopsies and fiber type of free‐ranging black bears (n = 7) have further shown preserved proportions of the myosin isoforms36 and fast‐ and slow‐twitch fibers between hibernating and active bears,34 which is associated with suppressed disuse atrophy.
Limited muscle loss during hibernation has been explained by findings suggesting periodic muscle activity during dormancy among free‐ranging black bears (n = 5).37 However, transection of nerves in hibernating captive brown bears (n = 8) revealed minimal change of muscle mass,38 which challenges the importance of muscle activity.
Measurements of protein content and nitrogen stable isotope enrichment show that protein catabolism in free‐ranging black bears (n = 12) is lower in winter than in summer.39 Plasma from hibernating bears incubated in the presence of rat skeletal muscle showed down‐regulation of the proteolytic rate by 40% compared to control plasma. Therefore, it is conceivable that a proteolytic inhibitor could influence pathways depressing muscle wasting during immobilization.40 Moreover, expressions of genes involved in the urea cycle are depressed in the hibernating state in parallel with muscle overexpression of amino acid transporters, suggesting facilitation of protein synthesis in captive black bears (n = 11). These results suggest that amino acids are redirected from catabolic pathways to protein biosynthesis.16
Further investigation of muscle responses to hibernation could be helpful for treating people suffering from immobilization or neuromuscular diseases. In order to influence the proteolytic pathway as during hibernation, peroxisome proliferator‐activated receptor‐γ coactivator (PGC‐1α; stimulates mitochondrial biogenesis and regulates muscle fiber type formation) could be a potential target. In a study made on ground squirrels in torpor, the animals exhibited a shift to slow‐twitch muscle fibers accompanied by up‐regulated activation of the endurance exercise pathway mediated by PGC‐1α.41
Bone remodeling
In humans, long‐term bed rest leads to unbalanced bone remodeling and skeletal changes leading to disuse osteoporosis.42 Despite months of disuse, hibernating free‐ranging black bears (n = 65) exhibit no loss of cortical bone geometry or mechanical properties.43, 44 Bone loss can also be assessed by measuring bone metabolism markers. Studies measuring various serum biochemical markers have identified several parameters that significantly differ between hibernation and the active state (Table 2).45, 46, 47, 48, 49, 50 Interestingly, hibernating bears seem to retain a consistent calcium level during hibernation,48, 50 even though bone turnover marker levels suggest unbalanced bone remodeling.
Table 2.
Alterations of serum parameters from active state to hibernating state
| First author; population | Total calcium | CTX | ICTP | PIIINP | PICP | BSALP | NTX | PTH | 1,25 (OH)D | 25 (OH)D | IGF‐I | Cortisol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donahue SW (2003); wild black bears (n = 17) | ↑ | → | ↑ | |||||||||
| Donahue SW (2003); captive black bears (n = 5) | ↑ | ↓ | → | ↑ | ||||||||
| Donahue SW (2006); captive black bears (n = 16) | → | → | ↓ | |||||||||
| Bradford RM (2010); captive black bears (n = 16) | ↓ | |||||||||||
| Seger RL (2011); wild black bears (n = 139) | → | ↑ | → | ↓ | ↑ | ↓ | ||||||
| Vestergaard P (2011); wild brown bears (n = 7) | → | → | → | ↓ | → | → | → |
All statistically significant (p < 0.05) alterations of serum levels are marked with the signs ↑ and ↓, respectively, indicating that the biochemical marker is up‐ or down‐regulated in the hibernating state compared to the active state. The parameters that did not significantly differ are marked with the sign →. Consistencies are seen for total calcium, ICTP, 25(OH)D, PTH, IGF‐I, and cortisol. Neither total serum calcium, 25(OH)D nor PTH changed significantly according to at least two different studies. On the other hand, IGF‐I was unambiguously down‐regulated and cortisol was up‐regulated in hibernation—features that promote bone loss in humans.
CTX = carboxy‐terminal telopeptides of collagen cross‐links; ICTP = cross‐linked c telopeptide of type 1 collagen; PIIINP = procollagen III N‐terminal propeptide; PICP = carboxy‐terminal propeptide of type collagen 1; BSALP = bone specific alkaline phosphatase; NTX = amino‐terminal telopeptides of collagen cross‐links; PTH, parathyroid hormone; 1,25(OH)D = 1,25‐dihydroxyvitamin D; 25(OH)D2 = 25‐hydroxy‐vitamin D2: IGF‐I = insulin‐like growth factor‐1.
Serum concentrations of PTH remain consistent year‐round;47, 48, 50 however, amino acid residue differences exist between human PTH and black bear PTH and induced cAMP activation of black bear PTH has been found in osteoblast cultures.51 These suggests that black bear PTH could be a potent osteoanabolic agent, important for maintaining bone mass during hibernation. Subcutaneous injection of black bear PTH in mice with dystrophin deficiency (n = 20) resulted in a sevenfold increase in bone volume fraction, as well as expanded osteoblast area and down‐regulated osteoclast area.51
Clarification of the impact hierarchy of the altered bone serum markers during hibernation could be a step in the direction of developing new tools to prevent and treat humans with osteoporosis. Some osteoanabolic factors, such as calcitonin52 and PTH,53 are in use in osteoporosis treatment. However, there is room for more efficient treatments and a possibility would be to explore the potential of biochemical markers found in bears.
The finding of multipotent stem cells with osteogenic capacity has raised interest for their role in bone regeneration. Adipose tissue‐derived stem cells from free‐ranging brown bears (n = 6) showed an inclination to undergo spontaneous and unstimulated osteogenesis and chondrogenesis in cultures,54 indicating an advantageous differentiation potential. Further research in osteoporosis could be meaningful with the aim to reestablish normal bone regenerative mechanisms.
Discussion
Similarities between human conditions and hibernation
Some human conditions are associated with physiological changes quite similar to those found in hibernating bears. Human myocardial hibernation is defined as reversible heart dysfunction during chronic ischemia, whereas myocardial stunning is characterized by prolonged reduced contractile function after reperfusion of severe ischemia. The underlying pathophysiology is controversial with only limited data available in humans.55 Some argue that myocardial hibernation is an adaptive response in which cardiac function and metabolism are down‐regulated to cope with the decreased energy supply during hypoperfusion,56 possibly mediated by decreased β‐adrenoceptor density.57 This cardiac adaption has similarities to the extreme bradycardia during respiratory sinus arrhythmia and reduced metabolic demands in hibernating bears.
During accidental severe hypothermia (less than 28°C), humans react with reduced heart rate, respiratory rate, blood pressure, and kidney function.58 Humans exposed to severe cold instinctively try to reduce heat loss by seeking some kind of cover. This is probably partly an autonomous mechanism associated with primitive and burrowing‐like behavior to avoid a cold environment, just like what is observed in hibernating animals.59
Winter depression and hypersomnia (prolonged sleep) are characterized by hypometabolism and decreased heart rate. These conditions could be secondary to an up‐regulated parasympathetic tone as a response to winter onset, just like in hibernation.60
Strengths and limitations
Most of the studies included in the present review used a prospective study design, in which every bear served as its own control. This method enabled an optimized comparison by eliminating bias based on individual characteristics (genetics, weight, activity level, etc.).
Several of the reviewed studies were limited by relatively small sample sizes and high variations within the population (sex, age, and pregnancy). Furthermore, we chose to include studies with both free‐ranging and captive bears and thus, differences in diet and hibernation patterns61—as well as bears from different geographic origins representing different climate zones and nutritional availability. This inclusion increases the variation between populations but also broadens the overall picture.
Conclusion
Hibernating bears have developed several survival strategies, which show promising implications for patients suffering a number of different diseases, including heart failure, atherosclerosis, kidney dysfunction, muscle wasting, and osteoporosis. Considering the limitations of traditional rodent animal models, a systematic experimental and clinical research approach based on bear physiology is warranted in order to investigate the possibility of biomimicry and isolating protective biochemical markers and genes for future patient treatments.
Acknowledgments
We received no specific funding for this paper.
References
- 1. Martin B, Ji S, Maudsley S, Mattson MP. Control laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci 2010; 107: 6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology 2014; 23: 413–420. [DOI] [PubMed] [Google Scholar]
- 3. Bolker J. Model organisms: there's more to life than rats and flies. Nature 2012; 491: 31–33. [DOI] [PubMed] [Google Scholar]
- 4. Holcombe RF. Crisis in medical research. Acad Med 1997; 72: 1–4. [PubMed] [Google Scholar]
- 5. Folk EG, Hunt JM, Folk MA. Further evidence for hibernation of bears. Int Assoc Bear Res Manage 1980; 4: 43–47. [Google Scholar]
- 6. Laske TG, Garshelis DL, Iaizzo PA. Monitoring the wild black bear's reaction to human and environmental stressors. BMC Physiol 2011; 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toien O, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science 2011; 331: 906–909. [DOI] [PubMed] [Google Scholar]
- 8. Nelson OL, Robbins CT. Cardiac function adaptations in hibernating grizzly bears (ursus arctos horribilis). J Comp Physiol B 2010; 180: 465–473. [DOI] [PubMed] [Google Scholar]
- 9. Nelson OL, Robbins CT, Wu Y, Granzier H. Titin isoform switching is a major cardiac adaptive response in hibernating grizzly bears. Am J Physiol Heart Circ Physiol 2008; 295: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laske TG, Harlow HJ, Garshelis DL, Iaizzo PA. Extreme respiratory sinus arrhythmia enables overwintering black bear survival—physiological insights and applications to human medicine. J Cardiovasc Transl Res 2010; 3: 559–569. [DOI] [PubMed] [Google Scholar]
- 11. Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol 2007; 74: 263–285. [DOI] [PubMed] [Google Scholar]
- 12. Hissa R, Siekkinen J, Hohtola E, Saarela S, Hakala A, Pudas J. Seasonal patterns in the physiology of the European brown bear (ursus arctos arctos) in Finland. Comp Biochem Physiol A Physiol 1994; 109: 781–791. [DOI] [PubMed] [Google Scholar]
- 13. Hellgren EC, Rogers LL, Seal US. Serum chemistry and hematology of black bears: physiological indices of habitat quality or seasonal patterns? J Mammal 1993; 74: 304–315. [Google Scholar]
- 14. Revsbech IG, Malte H, Frobert O, Evans A, Blanc S, Josefsson J, Fago A. Decrease in the red cell cofactor 2,3‐diphosphoglycerate increases hemoglobin oxygen affinity in the hibernating brown bear ursus arctos. Am J Physiol Regul Integr Comp Physiol 2013; 304: 43–49. [DOI] [PubMed] [Google Scholar]
- 15. Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Jr , Linke WA, Redfield MM. Sildenafil and B‐type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 2011; 124: 2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fedorov VB, Goropashnaya AV, Toien O, Stewart NC, Chang C, Wang H, Yan J, Showe LC, Showe MK, Barnes BM. Modulation of gene expression in heart and liver of hibernating black bears (ursus americanus). BMC Genom 2011; 12: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 2008; 32: 170–181. [DOI] [PubMed] [Google Scholar]
- 18. Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress‐induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA 2005; 102: 1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrows ND, Nelson OL, Robbins CT, Rourke BC. Increased cardiac alpha‐myosin heavy chain in left atria and decreased myocardial insulin‐like growth factor (igf‐I) expression accompany low heart rate in hibernating grizzly bears. Physiol Biochem Zool 2011; 84: 1–17. [DOI] [PubMed] [Google Scholar]
- 20. Stenvinkel P, Frobert O, Anderstam B, Palm F, Eriksson M, Bragfors‐Helin AC, Qureshi AR, Larsson T, Friebe A, Zedrosser A, et al. Metabolic changes in summer active and anuric hibernating free‐ranging brown bears (ursus arctos). PLoS One 2013; 8: e72934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arinell K, Sahdo B, Evans AL, Arnemo JM, Baandrup U, Frobert O. Brown bears (ursus arctos) seem resistant to atherosclerosis despite highly elevated plasma lipids during hibernation and active state. Clin Transl Sci 2012; 5: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torii R, Shiomi M, Ito T, Yamada S, Eguchi Y, Ikeda N. Cholesterol‐fed ovariectomized monkeys are good animal models for human atherosclerosis of postmenopausal women. Primates 2003; 44: 247–252. [DOI] [PubMed] [Google Scholar]
- 23. Miller AD, McDonough S. Interthalamic hematoma secondary to cerebrovascular atherosclerosis in an aged grizzly bear (ursus arctos horribilis) with primary cardiac schwannoma. J Zoo Wildl Med 2008; 39: 659–662. [DOI] [PubMed] [Google Scholar]
- 24. Liu S, Lorenzen ED, Fumagalli M, Li B, Harris K, Xiong Z, Zhou L, Korneliussen TS, Somel M, Babbitt C, et al. Population genomics reveal recent speciation and rapid evolutionary adaptation in polar bears. Cell 2014; 157: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berg von Linde M, Arevström L, Fröbert O. Population genomics reveal recent speciation and rapid evolutionary adaptation in polar bears. Cell http://www.cell.com/cell/comments/S0092–8674%2814%2900488–7. Accessed 14 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frobert O, Christensen K, Fahlman A, Brunberg S, Josefsson J, Sarndahl E, Swenson JE, Arnemo JM. Platelet function in brown bear (ursus arctos) compared to man. Thromb J 2010; 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahdo B, Evans AL, Arnemo JM, Frobert O, Sarndahl E, Blanc S. Body temperature during hibernation is highly correlated with a decrease in circulating innate immune cells in the brown bear (ursus arctos): a common feature among hibernators? Int J Med Sci 2013; 10: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erlenbach JA, Rode KD, Raubenheimer D, Robbins CT. Macronutrient optimization and energy maximization determine diets of brown bears. J Mammal 2014; 95: 160–168. [Google Scholar]
- 29. Johnson SA, Figueroa A, Navaei N, Wong A, Kalfon R, Ormsbee LT, Feresin RG, Elam ML, Hooshmand S, Payton ME, et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre‐ and stage 1‐hypertension: a randomized, double‐blind, placebo‐controlled clinical trial. J Acad Nutr Diet 2015; 115: 369–377. [DOI] [PubMed] [Google Scholar]
- 30. Brown DC, Mulhausen RO, Andrew DJ, Seal US. Renal function in anesthetized dormant and active bears. Am J Physiol 1971; 220: 293–298. [DOI] [PubMed] [Google Scholar]
- 31. Storm GL, Alt GL, Matula GJ, Nelson RA. Blood chemistry of black bears from pennsylvania during winter dormancy. J Wildl Dis 1988; 24: 515–521. [DOI] [PubMed] [Google Scholar]
- 32. Stenvinkel P, Jani AH, Johnson RJ. Hibernating bears (ursidae): metabolic magicians of definite interest for the nephrologist. Kidney Int 2013; 83: 207–212. [DOI] [PubMed] [Google Scholar]
- 33. Tinker DB, Harlow HJ, Beck TD. Protein use and muscle‐fiber changes in free‐ranging, hibernating black bears. Physiol Zool 1998; 71: 414–424. [DOI] [PubMed] [Google Scholar]
- 34. Hershey JD, Robbins CT, Nelson OL, Lin DC. Minimal seasonal alterations in the skeletal muscle of captive brown bears. Physiol Biochem Zool 2008; 81: 138–147. [DOI] [PubMed] [Google Scholar]
- 35. Lohuis TD, Harlow HJ, Beck TD, Iaizzo PA. Hibernating bears conserve muscle strength and maintain fatigue resistance. Physiol Biochem Zool 2007; 80: 257–269. [DOI] [PubMed] [Google Scholar]
- 36. Rourke BC, Cotton CJ, Harlow HJ, Caiozzo VJ. Maintenance of slow type I myosin protein and mRNA expression in overwintering prairie dogs (cynomys leucurus and ludovicianus) and black bears (ursus americanus). J Comp Physiol B 2006; 176: 709–720. [DOI] [PubMed] [Google Scholar]
- 37. Harlow HJ, Lohuis T, Anderson‐Sprecher RC, Beck TDI, Gettinger RD. Body surface temperature of hibernating black bears may be related to periodic muscle activity. J Mammal 2004; 85: 414–419. [Google Scholar]
- 38. Lin DC, Hershey JD, Mattoon JS, Robbins CT. Skeletal muscles of hibernating brown bears are unusually resistant to effects of denervation. J Exp Biol 2012; 215: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 39. Lohuis TD, Harlow HJ, Beck TD. Hibernating black bears (ursus americanus) experience skeletal muscle protein balance during winter anorexia. Comp Biochem Physiol B Biochem Mol Biol 2007; 147: 20–28. [DOI] [PubMed] [Google Scholar]
- 40. Fuster G, Busquets S, Almendro V, Lopez‐Soriano FJ, Argiles JM. Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy? Clin Nutr 2007; 26: 658–661. [DOI] [PubMed] [Google Scholar]
- 41. Xu R, Andres‐Mateos E, Mejias R, MacDonald EM, Leinwand LA, Merriman DK, Fink RH, Cohn RD. Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp Neurol 2013; 247: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takata S, Yasui N. Disuse osteoporosis. J Med Invest 2001; 48: 147–156. [PubMed] [Google Scholar]
- 43. McGee‐Lawrence ME, Wojda SJ, Barlow LN, Drummer TD, Bunnell K, Auger J, Black HL, Donahue SW. Six months of disuse during hibernation does not increase intracortical porosity or decrease cortical bone geometry, strength, or mineralization in black bear (ursus americanus) femurs. J Biomech 2009; 42: 1378–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGee ME, Maki AJ, Johnson SE, Nelson OL, Robbins CT, Donahue SW. Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (ursus arctos horribilis). Bone 2008; 42: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Donahue SW, Vaughan MR, Demers LM, Donahue HJ. Bone formation is not impaired by hibernation (disuse) in black bears ursus americanus. J Exp Biol 2003; 206: 4233–4239. [DOI] [PubMed] [Google Scholar]
- 46. Donahue SW, Vaughan MR, Demers LM, Donahue HJ. Serum markers of bone metabolism show bone loss in hibernating bears. Clin Orthop Relat Res 2003; 408: 295–301. [DOI] [PubMed] [Google Scholar]
- 47. Donahue SW, Galley SA, Vaughan MR, Patterson‐Buckendahl P, Demers LM, Vance JL, McGee ME. Parathyroid hormone may maintain bone formation in hibernating black bears (ursus americanus) to prevent disuse osteoporosis. J Exp Biol 2006; 209: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 48. Vestergaard P, Stoen OG, Swenson JE, Mosekilde L, Heickendorff L, Frobert O. Vitamin D status and bone and connective tissue turnover in brown bears (ursus arctos) during hibernation and the active state. PLoS One 2011; 6: e21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bradford RM. Exploration of the role of serum factors in maintaining bone mass during hibernation in black bears. Mich Technol Univ 2010; 1: 1–188. [Google Scholar]
- 50. Seger RL, Cross RA, Rosen CJ, Causey RC, Gundberg CM, Carpenter TO, Chen TC, Halteman WA, Holick MF, Jakubas WJ, et al. Investigating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating American black bear (ursus americanus). Bone 2011; 49: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 51. Gray SK, McGee‐Lawrence ME, Sanders JL, Condon KW, Tsai CJ, Donahue SW. Black bear parathyroid hormone has greater anabolic effects on trabecular bone in dystrophin‐deficient mice than in wild type mice. Bone 2012; 51: 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Munoz‐Torres M, Alonso G, Raya MP. Calcitonin therapy in osteoporosis. Treat Endocrinol 2004; 3: 117–132. [DOI] [PubMed] [Google Scholar]
- 53. Morley P, Whitfield JF, Willick GE. Parathyroid hormone: an anabolic treatment for osteoporosis. Curr Pharm Des 2001; 7: 671–687. [DOI] [PubMed] [Google Scholar]
- 54. Fink T, Rasmussen JG, Emmersen J, Pilgaard L, Fahlman A, Brunberg S, Josefsson J, Arnemo JM, Zachar V, Swenson JE, et al. Adipose‐derived stem cells from the brown bear (ursus arctos) spontaneously undergo chondrogenic and osteogenic differentiation in vitro. Stem Cell Res 2011; 7: 89–95. [DOI] [PubMed] [Google Scholar]
- 55. Luss H, Schafers M, Neumann J, Hammel D, Vahlhaus C, Baba HA, Janssen F, Scheld HH, Schober O, Breithardt G, et al. Biochemical mechanisms of hibernation and stunning in the human heart. Cardiovasc Res 2002; 56: 411–421. [DOI] [PubMed] [Google Scholar]
- 56. Hearse DJ. Myocardial hibernation. A form of endogenous protection? Eur Heart J 1997; 18: 2–7. [DOI] [PubMed] [Google Scholar]
- 57. Shan K, Bick RJ, Poindexter BJ, Nagueh SF, Shimoni S, Verani MS, Keng F, Reardon MJ, Letsou GV, Howell JF, et al. Altered adrenergic receptor density in myocardial hibernation in humans: a possible mechanism of depressed myocardial function. Circulation 2000; 102: 2599–2606. [DOI] [PubMed] [Google Scholar]
- 58. Locher T, Walpoth B, Pfluger D, Althaus U. Accidental hypothermia in Switzerland (1980–1987)–case reports and prognostic factors. Schweiz Med Wochenschr 1991; 121: 1020–1028. [PubMed] [Google Scholar]
- 59. Rothschild MA, Schneider V. “Terminal burrowing behaviour”—a phenomenon of lethal hypothermia. Int J Legal Med 1995; 107: 250–256. [DOI] [PubMed] [Google Scholar]
- 60. Austen ML, Wilson GV. Increased vagal tone during winter in subsyndromal seasonal affective disorder. Biol Psychiatry 2001; 50: 28–34. [DOI] [PubMed] [Google Scholar]
- 61. Evans AL, Sahlen V, Stoen OG, Fahlman A, Brunberg S, Madslien K, Frobert O, Swenson JE, Arnemo JM. Capture, anesthesia, and disturbance of free‐ranging brown bears (ursus arctos) during hibernation. PLoS One 2012; 7: e40520. [DOI] [PMC free article] [PubMed] [Google Scholar]


