Abstract
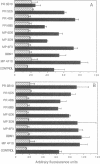
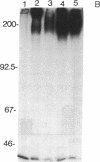
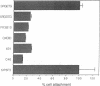
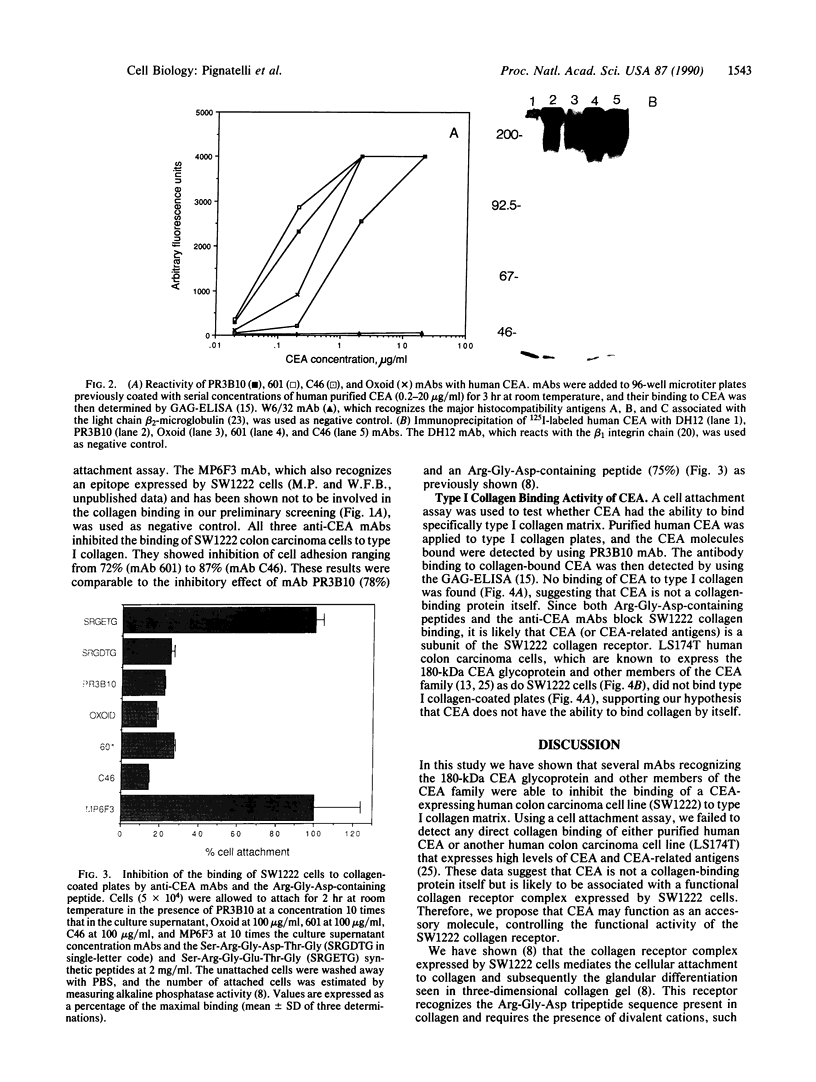
We have previously shown that a human colon carcinoma cell line (SW1222) expresses a collagen receptor recognizing the Arg-Gly-Asp tripeptide sequence found in collagen. This receptor mediates the cellular attachment to collagen and, subsequently, the glandular differentiation seen in a three-dimensional collagen gel culture. In a search to identify cell surface molecules mediating the adhesion and differentiation of SW1222 cells, we have screened a panel of monoclonal antibodies recognizing epithelial cell surface determinants for their ability to inhibit the collagen binding of SW1222 cells. We have found that four monoclonal antibodies recognizing the 180-kDa carcinoembryonic antigen (CEA) glycoprotein and other members of the CEA family inhibited (up to 87%) the binding of SW1222 cells to type I collagen matrix. Using a cell attachment assay, we have not detected any direct collagen binding of either purified CEA or another CEA-expressing human colon carcinoma cell line (LS174T). These data suggest that CEA is not a collagen-binding protein itself but is likely to be associated with the functional Arg-Gly-Asp collagen receptor expressed by SW1222 cells. We suggest that CEA may function as an accessory molecule, controlling the functional activity of the SW1222 collagen receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Kinoshita K., Nakane P. K., Brown W. R. Differential expression carcinoembryonic antigen and secretory component during colonic epithelial cell differentiation and in colonic carcinomas. Gastroenterology. 1987 Dec;93(6):1330–1338. doi: 10.1016/0016-5085(87)90263-0. [DOI] [PubMed] [Google Scholar]
- Alcover A., Alberini C., Acuto O., Clayton L. K., Transy C., Spagnoli G. C., Moingeon P., Lopez P., Reinherz E. L. Interdependence of CD3-Ti and CD2 activation pathways in human T lymphocytes. EMBO J. 1988 Jul;7(7):1973–1977. doi: 10.1002/j.1460-2075.1988.tb03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C. P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989 Apr 21;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Bockenstedt L. K., Goldsmith M. A., Dustin M., Olive D., Springer T. A., Weiss A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J Immunol. 1988 Sep 15;141(6):1904–1911. [PubMed] [Google Scholar]
- Bourdon M. A., Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989 Mar;108(3):1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Brown M. H., Cantrell D. A., Brattsand G., Crumpton M. J., Gullberg M. The CD2 antigen associates with the T-cell antigen receptor CD3 antigen complex on the surface of human T lymphocytes. Nature. 1989 Jun 15;339(6225):551–553. doi: 10.1038/339551a0. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol. 1986 Mar;102(3):688–696. doi: 10.1083/jcb.102.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Pytela R., Pierschbacher M. D., Klier F. G., Ruoslahti E., Reisfeld R. A. An Arg-Gly-Asp-directed receptor on the surface of human melanoma cells exists in an divalent cation-dependent functional complex with the disialoganglioside GD2. J Cell Biol. 1987 Sep;105(3):1163–1173. doi: 10.1083/jcb.105.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin H., Bodmer W. F. A sensitive micro-immunoassay using beta-galactosidase/anti-beta-galactosidase complexes. J Immunol Methods. 1987 Feb 26;97(1):19–27. doi: 10.1016/0022-1759(87)90100-1. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GOLD P., FREEDMAN S. O. DEMONSTRATION OF TUMOR-SPECIFIC ANTIGENS IN HUMAN COLONIC CARCINOMATA BY IMMUNOLOGICAL TOLERANCE AND ABSORPTION TECHNIQUES. J Exp Med. 1965 Mar 1;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jaspers M., de Strooper B., Spaepen M., van Leuven F., David G., van den Berghe H., Cassiman J. J. Post-translational modification of the beta-subunit of the human fibronectin receptor. FEBS Lett. 1988 Apr 25;231(2):402–406. doi: 10.1016/0014-5793(88)80859-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Lu M. L., Beacham D. A., Jacobson B. S. The identification and characterization of collagen receptors involved in HeLa cell-substratum adhesion. J Biol Chem. 1989 Aug 15;264(23):13546–13558. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Bodmer W. F. Genetics and biochemistry of collagen binding-triggered glandular differentiation in a human colon carcinoma cell line. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5561–5565. doi: 10.1073/pnas.85.15.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Bodmer W. F. Integrin-receptor-mediated differentiation and growth inhibition are enhanced by transforming growth factor-beta in colorectal tumour cells grown in collagen gel. Int J Cancer. 1989 Sep 15;44(3):518–523. doi: 10.1002/ijc.2910440324. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman P. I., Bodmer W. F. Control of differentiation in human colorectal carcinoma cell lines: epithelial-mesenchymal interactions. J Pathol. 1988 Nov;156(3):197–211. doi: 10.1002/path.1711560305. [DOI] [PubMed] [Google Scholar]
- Richman P. I., Bodmer W. F. Monoclonal antibodies to human colorectal epithelium: markers for differentiation and tumour characterization. Int J Cancer. 1987 Mar 15;39(3):317–328. doi: 10.1002/ijc.2910390309. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rogers G. T. Carcinoembryonic antigens and related glycoproteins. Molecular aspects and specificity. Biochim Biophys Acta. 1983 Dec 29;695(3-4):227–249. doi: 10.1016/0304-419x(83)90013-6. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Lechler R. I., Howland K., Bal V., Eckels D. D., Sekaly R., Long E. O., Taylor W. R., Lamb J. R. Structural model of HLA-DR1 restricted T cell antigen recognition. Cell. 1988 Feb 26;52(4):515–523. doi: 10.1016/0092-8674(88)90464-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Shirota K., Minassian H., Jothy S. Protein G-gold immunoelectron microscopy of colon carcinoma: the effect of tumor differentiation on carcinoembryonic antigen immunostaining. Exp Mol Pathol. 1988 Dec;49(3):305–315. doi: 10.1016/0014-4800(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Coligan J. E. Electron microscopy and physical characterization of the carcinoembryonic antigen. Biochemistry. 1975 Jun 3;14(11):2323–2330. doi: 10.1021/bi00682a008. [DOI] [PubMed] [Google Scholar]
- Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976 May 15;17(5):588–596. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Pande H., Paxton R. J., Shively L., Padma A., Simmer R. L., Todd C. W., Riggs A. D., Shively J. E. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc Natl Acad Sci U S A. 1987 May;84(9):2965–2969. doi: 10.1073/pnas.84.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Toribara N. W., Sack T. L., Gum J. R., Ho S. B., Shively J. E., Willson J. K., Kim Y. S. Heterogeneity in the induction and expression of carcinoembryonic antigen-related antigens in human colon cancer cell lines. Cancer Res. 1989 Jun 15;49(12):3321–3327. [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Ortlieb B., Friedrich R., von Kleist S. Isolation and characterization of cDNA clones encoding the human carcinoembryonic antigen reveal a highly conserved repeating structure. Proc Natl Acad Sci U S A. 1987 May;84(9):2960–2964. doi: 10.1073/pnas.84.9.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kleist S., Chavanel G., Burtin P. Identification of an antigen from normal human tissue that crossreacts with the carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2492–2494. doi: 10.1073/pnas.69.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]