Abstract
The interleukin (IL)-17/IL-17 receptor (IL-17R) complex has been shown to be important for the regulation of inflammation; however, its role in the regulation of tumor processes has recently emerged as a research focus. The present study demonstrated that oxaliplatin was able to increase the levels of IL-17/IL-17R in hepatocellular carcinoma (HCC) patients and cells lines, and that it had important roles in reducing the susceptibility of the cells to oxaliplatin-induced apoptosis. Furthermore, the expression of autophagy-related proteins was induced by IL-17/IL-17R and autophagy was shown to induce resistance to oxaliplatin in HCC. In addition, the janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway was shown to be an important pathway in the induction of autophagy in response to oxaliplatin. Autopjhagy was inhibited by 3-methyladenine and JAK2/STAT3 signaling was blocked by AG490, which induced apoptosis in SMMC7721 cells treated with oxaliplatin. The results of the present study may help to elucidate the mechanism underlying the role of IL-17/IL-17R-induced autophagy in the chemoresistance of HCC, as well as help to establish and develop measures to overcome chemoresistance in HCC.
Keywords: hepatocellular carcinoma, interleukin-17/interleukin-17 receptor, autophagy, janus kinase 2/signal transducer and activator of transcription 3
Introduction
Hepatocellular carcinoma (HCC) is a major malignancy worldwide and its incidence is increasing annually; it is the second most common cause of cancer-associated mortality (1). The majority of patients have a low survival rate as a result of locally advanced or metastatic diseases, and surgery is feasible for only a small percentage of patients with HCC. Therefore, chemotherapy is the optimal therapeutic strategy for inoperable HCC (2). Oxaliplatin has been widely used in chemotherapy to reduce tumor recurrence and prolong survival in patients with HCC because of its fewer side effects compared with other platinum drugs (3). However, chemoresistance to oxaliplatin in the form of suppressed HCC apoptosis is commonly observed (4).
Interleukin-17 (IL-17) is predominantly secreted by interleukin-17-producing T-helper (Th17) cells, which participate in the progression and pathogenesis of inflammatory diseases (3). The IL-17 receptor (IL-17R) is expressed on the surface of numerous cells, including macrophages, dendritic cells, epithelial cells, fibroblasts and T lymphocytes (5,6). Previous studies reported that IL-17-producing cells accumulated in tumors (7,8), and that patients with malignant serum effusions (9) or multiple myeloma (10) showed significantly higher serum levels of IL-17. Furthermore, patients with persistently higher levels of IL-17 demonstrated the requirement for longer courses of chemotherapy, since these patients comprised a significant proportion of all cases of recurrence (11). Typically, IL-17 does not engage with Toll/IL-1 receptor (TIR) domain-containing adaptors, such as MyD88, TIR domain-containing adapter protein inducing interferon-β or IL-1 receptor-associated kinases (12). Rather, IL-17 signals through nuclear factor (NF)-κB (13), mitogen-activated protein kinase (MAPK) (14) and phosphoinositide 3-kinase (PI3K) (14) signaling pathways. The janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway plays an important role in regulating a number of pathways associated with tumorigenesis, including cell cycle progression, apoptosis and tumor cell evasion of the immune system (15,16). In a previous study, phosphorylation of STAT3 was markedly increased as early as 3 h following IL-17 treatment and lasted for 24 h (17), which indicated that JAK2/STAT3 signaling may have important roles in tumor progression associated with IL-17.
Autophagy involves lysosomal-mediated degradation of cellular organelles and has been closely related to tumor occurrence and progression (18). A previous study reported that resistance to oxaliplatin in HepG2 cells could be recovered by inhibition of autophagy (19), which suggested opportunities for exploitation of autophagy as a therapeutic target in cancer.
Although the association between IL-17 and tumour chemotherapy has been previously investigated (20), the underlying mechanism remains unclear. Therefore, the present study aimed to elucidate the role of IL-17/IL-17R-induced autophagy in the resistance of HCC cells to oxaliplatin, and to determine the potential underlying mechanism.
Materials and methods
Patient samples and tissue processing
A series of HCC specimens were obtained from 30 patients with pathologically confirmed HCC at the Affiliated Hospital of North China University of Science and Technology (Tangshan, China). No patients received adjuvant chemotherapy, radiotherapy or surgery prior to admission. All patients were administered one course (2 weeks) of oxaliplatin, after which the concentration of IL-17 in sera and IL-17R mRNA levels were detected. In addition, matched normal hepatic tissues were obtained from 28 patients who were admitted to hospital due to wounds obtained in a fall or traffic accident. Peripheral blood samples (3 ml) were collected from all patients. HCC biopsy specimens for the detection of IL-17R mRNA expression levels by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were collected via paracentesis per cutem prior to and following oxaliplatin treatment or surgical resection. Serum IL-17 levels were determined using an ELISA. The present study was approved by the Institutional Review Board of North China University of Science and Technology. Informed consent was obtained from all patients prior to specimen collection.
Cell lines and culture conditions
The human SMMC-7721, L02 and HepG2 cells lines were maintained at 37°C in a humidified atmosphere containing 5% CO2 in high-glucose Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 units/ml penicillin and 100 mg/ml streptomycin.
ELISA assays
Serum levels of IL-17 (pg/ml) were measured using a solid phase sandwich ELISA assay according to the manufacturer's protocols (R&D Systems, Inc., Minneapolis, MN, USA).
RNA isolation and RT-qPCR
To examine IL-17R expression in HCC patients prior to and following oxaliplatin therapy, IL-17R mRNA expression levels in the tumor tissues were compared with matched normal tissues by RT-qPCR. Total RNA was extracted and reverse transcribed using an RNeasy kit (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA), according to the manufacturer's protocol. RNA (1 µg), along with 10X DNase I reaction buffer and 1 µg DNase I RNase-free was transferred to a 1.5 ml tube where the volume was adjusted to 10 µl using RNase-free water. After incubating for 30 min at 37°C, the DNase I was inactivated by the addition of 1 µl 25 mM EDTA. The mixture was subsequently heated for 10 min at 65°C. qPCR was performed in 20 µl reaction volumes containing 2.0 µl cDNA, 0.4 µl of each primer, 6.0 µl ddH2O, 0.4 µl ROX reference dye and 10 µl fluorescent SYBR Green (Takara Bio, Inc., Otsu, Japan). Amplification was performed in 96-well optical plates on a 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 30 sec incubation at 95°C, followed by 45 cycles of 95°C for 5 sec and 60°C for 60 sec. The primers used were as follows: IL-17R forward, 5′-CACTCACTCTACGCAACCTTAA-3′, reverse, 5′-AGATGCCCGTGATGAACC-3′; and GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-ATGGTGGTGAAGACGCCAGT-3′.
Each sample was analyzed in triplicate
The 2−ΔΔCq method of relative quantification was performed to calculate relative changes in the mRNA expression levels of target genes.
Cytokine inhibitor treatment
Cells were cultured in 6-well plates and treated with 20 µg/ml Oxa in the presence or absence of an anti-IL-17R antibody (10 µg/ml) or IL-17 (200 ng/ml) for 18 h. The apoptosis-related proteins BCL-2 and BAX were measured by western blotting. LY294002 (Beyotime Institute of Biotechnology, Haimen, China), a PI3K-specific inhibitor, AG490 (Beyotime Institute of Biotechnology), a JAK2 inhibitor, and 3-MA (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), an inhibitor of autophagy, were dissolved in dimethyl sulfoxide prior to use. These inhibitors were added to the culture medium 1 h prior to oxaliplatin treatment, with AG490 added at a dose of 15 µg/ml and LY294002 at 7.5 µg/ml. Cells were treated with 20 µg/ml oxaliplatin for 18 h. No cell cytotoxicity of these inhibitors, as assessed using a nuclear dye exclusion assay (21), was observed at the doses used in this study (data not shown).
Western blotting
Following treatment of the cells with oxaliplatin (20 µg/ml), the cells were lysed in whole-cell lysate (Wuhan Boster Biological Technology, Ltd., Wuhan, China) containing phenylmethylsulfonyl fluoride and a phosphatase inhibitor. Equal quantities of cell lysate (60 µg) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. After blocking in 5% evaporated milk for 1 h at 37°C, the membranes were incubated with the following primary antibodies: Anti-IL-17R (#D1Y4C), anti-B-cell lymphoma (BCL)-2 (#D55G8), anti-BCL-2-associated X protein (BAX; #D2E11), anti-microtubule-associated protein 1 light chain 3β (LC3B; #D11), anti-JAK2 (#D2E12), anti-phosphorylated (p)-JAK2 (#D15E2), anti-STAT3 (#D3Z2G), anti-p-STAT3 (#6E4) (all 1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-Beclin-1 (dilution, 1:500; #B6061; Sigma-Aldrich; Merck Millipore). GAPDH was used as a loading control and was detected using an anti-GAPDH antibody (dilution, 1:5,000; #AP0066; Bioworld Technology, Inc., St. Louis Park, MN, USA). The membrane was incubated for 1 h at 37°C with goat anti-mouse immunoglobulin (Ig)G (dilution, 1:10,000; #ab6785) and goat anti-rabbit IgG (dilution, 1:10,000; #ab6721) (Abcam, Cambridge, UK). After washing the membrane for 45 min with cleaning solution, proteins were detected using an enhanced chemiluminescence system and graphs were analyzed using Image Lab software v2.5.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each experiment was repeated three times.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Graphs were analyzed using the Image Lab system. Data are expressed as the mean ± standard deviation of the values from three independent experiments. Statistical analyses were conducted using either the Student's t-test or one-way analysis of variance in comparison with corresponding controls. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of IL-17 and IL-17R in HCC patients
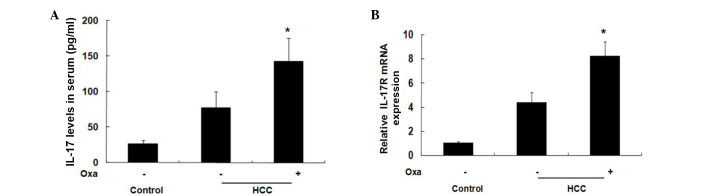
IL-17 has a role in numerous autoimmune and inflammatory conditions, including rheumatoid arthritis, multiple sclerosis, psoriasis, Crohn's disease and systemic lupus erythematosus, through combining with IL-17R (22). However, evidence has shown that IL-17 may also contribute to disease progression and treatment response in patients with tumors (23). In the present study, the levels of IL-17 were significantly increased in oxaliplatin-treated HCC patients, as compared with untreated HCC patients. Prior to treatment, the serum levels of IL-17 were 77.36±22.90 pg/ml, but were significantly increased up to 142.41±33.25 pg/ml after one course of treatment (2 weeks) (P<0.05; Fig. 1A). Furthermore, the mRNA expression levels of IL-17R in oxaliplatin-treated HCC biopsy specimens were significantly higher compared with untreated HCC biopsy specimens (P<0.05; Fig. 1B). These results suggest that oxaliplatin increases the expression of IL-17/IL-17R in patients with HCC, and that there is an association between oxaliplatin-induced apoptosis and IL-17/IL-17R.
Figure 1.
IL-17 and IL-17R expression in HCC patients. (A) Serum samples from 30 HCC patients prior to and following Oxa treatment, as well as 30 samples from healthy controls, were assayed using an IL-17 ELISA. (B) Tissues samples from 30 HCC patients prior to and following Oxa treatment, as well as 30 samples from controls, were assayed by reverse transcription-quantitative polymerase chain reaction. *P<0.05 vs. Oxa-untreated group. IL-17, interleukin-17; IL-17R, IL-17 receptor; HCC, hepatocellular carcinoma; Oxa, oxaliplatin.
Oxaliplatin induces the expression of IL-17R in HCC cells
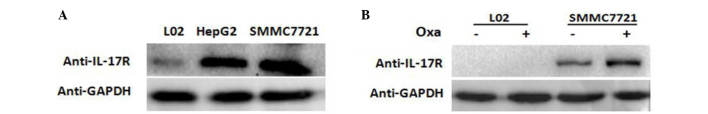
L02, HepG2 and SMMC-7721 cell lines were cultured in the presence of oxaliplatin and western blot analysis was performed to measure the amount of IL-17R protein in each cell line. The results showed that IL-17R was expressed in HepG2 and SMMC-7721 cells, but not in L02 cells (Fig. 2A).
Figure 2.
Expression of IL-17R in hepatocarcinoma cells. (A) IL-17R expression L02, HepG2 and SMMC-7721 cells was detected by western blot analysis. (B) After culturing L02 and SMMC-7721 cells for 18 h in the presence of 20 µg/ml Oxa, the expression of IL-17R was measured by western blotting. GAPDH was used as an internal control. IL-17R, interleukin-17 receptor; Oxa, oxaliplatin.
In order to confirm the changes in IL-17R expression following oxaliplatin treatment of SMMC-7721 cells, oxaliplatin (20 µg/ml) was added to SMMC-7721 and L02 cells for 18 h. Western blotting showed that the expression of IL-17R increased markedly in oxaliplatin-treated SMMC-7721 cells compared with the untreated cells (Fig. 2B). These results suggest that IL-17 is expressed in HCC cells and is increased following oxaliplatin treatment.
IL-17/IL-17R inhibits oxaliplatin-induced apoptosis of SMMC-7721 cells
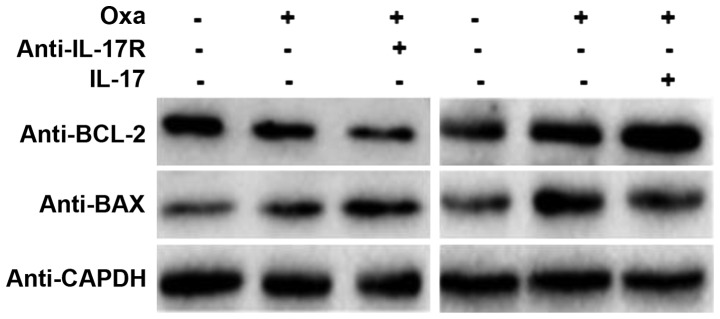
As shown in Fig. 2, IL-17R was expressed in SMMC-7721 cells. Furthermore, expression of IL-17R in SMMC-7721 cells increased markedly following oxaliplatin treatment. However, the role of IL-17R oxaliplatin-induced in these cells is unknown. As a mechanism for programmed cell death, apoptosis is regulated by the BCL-2 family proteins, including BAX, which control the sensitivity of cells to apoptotic stresses. BCL-2 is an anti-apoptosis gene, whereas BAX is an apoptosis-promoting matrix gene (24). In the present study, IL-17R-blocking and IL-17-promoting assays were designed to observe the effect of IL-17/IL-17R on oxaliplatin-induced apoptosis. Western blotting showed that BCL-2 protein expression was decreased and BAX protein expression was increased in oxaliplatin-treated SMMC-7721 cells, as compared with the untreated cells, which indicated that apoptosis was induced by oxaliplatin treatment (Fig. 3). When the IL-17R on the surface of SMMC-7721 cells was blocked by a neutralizing anti-IL-17R antibody (10 µg/ml; #MAB177; R&D Systems, Inc.), the expression of BCL-2 was decreased and that of BAX was increased (Fig. 3). Conversely, the expression of BCL-2 was increased and that of BAX was decreased in IL-17-promoting SMMC7721 cells. These results suggest that IL-17/IL-17R may inhibit HCC apoptosis and that blocking IL-17R is able to upregulate the susceptibility of HCC cells to oxaliplatin-induced apoptosis.
Figure 3.
Effect of IL-17/IL-17R on the apoptosis of SMMC-7721 cells. SMMC-7721 cells were treated with 20 µg/ml Oxa in the presence or absence of an anti-IL-17R antibody (10 µg/ml) or IL-17 (200 ng/ml) for 18 h. The apoptosis-related proteins BCL-2 and BAX were measured by western blotting. GAPDH was used as an internal control. Oxa, oxaliplatin; IL-17, interleukin-17; IL-17R, IL-17 receptor; BCL-2, B-cell lymphoma-2; BAX, BCL-2-associated X protein.
IL-17/IL-17R induces autophagy in SMMC-7721 cells
Increasingly, studies have focused on the effect of autophagy on tumor progression (25,26). Autophagy is a reversible process that regulates tumor survival or death; thus, it is closely associated with tumor progression (27). Furthermore, autophagy during chemotherapy has been shown to induce chemoresistance (28), while sensitivity to chemotherapy was increased when autophagy was inhibited (29,30).
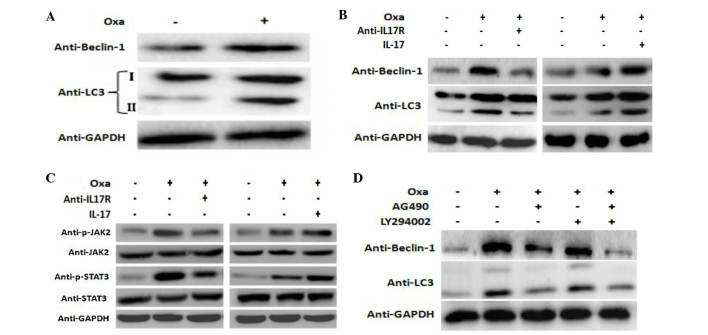
It is well known that decreased susceptibility to apoptosis during chemotherapy is the main mechanism of chemoresistance (31,32). LC3 is a specific marker of autophagosomes in mammalian cells, and the conversion of the soluble form of LC3 (LC3-I) to the autophagosome-associated form (LC3-II) is a characteristic of autophagy (33–35). Furthermore, Beclin-1 is another important autophagy gene. In the present study, Beclin-1 and LC3-II expression in SMMC-7721 cells was increased following treatment with oxaliplatin (Fig. 4A). In addition, the expression of Beclin-1 and LC3-II was markedly attenuated following blocking of IL-17R with a neutralizing antibody, and was increased accordingly following exposure to IL-17 (Fig. 4B).
Figure 4.
IL-17/IL-17R induces autophagy via the JAK2/STAT3 signaling pathway. SMMC-7721 cells were treated with 20 µg/ml Oxa in the presence or absence of (A) anti-IL-17R (10 µg/ml) or (B) IL-17 (200 ng/ml) for 18 h. (C) The activation of JAK2 and STAT3 was detected by western blotting. (D) LY294002 (10 µg/ml) and AG490 (15 µg/ml) were added to the culture medium for 1 h prior to Oxa treatment. LC3 and Beclin-1 protein expression was determined by western blot analysis. GAPDH was used as an internal control. Oxa, oxaliplatin; IL-17, interleukin-17; IL-17R, IL-17 receptor; JAK2, janus kinase 2; STAT3, signal transducer and activator of transcription 3; LC3, microtubule-associated protein 1 light chain.
IL-17/IL-17R induces autophagy in SMMC-7721 cells through the JAK2/STAT3 signaling pathway
IL-17 mediates cellular activities through various signal transduction pathways, including NF-κB, MAPK and PI3K (14). The present study investigated the potential role of the JAK2/STAT3 signaling pathway in IL-17-mediated autophagy. In the present study, the levels of p-JAK2 and p-STAT3 were increased in oxaliplatin-stimulated cells compared with the control cells, while they were decreased following blocking of IL-17R. Conversely, treatment with IL-17 (Sigma-Aldrich; Merck Millipore) increased the levels of p-JAK2 and p-STAT3 in SMMC-7721 cells (Fig. 4C). These results suggest that IL-17/IL-17R mediate cellular responses through the JAK2/STAT3 signaling pathway.
In order to investigate the relationship between IL17/IL17R and JAK2/STAT3 and oxaliplatin-induced autophagy, blocking experiments were performed. AG490, which is a JAK inhibitor, is able to specifically block the activation of the JAK2/STAT3 signaling pathway, as tyrosine phosphorylation of STAT3 is dependent on JAK activity (36). Furthermore, since the PI3K pathway has a crucial role in autophagy (37–39), the PI3K signaling pathway was used as a control pathway to investigate the JAK2/STAT3 pathway. LY294002, which is reported to inhibit AKT activation in a dose-dependent manner (40), was used and compared with the effect of AG490. SMMC-7721 cells were incubated with AG490 (15 µg/ml) or LY294002 (10 µg/ml) for 1 h prior to oxaliplatin treatment, after which autophagy-related proteins were detected by western blotting. Notably, a marked decrease in the expression of both Beclin-1 and LC3 II proteins was observed in AG490-treated cells, while only a negligible change was observed in LY294002-treated cells (Fig. 4D). These results suggest that inhibition of the JAK2/STAT3 signaling pathway suppresses oxaliplatin-induced autophagy to a greater extent than inhibition of the PI3K pathway, which further indicates that the JAK2/STAT3 signaling pathway may have an important role in oxaliplatin-induced autophagy.
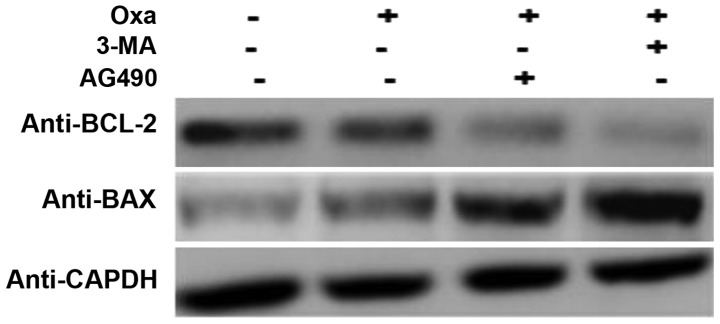
In order to further confirm the role of IL-17/IL-17R and the JAK2/STAT3 signaling pathway in the induction of autophagy and inhibition of apoptosis, SMMC-7721 cells were treated with 3-methyladenine (3-MA; 10 mM; Sigma-Aldrich; Merck Millipore), which is a known inhibitor of autophagy (41), and AG490 to inhibit the JAK2/STAT3 signaling pathway. Compared with the cells treated with oxaliplatin only, the AG490- and oxaliplatin-treated cells expressed less BCL-2 and more BAX, which indicated that apoptosis was induced to a greater extent in these cells. There was no difference in the expression of BCL-2 and BAX following treatment with AG490 and 3-MA (Fig. 5). These results suggest that autophagy in oxaliplatin-treated cells is induced through the JAK2/STAT3 signaling pathway.
Figure 5.
IL-17/IL-17R downregulate apoptosis by inducing autophagy. 3-MA (10 mM) and AG490 (15 µg/ml) were added to the culture medium for 1 h prior to Oxa treatment, after which SMMC-7721 cells were treated with 20 µg/ml Oxa for 18 h. BCL-2 and BAX protein expression was determined by western blot analysis. GAPDH was used as an internal control. Oxa, oxaliplatin; IL-17, interleukin-17; IL-17R, IL-17 receptor; BCL-2, B-cell lymphoma-2; BAX, BCL-2-associated X protein; 3-MA, 3-methyladenine.
Together, the results of the present study suggest that autophagy is activated in HCC cells following oxaliplatin treatment via IL17/IL17R-JAK2/STAT3, and that this may be involved in the chemoresistance of HCC to oxaliplatin.
Discussion
IL-17, which is the hallmark cytokine of the newly-defined Th17 cell subset, serves an important role in inflammatory diseases (42). Since chronic inflammation has been associated with tumor invasion, migration and metastasis (43), the significance of IL-17 in tumor progression has received increasing attention. The receptor of IL-17, IL-17R, is a common signaling subunit used by multiple ligands (44). Researches have begun to investigate the unusual functional motifs and novel proximal signaling mediators employed by the IL-17R family to mediate downstream events (7,45). There is evidence that IL-17 may emerge as a novel prognostic marker in HCC.
In the present study, it was demonstrated that autophagy downregulated oxaliplatin-induced HCC apoptosis through the IL-17/IL-17R-JAK2/STAT3 signaling pathway. IL-17 and IL-17R were markedly increased in oxaliplatin-treated HCC patients. Previous studies reported that IL-17 may exert pro-tumor or antitumor effects in various tumor contexts (46–48). The explanation for this discrepancy remains unknown. The present study aimed to determine the role that IL-17/IL-17R plays in the response of HCC to chemotherapy. Western blot analysis demonstrated that the expression of IL-17R was upregulated in HepG2 and SMMC-7721 cell lines, which was consistent with the report that IL-17-producing cells accumulate in various cancers, including HCC (8). In addition, IL-17/IL-17R expression was shown to increase in oxaliplatin-treated SMMC-7721 cells, which was associated with downregulation of apoptosis, as demonstrated by the detection of BCL-2 and BAX expression by western blotting.
Previous studies have focused on the relationship between autophagy and tumors (49,50). The role of autophagy in tumors is complex, as it has been associated with both tumor suppression and therapeutic resistance in advanced tumors (51). Inhibition of autophagy acted synergistically with chemotherapy in a mouse model of lymphoma (52). Autophagy was considered a temporary survival mechanism through the interaction of autophagy-related Beclin-1 and anti-apoptotic BCL-2 (53); thus, autophagy may be induced upon chemotherapy as a survival mechanism. In the present study, the reduced sensitivity of HCC cells to apoptosis may have been related to the induction of autophagy. Therefore, the expression of autophagy-related proteins, including LC3B and Beclin-1, was detected in oxaliplatin-treated SMMC-7721 cells. It was determined that oxaliplatin was able to induce autophagy in HCC and that autophagy was dependent on IL-17/IL-17R. To delineate the potential mechanism underlying IL-17/IL-17R-induced autophagy, the activation status of JAK2 and STAT3 under the interference of IL-17/IL-17R was observed. It was found that IL-17/IL-17R increased the phosphorylation of JAK2 and STAT3, which was consistent with the fact that STAT3 controls Th17 cell differentiation (54). Previous studies have shown that the PI3K signaling pathway has a crucial role in autophagy (55–57). Therefore, to confirm the roles of the PI3K and/or the JAK2/STAT3 signaling pathway in autophagy activation in oxaliplatin-treated HCC cells, SMMC-7721 cells were pre-treated with both LY294002 and AG490 to block PI3K and JAK2/STAT3. According to the western blot result, autophagy was inhibited to a greater extent in AG490-treated cells, which suggested that the JAK2/STAT3 signaling pathway was predominantly involved. 3-MA is a specific autophagy inhibitor, and pre-treatment of SMMC-7721 cells with 3-MA induced apoptosis to a greater extent than treatment with oxaliplatin alone by increasing BAX and decreasing BCL-2 expression. Similarly, cells treated with AG490 expressed less BCL-2 and more BAX, thus confirming that autophagy was induced by the JAK2/STAT3 signaling pathway.
Consistent with the results of the present study, Huang et al (58) reported that a JAK inhibitor was able to effectively suppress IL-17A-induced gene expression in human bronchial epithelial cells. Sun et al (59) suggested that STAT3 signaling was a key pathway that mediates immune suppression in the tumor microenvironment, and that aberrantly activated STAT3 in HCC cells resulted in the upregulation of cytokines, including IL-17. Cross-Knorr et al (60) showed that oxaliplatin was able to enhance the apoptosis of cancer cells by disrupting survival signaling via the JAK/STAT pathway at the receptor level in stage II colon cancer patients. Together, these findings suggested the importance of the JAK2/STAT3 signaling in the regulation of IL-17 signaling.
Previous reports have also indicated that pharmacological inhibitors of JAKs are able to limit IL-17 signaling (61), although these results should be interpreted with caution because of the non-specific effects of such compounds (62). Furthermore, IL-17-induced activation of STAT factors, which could promote cytokines secretion (including IL-6), have not been satisfactorily disproved (7). Therefore, whether IL-17/IL-17R mediates oxaliplatin-induced autophagy directly or through other pathways requires further analysis.
In conclusion, the present study demonstrated that autophagy inhibited oxaliplatin-induced HCC apoptosis via the IL-17/IL-17R-JAK2/STAT3 signaling pathway. These results suggested that blocking IL-17/IL-17R may be considered a novel therapy for chemoresistant HCCs.
Acknowledgements
This study was supported by the Natural Science Foundation of Hebei Province (grant no. 2016209007).
References
- 1.Zhao Y, Wang Q, Deng X, Shi P, Wang Z. Quantitative assessment of the association between GSTP1 gene Ile105Val polymorphism and susceptibility to hepatocellular carcinoma. Tumor Biol. 2013;34:2121–2126. doi: 10.1007/s13277-013-0695-1. [DOI] [PubMed] [Google Scholar]
- 2.Louaf S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, Asnacios A, Hannoun L, Poynard T, Taïeb J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): Results of a phase II study. Cancer. 2007;109:1384–1390. doi: 10.1002/cncr.22532. [DOI] [PubMed] [Google Scholar]
- 3.Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396–403. doi: 10.1111/j.1365-2893.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 4.Du H, Yang W, Chen L, Shi M, Seewoo V, Wang J, Lin A, Liu Z, Qiu W. Role of autophagy in resistance to oxaliplatin in hepatocellular carcinoma cells. Oncol Rep. 2012;27:143–150. doi: 10.3892/or.2011.1464. [DOI] [PubMed] [Google Scholar]
- 5.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: Implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23:613–619. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, et al. Contribution of IL-17-producing gamma delta T cells to the ecacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.201002692084c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett. 2012;148:144–150. doi: 10.1016/j.imlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Lemancewicz D, Bolkun L, Jablonska E, Czeczuga-Semeniuk E, Kostur A, Kloczko J, Dzieciol J. The role of interleukin-17A and interleukin-17E in multiple myeloma patients. Med Sci Monit. 2012;18:BR54–BR59. doi: 10.12659/MSM.882204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Droeser RA, Güth U, Eppenberger-Castori S, Stadlmann S, Hirt C, Terracciano L, Singer G. High IL-17-positive tumor immune cell infiltretion is indicative for chemosensitivity of ovarian carcinoma. J Cancer Res Clin Oncol. 2013;139:1295–1302. doi: 10.1007/s00432-013-1441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Nat Acad Sci USA. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korn T. Th17 cells: Effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaffen SL. Structure and signaling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okinaga T, Ariyoshi W, Akifusa S. Essential role of JAK/STAT pathway in the induction of cell cycle arrest in macrophages infected with periodontopathic bacterium Aggregatibacter actinomycetemcomitans. Med Microbiol Immunol. 2013;202:167–74. doi: 10.1007/s00430-012-0282-x. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Jiao B, Yao M, Shi X, Zheng Z, Li S, Chen L. ISG12a inhibits HCV replication and potentiates the anti-HCV activity of IFN-α through activation of the Jak/STAT signaling pathway independent of autophagy and apoptosis. Virus Res. 2016;10:S0168–S01702. doi: 10.1016/j.virusres.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, An Y, Wang Y, Zhang C, Zhang H, Huang C, Jiang H, Wang X, Li X. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2013;29:2019–2024. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
- 19.Harris SJ, Ciuclan L, Finan PM, Wymann MP, Walker C, Westwick J, Ward SG, Thomas MJ. Genetic ablation of PI3Kγ results in defective IL-17RA signalling in T lymphocytes and increased IL-17 levels. Eur J Immunol. 2012;42:3394–3404. doi: 10.1002/eji.201242463. [DOI] [PubMed] [Google Scholar]
- 20.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 21.Fortmüller K, Alt K, Gierschner D, Wolf P, Baum V, Freudenberg N, Wetterauer U, Elsässer-Beile U, Bühler P. Effective targeting of prostate cancer by lymphocytes redirected by a PSMAxCD3 bispecific single-chain diabody. Prostate. 2011;71:588–596. doi: 10.1002/pros.21274. [DOI] [PubMed] [Google Scholar]
- 22.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: A TGF-β-dependent immunosuppressive activity? Trends Mol Med. 2012;18:742–749. doi: 10.1016/j.molmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Anilkumar U, Prehn JHM. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front Cell Neurosci. 2014;30:281. doi: 10.3389/fncel.2014.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Lv SX. The effect of JAK2 knockout on inhibition of liver tumor growth by inducing apoptosis, autophagy and anti-proliferation via STATs and PI3K/AKT signaling pathways. Biomed Pharmacother. 2016;84:1202–1212. doi: 10.1016/j.biopha.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 26.Tan YQ, Zhang J, Zhou G. Autophagy and its implication in human oral diseases. Autophagy. 2016;20:0. doi: 10.1080/15548627.2016.1234563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J, Gong Z, Shen HM. The role of autophagy in liver cancer: Molecular mechanisms and potential therapeutic targets. Biochim Biophys Acta. 2013;1836:15–26. doi: 10.1016/j.bbcan.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2011;28:105–111. doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- 29.Golden EB, Cho HY, Jahanian A, Hofman FM, Louie SG, Schönthal AH, Chen TC. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus. 2014;37:E12. doi: 10.3171/2014.9.FOCUS14504. [DOI] [PubMed] [Google Scholar]
- 30.Wu W, Li W, Zhou Y, Zhang C. Inhibition of beclin1 affects the chemotherapeutic sensitivity of osteosarcoma. Int J Clin Exp Pathol. 2014;7:7114–7122. [PMC free article] [PubMed] [Google Scholar]
- 31.Moore N, Houghton J, Lyle S. Slow-cycling therapy-resistant cancer cells. Stem Cells Dev. 2012;21:1822–1830. doi: 10.1089/scd.2011.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jungwirth U, Xanthos DN, Gojo J, Bytzek AK, Körner W, Heffeter P, Abramkin SA, Jakupec MA, Hartinger CG, Windberger U, et al. Anticancer activity of methyl-substituted oxaliplatin analogs. Mol Pharmacol. 2012;81:719–728. doi: 10.1124/mol.111.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12:226. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou YY, Li Y, Jiang WQ, Zhou LF. MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep. 2015;35:e0199. doi: 10.1042/BSR20140141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan L, Wei S, Wang J, Liu X. Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. J Agric Food Chem. 2014;62:5390–5400. doi: 10.1021/jf500903g. [DOI] [PubMed] [Google Scholar]
- 38.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A, Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX, Ye WC. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 2013;34:1331–1342. doi: 10.1093/carcin/bgt060. [DOI] [PubMed] [Google Scholar]
- 40.Bai R, Ding T, Zhao J, Liu S, Zhang L, Lan X, Yu Y, Yin L. The effect of PI3K inhibitor LY294002 and gemcitabine hydrochloride combined with ionizing radiation on the formation of vasculogenic mimicry of Panc-1 cells in vitro and in vivo. Neoplasma. 2016;63:80–92. doi: 10.4149/neo_2016_010. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Yang D, Wang W, Piao S, Zhou J, Saiyin W, Zheng C, Sun H, Li Y. Inhibition of autophagy by 3-MA enhances IL-24- induced apoptosis in human oral squamous cell carcinoma cells. J Exp Clin Cancer Res. 2015;34:97. doi: 10.1186/s13046-015-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada H. Th17 cells in human rheumatoid arthritis. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:249–255. doi: 10.2177/jsci.32.249. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 43.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novatchkova M, Leibbrandt A, Werzowa J, Neubüser A, Eisenhaberemail F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 45.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Weng W, Xu W, Wang Y, Yu W, Tang X, Ma L, Pan Q, Wang J, Sun F. Prognostic significance of interleukin 17 in cancer: A meta-analysis. Int J Clin Exp Med. 2014;7:3258–3269. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hambright HG, Ghosh R. Autophagy: In the cROSshairs of cancer. Biochem Pharmacol. doi: 10.1016/j.bcp.2016.10.006. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marin JJ, Lozano E, Perez MJ. Lack of mitochondrial DNA impairs chemical hypoxia-induced autophagy in liver tumor cells through ROS-AMPK-ULK1 signaling dysregulation independently of HIF-1α. Free Radic Biol Med. 2016;101:71–84. doi: 10.1016/j.freeradbiomed.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Weiland A, Roswall P, Hatzihristidis TC, Pietras K, Ostman A, Strell C. Fibroblast-dependependent regulation of the stem cell properties of cancer cells. Neoplasma. 2012;59:719–727. doi: 10.4149/neo_2012_091. [DOI] [PubMed] [Google Scholar]
- 52.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan J, Zhang Y, Sheng Y, Fu X, Cheng H, Zhou R. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy. 2015;11:1081–1098. doi: 10.1080/15548627.2015.1040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Y, Li Z, Yin Y, Lan H, Wang J, Zhao J, Feng J, Li Y, Zhang W. Ghrelin inhibits the differentiation of T helper 17 cells through mTOR/STAT3 signaling pathway. PLoS One. 2015;10:e0117081. doi: 10.1371/journal.pone.0117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li P, Shi J, He Q, Hu Q, Wang YY, Zhang LJ, Chan WT, Chen WX. Streptococcus pneumoniae Induces autophagy through the inhibition of the PI3K-I/Akt/mTOR pathway and ROS hypergeneration in A549 cells. PLoS One. 2015;10:e0122753. doi: 10.1371/journal.pone.0122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aveleira CA, Botelho M, Carmo-Silva S, Pascoal JF, Ferreira-Marques M, Nóbrega C, Cortes L, Valero J, Sousa-Ferreira L, Álvaro AR, et al. Neuropeptide Y stimulates autophagy in hypothalamic neurons. Proc Natl Acad Sci USA. 2015;112:E1642–E1651. doi: 10.1073/pnas.1416609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang P, Guo QS, Wang ZW, Qian HX. HBx induces HepG-2 cells autophagy through PI3K/Akt-mTOR pathway. Mol Cell Biochem. 2013;372:161–168. doi: 10.1007/s11010-012-1457-x. [DOI] [PubMed] [Google Scholar]
- 58.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-κappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 59.Sun X, Zhang J, Hou Z, Han Q, Zhang C, Tian Z. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. Cell Cycle. 2015;14:243–252. doi: 10.4161/15384101.2014.977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cross-Knorr S, Lu S, Perez K, Guevara S, Brilliant K, Pisano C, Quesenberry PJ, Resnick MB, Chatterjee D. RKIP phosphorylation and STAT3 activation is inhibited by oxaliplatin and camptothecin and are associated with poor prognosis in stage II colon cancer patients. BMC Cancer. 2013;13:463. doi: 10.1186/1471-2407-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, Kim HY. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7:R139–R148. doi: 10.1186/ar1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:51. doi: 10.1186/1756-9966-29-51. [DOI] [PMC free article] [PubMed] [Google Scholar]