Abstract
A correlation between the lymphocyte-to-monocyte ratio (LMR) and the survival of patients with hematological malignancies has been reported previously. However, there have been few studies investigating the prognostic significance of LMR in patients with solid tumors. The aim of the present study was to evaluate the prognostic significance of preoperative LMR in patients with colorectal cancer (CRC). A total of 189 patients undergoing potentially curative surgery for CRC were enrolled. The LMR was calculated from preoperative blood samples by dividing absolute lymphocyte count by absolute monocyte count. A cut-off value of 4.8 was set based on the receiver operating characteristic curve; 116 patients were classified as high-LMR, and 73 patients classified as low-LMR. The high-LMR group exhibited significantly better relapse-free survival (P=0.0018) and overall survival (P=0.0127) rates than the low-LMR group. According to the multivariate analysis of survival, preoperative LMR was identified as an independent prognostic factor for relapse-free survival (P=0.041) and overall survival (P=0.031). Therefore, preoperative LMR is a useful prognostic marker in patients with CRC.
Keywords: colorectal cancer, prognosis, lymphocyte-to-monocyte ratio, inflammation, biomarker
Introduction
Colorectal cancer (CRC) is the third most common cancer and fourth leading cause of cancer-associated mortality worldwide (1). Despite advances in surgical procedures and adjuvant chemotherapy, 20–25% of patients still experience relapse following curative surgery (2). The Union for International Cancer Control (UICC) tumor node metastasis (TNM) staging system (3) is currently the most reliable indicator of patient prognosis and is widely used amongst practitioners. However, there are differences in patient prognosis even within the same TNM stage. Therefore, more reliable markers are required to improve predictions of cancer recurrence and patient survival.
It has previously been reported that inflammation is important in determining cancer progression (4,5). Inflammation-based indices, such as the C-reactive protein level, Glasgow prognostic score, and neutrophil-to-lymphocyte ratio; are useful for predicting the prognosis of patients with CRC as well as various other types of cancer (6–9). Recent studies investigating various types of malignancies have demonstrated a correlation between the lymphocyte-to-monocyte ratio (LMR), which also reflects the degree of systemic inflammation, and patient survival (10–14). However, the prognostic value of the LMR has mainly been investigated in patients with hematological malignancies, with few reports focusing on patients with solid tumors. Therefore, the aim of this retrospective study was to evaluate the prognostic significance of preoperative LMR in patients with CRC who are able to undergo potentially curative surgery.
Materials and methods
Patients
A total of 189 patients with CRC were enrolled. All patients underwent potentially curative surgery for CRC in the Department of Surgical Oncology, Osaka City University, between January 2007 and December 2009. Patients who received preoperative therapy, underwent emergency surgery for perforation/obstruction, or who had inflammatory bowel disease were excluded from the study.
The patient characteristics are presented in Table I. Included in the study were 107 males and 82 females, and median patient age at initial surgery was 68 years old (range, 26–86 years old). A total of 112 patients had primary tumors located in the colon and 77 had primary tumors located in the rectum. Resected specimens were pathologically classified according to the UICC TNM classification of malignant tumors, ver. 7 (3). The distribution of cancer stages was as follows: stage I, 63; stage II, 65; stage III, 61 patients. All patients underwent regular physical examinations and blood tests. The levels of tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were measured, and mandatory screening was performed using colonoscopy and computed tomography until December 2014 (60 months following surgery) or patient mortality.
Table I.
Patient characteristics.
| Characteristic | No. patients |
|---|---|
| Gender | |
| Male | 107 |
| Female | 82 |
| Age, years | |
| Median (range) | 68 (26–86) |
| Location of primary tumor | |
| Colon | 112 |
| Rectum | 77 |
| Tumor depth | |
| T1-3 | 150 |
| T4 | 39 |
| Tumor diameter, cm | |
| Median (range) | 4.0 (0.2–11.0) |
| Histological type | |
| Well or moderately differentiated | 169 |
| Poorly differentiated or mucinous | 16 |
| Lymphatic involvement | |
| Negative | 76 |
| Positive | 113 |
| Venous involvement | |
| Negative | 163 |
| Positive | 21 |
| Lymph node metastases | |
| Negative | 126 |
| Positive | 63 |
| Stagea | |
| I | 63 |
| II | 65 |
| III | 61 |
| Lymphocyte count, per mm3 | |
| Median (range) | 1690 (432–3891) |
| Monocyte count, per mm3 | |
| Median (range) | 324 (28–792) |
According to the Union for International Cancer Control Tumor- Node-Metastasis Classification of Malignant Tumors (3).
Blood sample analysis
Preoperative blood samples were obtained at the time of diagnosis prior to surgery. The differential white blood cell count was analyzed using the Sysmex XE-5000 automated hematology analyzer™ (Sysmex, Kobe, Japan) following the manufacturer protocol. LMR was calculated from the preoperative blood samples by dividing the absolute lymphocyte count by the absolute monocyte count.
Statistical analysis
A receiver operating characteristic (ROC) curve was used to determine an appropriate cut-off value. All patients were classified into two groups according to the preoperative LMR. The significance of associations between preoperative LMR and clinicopathological characteristics was analyzed using χ2 test and Fisher's exact test. Duration of survival was calculated according to the Kaplan-Meier method. Differences between survival curves were assessed with the log-rank test. A multivariate analysis was performed according to the Cox proportional hazards model, and all statistical analyses were performed using the SPSS software package (SPSS Inc., Tokyo, Japan). P<0.05 was considered to indicate a statistically significant difference.
Ethical considerations
The current study conformed to the provisions of the Declaration of Helsinki and was approved by the ethics committee of Osaka City University. All patients were informed of the investigational nature of this study and provided written informed consent.
Results
Survival analysis according to the lymphocyte/monocyte count
The median preoperative lymphocyte count was 1,690/mm3 (range, 432–3,891), and 1,000 mm3 was set as the cut-off value, in accordance with previous studies (15). Following the lymphocyte count, 171 patients were placed into the high-lymphocyte group and 18 patients into the low-lymphocyte group. Relapse-free survival rate and overall survival rate were significantly lower in the low-lymphocyte group compared with the high-lymphocyte group (Fig. 1, relapse-free survival; P=0.0131; Fig. 2, overall survival; P=0.0314).
Figure 1.
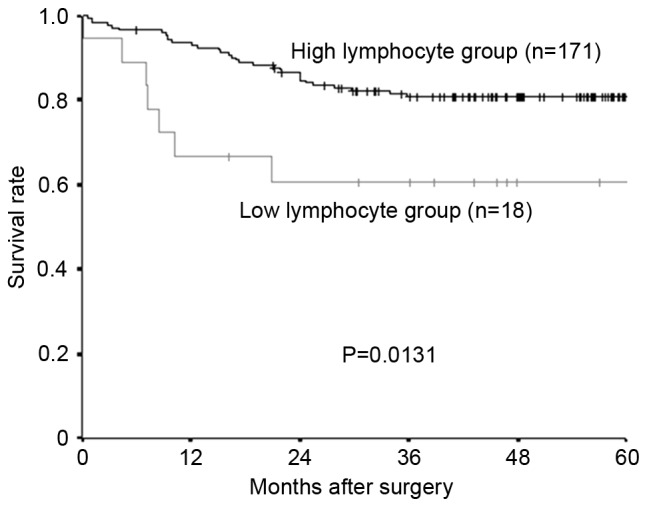
Kaplan-Meier survival curves for the relapse-free survival of different lymphocyte groups. The relapse-free survival rate was significantly lower in the low-lymphocyte group compared with the high-lymphocyte group (P=0.0131).
Figure 2.
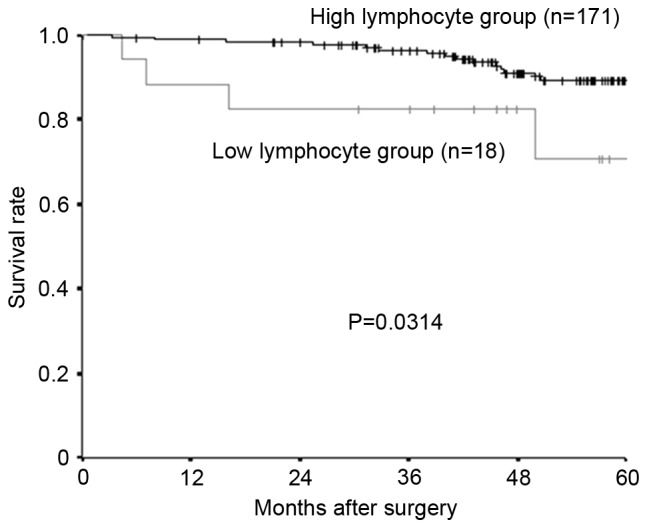
Kaplan-Meier survival curves for the overall survival. The overall survival rate was significantly lower in the low-lymphocyte group than in the high-lymphocyte group (P=0.0314).
The median preoperative monocyte count was 324/mm3 (range, 28–792), and 300 was set as the cut-off value, based on previous reports (16). A total of 76 patients were placed into the high-monocyte group and 113 patients into the low-monocyte group. Both the relapse-free survival rate and the overall survival rate were significantly lower in the high-monocyte group compared with the low-monocyte group (Fig. 3, relapse-free survival; P=0.0243; Fig. 4, overall survival; P=0.0444).
Figure 3.
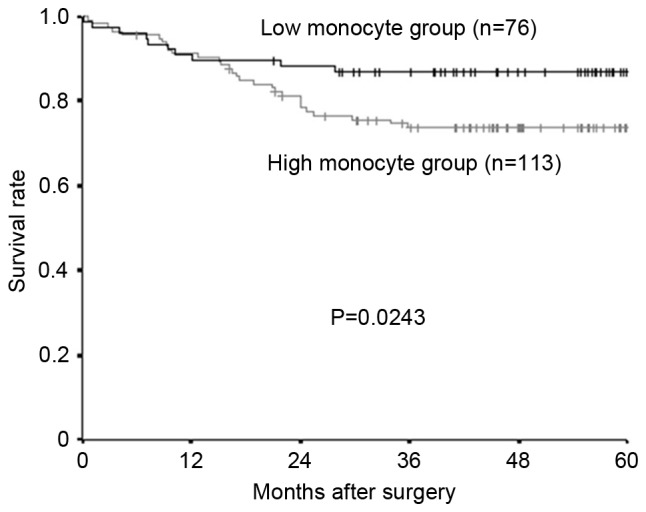
Kaplan-Meier survival curves for the relapse-free survival of different monocyte groups. The relapse-free survival rate was significantly lower in the high-monocyte group compared with the low-monocyte group (P=0.0243).
Figure 4.
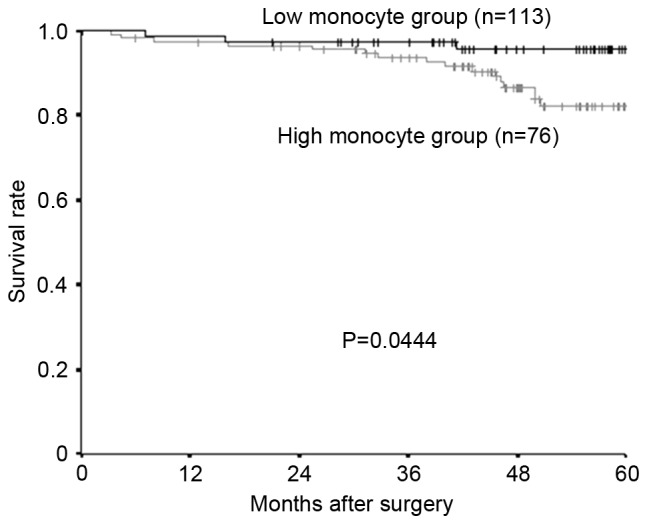
Kaplan-Meier survival curves for the overall survival of different monocyte groups. The overall survival rate was significantly worse in the high-monocyte group than in the low-monocyte group (P=0.0444).
Cut-off value for the LMR
The median preoperative LMR was 5.429 (range, 1.494–57.500). The continuous variable LMR was used as the test variable and the 5-year rate survival as the state variable. The cut-off value for the preoperative LMR was investigated using the ROC curve, and was determined as 4.8 (the sensitivity was 64.1% and the specificity was 63.2%; Fig. 5). Based on this cut-off value, 116 patients were classified into the high-LMR group and 73 patients were classified into the low-LMR group.
Figure 5.
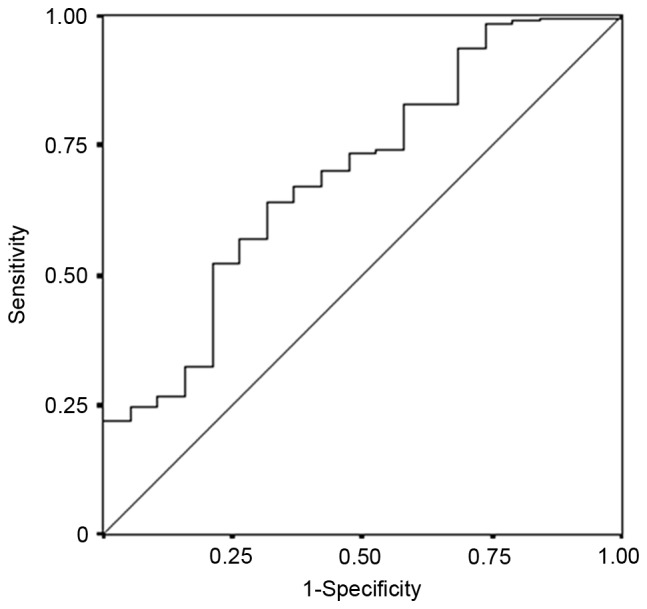
The results of a receiver operating characteristic curve analysis of the LMR in patients with colorectal cancer who underwent potentially curative surgery. Area under the curve, 0.694; 95% confidence interval, 0.568–0.819; P=0.006. LMR, lymphocyte-to-monocyte ratio.
Correlation between the LMR and clinicopathological parameters
The associations between preoperative LMR and clinicopathological parameters are shown in Table II. The only significant relationship identified was between preoperative LMR and lymphatic involvement (P=0.013, Table II).
Table II.
Associations between preoperative LMR and clinicopathological factors.
| LMR | |||
|---|---|---|---|
| Factors | High | Low | P-value |
| Age, years | 0.073 | ||
| <70 | 70 | 34 | |
| ≥70 | 46 | 39 | |
| Gender | 0.099 | ||
| Male | 60 | 47 | |
| Female | 56 | 26 | |
| Tumor location | 0.880 | ||
| Colon | 68 | 44 | |
| Rectum | 48 | 29 | |
| Tumor depth | 0.196 | ||
| T1-3 | 96 | 54 | |
| T4 | 20 | 19 | |
| Tumor diameter, cm | 0.063 | ||
| <5 | 75 | 37 | |
| ≥5 | 38 | 34 | |
| Histological type | 0.789 | ||
| Well/moderately differentiated | 105 | 64 | |
| Poorly differentiated/mucinous | 9 | 7 | |
| Lymphatic involvement | 0.013 | ||
| Negative | 52 | 19 | |
| Positive | 61 | 52 | |
| Venous involvement | 0.352 | ||
| Negative | 98 | 65 | |
| Positive | 15 | 6 | |
| Lymph node metastasis | 0.156 | ||
| Negative | 82 | 44 | |
| Positive | 34 | 29 | |
| Preoperative CEA (>5 ng/ml) | 1.000 | ||
| Negative | 77 | 47 | |
| Positive | 38 | 24 | |
| Stagea | 0.139 | ||
| I | 44 | 19 | |
| II | 40 | 25 | |
| III | 32 | 29 | |
| Adjuvant chemotherapy | 0.881 | ||
| No | 64 | 39 | |
| Yes | 52 | 34 | |
LMR, lymphocyte-to-monocyte ratio; CEA, carcinoembryonic antigen.
According to the Union for International Cancer Control Tumor-Node-Metastasis Classification of Malignant Tumors (3). Bold indicates a statistically significant difference (P<0.05).
Survival analysis according to the LMR
The relapse-free survival rate was significantly lower in the low-LMR group compared with that of the high-LMR group (P=0.0018; Fig. 6), as was overall survival rate (P=0.0127; Fig. 7).
Figure 6.
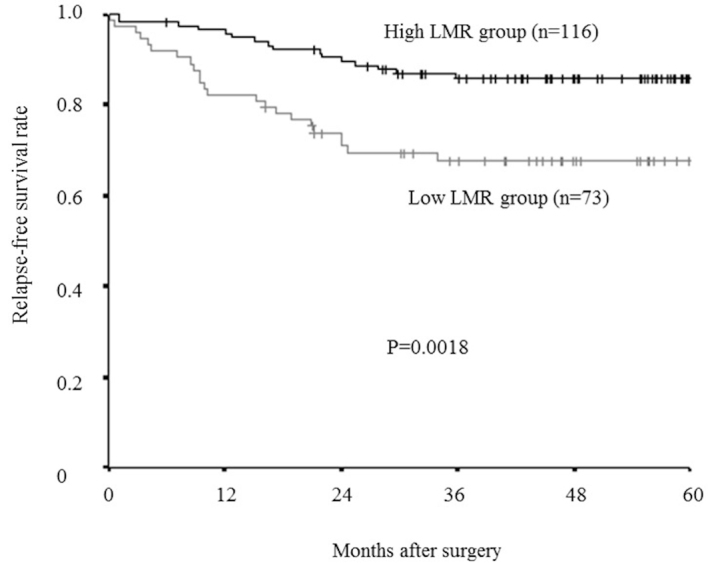
Kaplan-Meier survival curves for the relapse-free survival of different LMR groups. The relapse-free survival rate was significantly lower in the low-LMR group compared with the high-LMR group (P=0.0018). LMR, lymphocyte-to-monocyte ratio.
Figure 7.
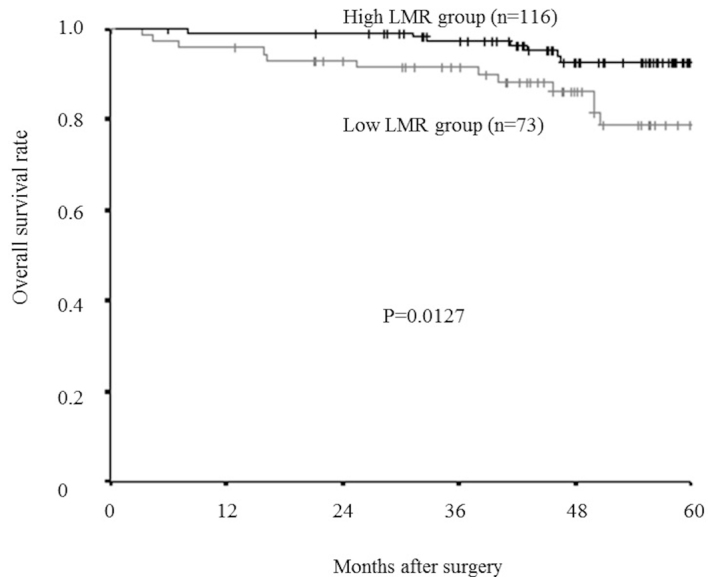
Kaplan-Meier survival curves for the overall survival rate of different LMR groups. The overall survival rate was significantly lower in the low-LMR group than in the high-LMR group (P=0.0127). LMR, lymphocyte-to-monocyte ratio.
Furthermore, even in an analysis limited to the patients with a normal lymphocyte count (>1,000/mm3), relapse-free survival rate was significantly lower in the low-LMR group than in the high-LMR group (P=0.0071; Fig. 8), and overall survival rate tended to be lower in the low-LMR group than in the high-LMR group, though this difference was not significant. (P=0.0792; Fig. 9).
Figure 8.
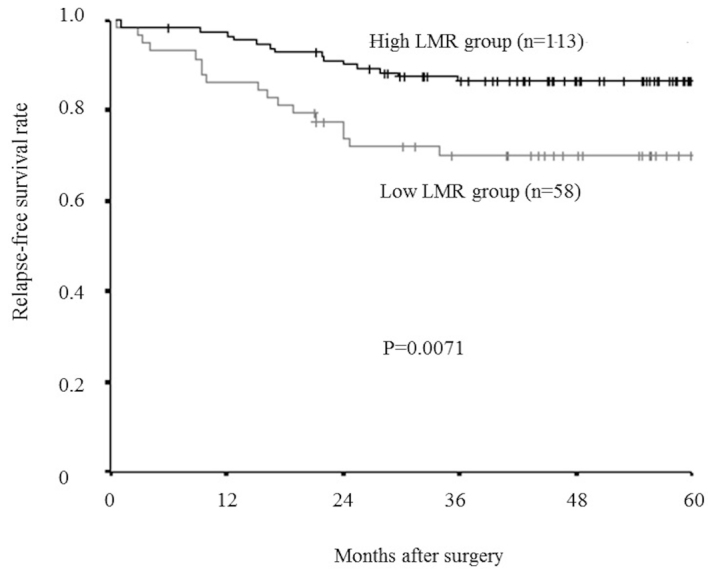
Kaplan-Meier survival curves for the relapse-free survival in an analysis limited to the patients with a normal lymphocyte count. The relapse-free survival rate was significantly lower in the low-LMR group than in the high-LMR group (P=0.0071). LMR, lymphocyte-to-monocyte ratio.
Figure 9.
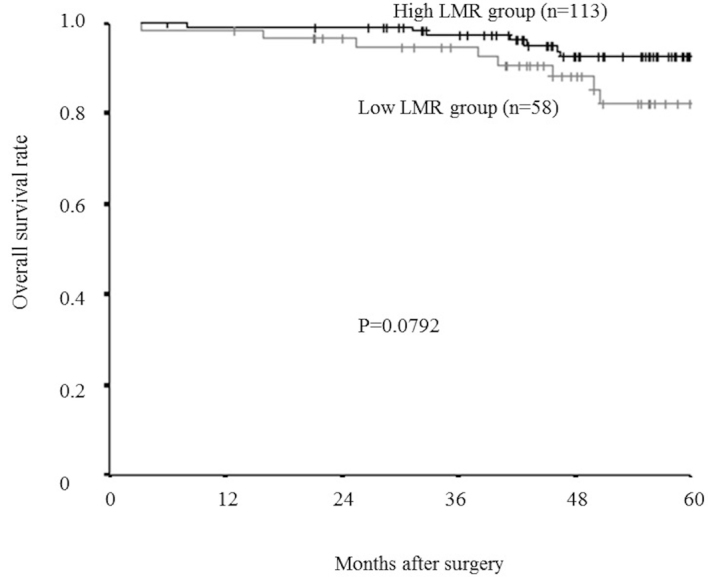
Kaplan-Meier survival curves for the overall survival in an analysis limited to the patients with a normal lymphocyte count. The overall survival rate tended to be lower in the low-LMR group compared to the high-LMR group, however the difference was not significant (P=0.0792). LMR, lymphocyte-to-monocyte ratio.
Prognostic factors influencing relapse-free/overall survival
The associations between relapse-free survival and various clinicopathological factors are presented in Table III. According to a univariate analysis, there were significant correlations between relapse-free survival and tumor diameter, histological type, lymphatic involvement, lymph node metastasis, preoperative CEA levels and the preoperative LMR (all P<0.05). Multivariate analysis indicated that only preoperative LMR was an independent risk factor for a poor relapse-free survival. The associations between overall survival and various clinicopathological factors are presented in Table IV. According to a univariate analysis, there were significant correlations between overall survival and both lymph node metastasis and preoperative LMR. In addition, a multivariate analysis indicated that lymph node metastasis and preoperative LMR were independent risk factors for poor overall survival.
Table III.
Associations between relapse-free survival and various clinicopathological factors.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factors | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value |
| Age (>70 years vs. ≤70 years) | 1.433 | 0.764–2.685 | 0.262 | |||
| Gender (female vs. male) | 1.575 | 0.809–3.064 | 0.181 | |||
| Location of primary tumor (rectum vs. colon) | 1.545 | 0.824–2.985 | 0.175 | |||
| Tumor depth (T4 vs. T1-3) | 1.396 | 0.680–2.865 | 0.363 | |||
| Tumor diameter (>5 cm vs. ≤5 cm) | 2.421 | 1.256–4.669 | 0.008 | 1.259 | 0.597–2.655 | 0.546 |
| Histological type (poor, mucinous vs. well, moderately) | 2.777 | 1.222–6.308 | 0.015 | 1.607 | 0.678–3.805 | 0.281 |
| Lymphatic involvement (positive vs. negative) | 3.211 | 1.417–7.276 | 0.005 | 1.946 | 0.676–5.600 | 0.217 |
| Venous involvement (positive vs. negative) | 1.782 | 0.787–4.039 | 0.166 | |||
| Lymph node metastasis (positive vs. negative) | 3.362 | 1.775–6.366 | <0.001 | 2.041 | 0.904–4.606 | 0.086 |
| Preoperative CEA (>5 ng/ml vs. ≤5 ng/ml) | 2.590 | 1.369–4.899 | 0.003 | 1.923 | 0.913–4.054 | 0.086 |
| Preoperative LMR (≥4.8 vs. <4.8) | 2.657 | 1.403–5.033 | 0.003 | 2.051 | 1.028–4.090 | 0.041 |
CI, confidence interval; CEA, carcinoembryonic antigen; LMR, lymphocyte-to-monocyte ratio. Bold denotes a statistically significant result (P<0.05).
Table IV.
Correlations between overall survival and various clinicopathological factors.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factors | Hazard Ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Age (>70 years vs. ≤70 years) | 1.386 | 0.562–3.416 | 0.479 | |||
| Gender (female vs. male) | 1.713 | 0.651–4.508 | 0.275 | |||
| Location of primary tumor (rectum vs. colon) | 1.909 | 0.767–4.748 | 0.164 | |||
| Tumor depth (T4 vs. T1-3) | 0.689 | 0.201–2.365 | 0.553 | |||
| Tumor diameter (>5 cm vs. ≤5 cm) | 1.224 | 0.492–3.047 | 0.664 | |||
| Histological type (poor, mucinous vs. well, moderate) | 1.172 | 0.271–5.078 | 0.832 | |||
| Lymphatic involvement (positive vs. negative) | 2.379 | 0.790–7.169 | 0.124 | |||
| Venous involvement (positive vs. negative) | 2.663 | 0.959–7.396 | 0.060 | |||
| Lymph node metastasis (positive vs. negative) | 2.814 | 1.132–6.996 | 0.026 | 2.533 | 1.014–6.328 | 0.047 |
| Preoperative CEA (>5 ng/ml vs. ≤5 ng/ml) | 2.008 | 0.815–4.946 | 0.130 | |||
| The preoperative LMR (≥4.8 vs. <4.8) | 3.081 | 1.212–7.830 | 0.018 | 2.805 | 1.098–7.163 | 0.031 |
CI, confidence interval; CEA, carcinoembryonic antigen; LMR, lymphocyte-to-monocyte ratio. Bold denotes a statistically significant result (P<0.05).
Discussion
Inflammation and cancer are closely related. Inflammation is caused not only by the systemic reaction of the host to the tumor, but also by inflammatory cytokines and chemokines released by cancer cells, and tumor-associated leukocytes that cause tumor growth, invasion, metastasis and suppression of the host immune system (4,5,17,18). Thus, inflammation reflects cancer progression, and the significance of systemic inflammatory markers in predicting the survival of patients with CRC, as well as other malignancies, has previously been reported. LMR, which consists of the peripheral lymphocyte and monocyte counts, also reflects systemic inflammation.
Lymphocytes serve an important role in tumor suppression. Previous studies have demonstrated a correlation between lymphopenia and poor prognosis of patients with various types of cancer (15,19). Lymphocytes induce cytotoxic cell death and produce cytokines that inhibit cancer cell proliferation and metastatic activity (4,20,21). The absolute peripheral lymphocyte count is assumed to reflect the degree of responsiveness of the entire immune system of a patient (15,22). Therefore, a low peripheral lymphocyte count may result in a weak and insufficient immunological reaction to the tumor, thus promoting tumor progression and metastasis (23).
By contrast, monocytes play an important role in tumor progression (4,24). A correlation between monocytosis and poor prognosis in different types of cancer has previously been observed (16,25–27). Monocytes represent a source of chemokines and cytokines that contribute to inflammation (28). Inflammation in the cancer microenvironment promotes tumor progression and metastasis (29). Moreover, tumor-associated macrophages (TAMs), derived from circulating monocytes (30), cause migration, intravasation, tumor cell invasion, tumor-associated angiogenesis, and the suppression of the anti-tumor immune system (4,31–33). The absolute peripheral monocyte count reflects the formation and/or presence of TAMs (34), thus a high peripheral monocyte count is responsible for a high tumor burden. Therefore, low LMR reflects insufficient antitumor immunity and an elevated tumor burden, and is associated with poor patient prognosis.
In previous studies, both the lymphocyte and the monocyte count have been reported as prognostic factors for the survival of patients with various malignancies, and this was confirmed in the present study. Furthermore, the prognostic significance of LMR was investigated, and was demonstrated to be an accurate prognostic marker. Even in an analysis limited to patients with a normal lymphocyte count, the LMR identified patients with a poor prognosis. Therefore, the LMR is considered to be a more accurate prognostic marker than the lymphocyte count alone.
The cut-off value for the LMR used in the present study was different from that of previous studies. A cut-off value of 4.8 was set based on a ROC analysis, and was higher than cut-off values used in previous studies, which ranged between 2.14–4.19 (12–14,35). Thus, although LMR is a useful prognostic marker for various solid tumors, the optimum cut-off value for the LMR may differ according to the organ, stage or end point (such as disease-free survival, progression-free survival or overall survival).
There are several limitations associated with the present study: i) A relatively small number of patients were evaluated; ii) the study design was retrospective; iii) the results obtained were not validated in another population; iv) potential confounding factors, such as infection, ischemia or acute coronary disease, which may affect the white blood cell count, were not assessed; v) the optimum cut-off value for the preoperative LMR remains unknown, although 4.8 was set as the cut-off value using the results of a ROC analysis. Therefore, a large prospective study is required to confirm the findings of the current study.
In conclusion, preoperative LMR may be a useful prognostic marker in patients with CRC able to undergo potentially curative surgery, and assessing the LMR may enable more informed decisions regarding choice of therapeutic strategies to be made. It is quick and easy to obtain a peripheral blood cell count, therefore, measuring preoperative LMR may become a novel clinical biomarker for patients with CRC.
Acknowledgements
The authors thank Brian Quinn, who provided medical writing services on behalf of JMC, Ltd.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC Cancer Staging Manual. 7th. Springer; New York: 2010. pp. 237–246. [Google Scholar]
- 3.Sobin LH, Gospodarowicz MK, Wittekind C. UICC. TNM Classification of Malignant Tumors. 7. Wiley-Liss; New York: 2009. [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Kimura K, Amano R, Kubo N, Tanaka H, et al. Elevated preoperative serum C-reactive protein levels are associated with poor survival in patients with colorectal cancer. Hepatogastroenterology. 2014;61:2236–2240. [PubMed] [Google Scholar]
- 7.Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K. A high preoperative neutrophil- to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–3294. [PubMed] [Google Scholar]
- 8.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 9.Maeda K, Shibutani M, Otani H, Nagahara H, Sugano K, Ikeya T, Amano R, Kimura K, Sakurai K, Kubo N, et al. Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer Res. 2013;33:5567–5573. [PubMed] [Google Scholar]
- 10.Li YL, Pan YY, Jiao Y, Ning J, Fan YG, Zhai ZM. Peripheral blood lymphocyte/monocyte ratio predicts outcome for patients with diffuse large B cell lymphoma after standard first-line regimens. Ann Hematol. 2014;93:617–626. doi: 10.1007/s00277-013-1916-9. [DOI] [PubMed] [Google Scholar]
- 11.Porrata LF, Ristow K, Habermann TM, Witzig TE, Colgan JP, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski GS, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in nodular lymphocyte-predominant Hodgkin lymphoma. Br J Haematol. 2012;157:321–330. doi: 10.1111/j.1365-2141.2012.09067.x. [DOI] [PubMed] [Google Scholar]
- 12.Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Feng JF. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2015;8:137–145. doi: 10.2147/OTT.S73794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: Based on a large cohort study. PLoS One. 2014;9:e108062. doi: 10.1371/journal.pone.0108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cézé N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305–1313. doi: 10.1007/s00280-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755–764. doi: 10.1016/j.surg.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 18.Guthrie GJ, Roxburgh CS, Horgan PG, McMillan DC. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev. 2013;39:89–96. doi: 10.1016/j.ctrv.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terzié J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 21.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2010;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:64. doi: 10.1186/1471-2407-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 24.Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J. 2013;27:3017–3029. doi: 10.1096/fj.12-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Tominaga M, Okunaga R, Shibata K, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11:596–602. doi: 10.1007/s11605-007-0140-0. [DOI] [PubMed] [Google Scholar]
- 26.Haruki K, Shiba H, Fujiwara Y, Furukawa K, Wakiyama S, Ogawa M, Ishida Y, Misawa T, Yanaga K. Perioperative change in peripheral blood monocyte count may predict prognosis in patients with colorectal liver metastasis after hepatic resection. J Surg Oncol. 2012;106:31–35. doi: 10.1002/jso.23033. [DOI] [PubMed] [Google Scholar]
- 27.Lee YY, Choi CH, Sung CO, Do IG, Huh S, Song T, Kim MK, Kim HJ, Kim TJ, Lee JW, et al. Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: Comparison with SCC-Ag level. Gynecol Oncol. 2012;124:92–97. doi: 10.1016/j.ygyno.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM, Kusmartsev S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–1119. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 29.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 31.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 32.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Lievense LA, Bezemer K, Aerts JG, Hegmans JP. Tumor-associated macrophages in thoracic malignancies. Lung Cancer. 2013;80:256–262. doi: 10.1016/j.lungcan.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Friesenbichler J, Trajanoski S, Stojakovic T, Eberhard K, Leithner A, Pichler M. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer. 2014;135:362–370. doi: 10.1002/ijc.28677. [DOI] [PubMed] [Google Scholar]
- 35.Go SI, Kim RB, Song HN, Kang MH, Lee US, Choi HJ, Lee SJ, Cho YJ, Jeong YY, Kim HC, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014;31:323. doi: 10.1007/s12032-014-0323-y. [DOI] [PubMed] [Google Scholar]


